Chronic obstructive pulmonary disease (COPD) and lung cancer screening
Introduction
Based on the results of the National Lung Screening Trial (NLST) (1), showing a 20% reduction in lung cancer mortality, annual computed tomography (CT) is now widely recommended in the USA (2,3). Eligibility for funded lung cancer screening is now based primarily on the inclusion criteria of the NLST, although this was not the original intent of the study. It is widely accepted that for high risk smokers (>55 years old and >30 pack years exposure), the benefits of CT screening are linearly related to their underlying risk of lung cancer (4,5). However, we have recently challenged this assumption (6). Post-hoc analysis of the results of the NLST indicates that the presence of chronic obstructive pulmonary disease (COPD) has a major effect on outcomes in screening (7). This review outlines just how the presence of COPD affects outcomes in CT-based lung cancer screening and why it is critical to view overall outcomes of screening, not just the risk of lung cancer, as the basis on which to best assess both the harms and benefits of screening (8). Comments made in this review are supported by results from analyzing data from the NLST, specifically a post-hoc analysis of data collected in the American College of Radiology Imaging Network (ACRIN) subgroup, where they had the foresight to undertake baseline pulmonary function testing and blood sampling for biomarker analysis (9).
Defining the presence of COPD in the NLST
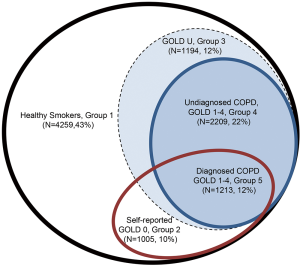
In the NLST-ACRIN sub-study of 18,674 NLST participants, we have shown that in this screening population approximately 35% have airflow limitation based on pre-bronchodilator spirometry (10). Consistent with many other cohort studies (11), for 70% of those with airflow limitation, their “COPD” was unrecognized and therefore not previously diagnosed. Conversely, we have found that about 50% of those who reported having COPD, chronic bronchitis, emphysema or adult asthma, no airflow limitation was evident on pulmonary function testing (Figure 1). While this group report symptoms consistent with “airways disease”, their pulmonary function testing did not meet spirometric criteria for airflow limitation based on Global Initiative for Obstructive Lung Disease (GOLD) where a ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC) of less than 0.70 is required (12). This group of “at risk smokers”, with symptomatic airways disease but no airflow limitation, have been previously defined as having GOLD 0 and represented 10% of the NLST-ACRIN cohort (13,14) (Figure 1). While studies show smokers with GOLD 0 are not at greater risk of developing COPD, they do have a poorer quality of life and higher all-cause mortality than those with normal lung function and no airways disease (13). Another subgroup of smokers to be defined by spirometry measurements are those with a reduced FEV1% predicted but preserved FEV1/FVC ratio (15). This group most likely reflects one or a combination of, mixed airways disease (combined obstructive and restrictive pattern), obese patients where reduced chest wall expansion limits FVC (restrictive alone), and those with mild COPD but for whom their FVC was sub-optimally performed, falsely elevating their FEV1/FVC ratio into the normal range (over 0.70). These “restrictive” groups have been collectively defined as GOLD Undefined (GOLD U) and are characterised by an elevated body mass index and higher prevalence of diabetes (15). In Figure 1, we show the breakdown of these groups in the NLST-ACRIN biomarker sub-study where both spirometry and blood for DNA studies were collected (10). As we outline below, the presence of airflow limitation (COPD GOLD 1–4 criteria, 34%) or COPD-related phenotypes (GOLD 0 or GOLD U, 10% and 12% respectively), has important implications in both the development of lung cancer and the utility of CT-based lung cancer screening.
While the presence of emphysema remains a strong risk predictor for future lung cancer (16-18), this review will not discuss the relevance of emphysema, or its severity, in lung cancer for several reasons. First, there exists little agreement on how to score the presence of emphysema, grade its severity and just what constitutes “normal” aging in contrast to smoking-related changes (16-18). Furthermore, there remains debate as to whether semi-quantitative or quantitative measures of emphysema severity are most reproducible and reflective of an increased lung cancer risk. In the NLST, the presence of emphysema on imaging [CT and chest X-ray (CXR) arms] was recorded as “yes” or “no”, making comparative analysis and meaningful interpretation difficult. Second, there are no age-adjusted normal values to measure the severity of emphysema. Third and most importantly, we remain concerned that the relationship between CT-based emphysema severity and risk of lung cancer has been shown to be non-linear thus limiting its utility in assigning risk of lung cancer relative to other risk variables for lung cancer (19).
Relationship between airflow limitation and risk of lung cancer
Numerous studies have shown that the presence of airflow limitation confers between a 2–6-fold increase in the risk of lung cancer depending on the study design and definition of COPD (20-23). Notably, spirometry-defined COPD confers a greater risk of lung cancer relative to the risk associated with self-reported COPD, particularly when compared to “healthy smokers” confirmed with spirometry, and matched for age and smoking history (19,21). We have shown that between 15–30% of randomly selected smokers over 40 years old have underlying COPD based on spirometry, whereas 60–85% of unscreened lung cancer case series have features of COPD, whether based on spirometry alone or combined with image-based emphysema (22-25). In a large prospective study by Mannino and colleagues, a clear linear dose-response relationship was found between increasing severity of airflow limitation and risk of lung cancer (21). In addition, this study showed that a restrictive pattern was also independently associated with an increased risk of lung cancer. We have replicated this finding showing that with increasing severity of airflow limitation, the risk of lung cancer increased in the NLST-ACRIN sub-study (Figure 2) (19). Indeed, we also replicated the findings of a 1977 study by Burrows and colleagues showing that a reduced FEV% predicted conferred a greater risk than that of age and pack years (20). The increase in lung cancer risk associated with a reduction in FEV1%predicted occurs somewhere between 80–90% of predicted, indicating the increase in risk for lung cancer occurs well before symptoms of airflow limitation are clinically evident or detected (19,23). This has relevance to the current interest in assessing the risk of lung cancer in high risk smokers eligible for CT-based lung cancer screening (26-28), yet spirometric screening of asymptomatic smokers for the presence of airflow limitation is not recommended. The currently accepted paradigm is that those at greatest risk of lung cancer get the most benefit from lung cancer screening because more lung cancer cases are identified in this group compared to those at lower risk (29,30). Without spirometry, this risk-based approach to lung cancer screening completely overlooks the relevance of pre-existing COPD in those developing lung cancer (5,26). Compared to those with “normal lungs”, we have shown in a post-hoc analysis of the NLST-ACRIN sub-study that those with COPD have both a greater risk of dying of lung cancer and a greater likelihood of dying of a non-lung cancer complication of smoking (6). These include dying of cardiovascular disease, respiratory disease and other cancers (see Figure 3), which represents the “competing cause of death” effect (discussed further below).
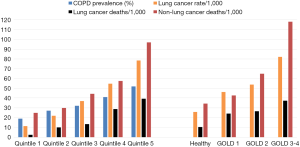
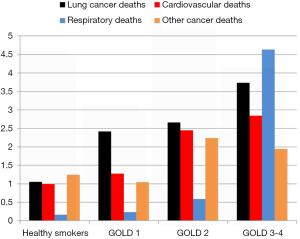
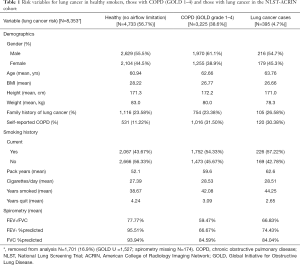
A second aspect that is poorly understood is the relationship between risk of lung cancer and the likelihood of having airflow limitation (31). While the lung cancer risk model (PLCOm2012) developed by Tammemagi and colleagues from the Prostate, Lung, Colorectal and Ovarian Cancer Screening study (PLCO) has been validated in the NLST, its predictive utility is considerably lessened in the NLST participants relative to the PLCO (5,30). This is because in the NLST, where only older and more heavily exposed smokers were recruited, the importance of the age and smoking exposure variables in the model was attenuated. This is due in part to the observation that for both duration of smoking (>30 years), and smoking exposure (>30 pack years), the dose relationship with risk of lung cancer flattens (29,30). What is more important is that as the risk of lung cancer increases using the PLCOm2012 model, we have found the likelihood of having COPD increases in a linear fashion (Figure 2) (31). Indeed in a receiver-operator-curve analysis of the NLST-ACRIN subgroup we found the PLCOm2012 model was only marginally less predictive of identifying who had COPD (airflow limitation on spirometry), as who developed lung cancer (area-under-the-curve of 0.65 and 0.67 respectively). This raises the interesting proposition that COPD objectively ascertained using spirometry, in contrast to self-reported disease as is used in current clinical models, would add to the predictive utility of defining smokers at greatest risk of lung cancer (24-26). Indeed Tammemagi and colleagues have shown in a Canadian study that adding spirometry improves the clinical risk model for lung cancer (27). That the Brock PLCOm2012 model for lung cancer risk also predicts the presence of COPD is not surprising as age, pack years, low BMI and self-reported COPD are also risk factors for having COPD (32) (Table 1). This is critical to the issue of competing cause of death where airflow limitation has an important impact on factors affecting outcomes of lung cancer screening, in particular all-cause mortality and operability (6,33). We note that with increasing airflow limitation (worsening COPD), increase in lung cancer mortality is accompanied by an increase in cardiovascular deaths, respiratory deaths and death from other cancers (Figure 3). In moderate COPD (GOLD 2), the increase in risk of lung cancer is associated with an increased risk in cardiovascular deaths and deaths from other cancers. For severe and very severe COPD (GOLD 3–4), there is also a substantial increase in respiratory deaths (Figure 3). As we outline below, there are many factors that affect surviving from lung cancer and we suggest that COPD represents the single most important co-morbid disease in this setting. By using a risk-based approach to optimize screening, such as a minimum risk-based cut-off for screening [≥1.51% 6-year risk of lung cancer based on the PLCOm2012 model (28)], the attenuating benefit of CT screening those at greatest risk goes unrecognized (Figure 4). The following sections examine cause-specific mortality in an NLST subset that encompasses a total of 699 deaths (18% of all NLST deaths) over the 6–7 years of follow-up in the NLST. Lung cancer accounted for 189 deaths (27% of total deaths), where we calculate 17 fewer lung cancer deaths in the CT arm versus CXR (17/189). As this represents only 9% of all lung cancer deaths, we do not believe screening substantially alters our observations pertaining to comorbidity and the competing cause of death effect.
Full table
Airflow limitation and competing cause of death effect
In an ideal world, screening for lung cancer should save lives by reducing all- cause mortality, not just reduce lung cancer mortality (34). In the full NLST results, while lung cancer mortality in the CT arm was reduced by 17–20% relative to that in the CXR arm, mortality from respiratory disease was also reduced by 22% in the CT arm (1). However, overall mortality reduction was only 6–7% in the CT arm indicating the reduction in deaths from lung cancer were attenuated by non-lung cancer related deaths. A competing cause of death effect is present when there is a “failure to achieve improved life expectancy by preventing death from one disease (in this case lung cancer) due to death from another cause” (35-37). This is highly relevant to CT-based screening for lung cancer because lung cancer accounted for only 24% of all deaths in the NLST while comparable mortality was observed for cardiovascular disease (25%), other cancers (22%) and less for respiratory deaths (10%) (1). This is highly relevant because all-cause mortality during CT screening is comparatively high, relative to other screening programmes. Those eligible for lung cancer screening are older current or former smokers with a high pack year burden, with over 50% of screening participants having some “respiratory impairment” (Figure 1). We show that in the NLST-ACRIN sub-group analyses, 43% had normal lung function, 10% have GOLD 0, 12% have GOLD U, 22% have undiagnosed COPD and 12% have diagnosed COPD. This means over 50% of those in the NLST-ACRIN sub-study had risk factors for premature mortality in strong contrast to breast or colon cancer screening where co-morbid disease is much less frequent (38,39) (Table 2). We suggest that smoking is an important feature of this greater mortality because the observed prevalence of comorbid disease was greater in the screening participants of the NLST (≥30 pack years) relative to those in the PLCO study which included never smokers of a similar age range to that in the NLST (40). Indeed, we show that several comorbid diseases are many fold more prevalent in populations at high risk of lung cancer (i.e., NLST) including chronic lung disease (4–5 folds), diabetes (2–3 folds) and heart disease (2–4 folds), relative to populations at risk of breast or colon cancer (Table 2). This means comorbid disease in lung cancer screening participants, especially COPD, may play a large role in non-lung cancer related deaths that may underlie overtreatment.

Full table
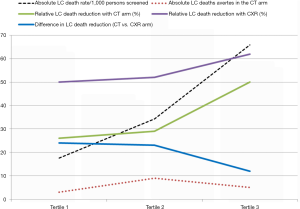
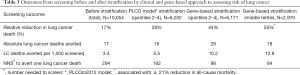
We have shown that regardless of whether the NLST-ACRIN sub-study participants are stratified by the severity of airflow limitation (GOLD grade), or risk of lung cancer according to the PLCOm2012 risk model (Figures 2,3), the mortality from non-lung cancer causes increases at a greater rate than for lung cancer. This difference in mortality, observed in Figure 2, is reflected by a divergence in mortality rate according to worsening COPD or increasing lung cancer risk. We suggest that this increase in non-lung cancer deaths relative to lung cancer deaths, is relevant to outcomes from CT screening because the non- lung cancer deaths offset the benefits of the screening intervention (i.e., non-lung cancer deaths attenuate the reduction in lung cancer deaths). Although the lung cancer incidence is greatest in these high risk smokers, the reduction in lung cancer mortality with CT screening relative to CXR is reduced due to deaths from non-lung cancer causes (Figure 4). Therefore in these highest risk groups overtreatment occurs reducing the efficiency of CT-based screening (41,42). This is particularly the case when absolute reductions in lung cancer deaths are analysed according to the number of smokers screened (lung cancer deaths averted/1,000 persons screened, Figure 4). We suggest that this competing cause of death effect has a major influence on reducing the utility of CT screening in those at greatest risk of lung cancer. Given the accepted strategy that excluding smokers at low risk achieves greater screening utility (or efficiency) (5,28), we propose excluding those at highest risk due to the competing cause of death effect is equally valid. We find that limiting screening to those of intermediate risk achieves even greater benefit (Table 3). We have shown this effect, whether the intermediate risk group is defined by those in the middle tertile (comprising 33%) or middle 3 quintiles (comprising 60%) of those in the intermediate risk category. Indeed, based on our analysis of the NLST-ACRIN sub-study, an overall 17% reduction in lung cancer deaths with CT screening (3.4 lung cancer deaths averted/1,000 persons screened) increases to a 28% reduction in lung cancer mortality (5.5/1,000 persons screened) for the middle 2nd–4th quintiles (Table 3). This enrichment in reducing lung cancer deaths modestly improves efficiency and cost-effectiveness of lung cancer screening by excluding low risk smokers in the bottom quintile where lung cancer rates are low and excluding very high risk smokers where there is potential for overtreatment.
Full table
COPD and effects on histology and operability
Past epidemiological studies have shown that in unscreened lung cancer, COPD is associated with small cell and squamous cell histological subtypes (43). In the NLST-ACRIN study we confirmed this finding and also showed that COPD was associated with more non-small cell (undifferentiated) lung cancers (10). This may have a subtle effect on outcomes. We have recently shown using a meta- analytical approach that non-small cell cancer diagnosed in the unscreened setting has a comparable 5-year survival following surgery whether the lung cancer patient had COPD or no COPD, based on pre-surgical pulmonary function testing (44). We confirmed this finding in a post-hoc analysis of the NLST where 5-year survival was no different between those with and without COPD (44). One important finding from this study was that the more indolent adenocarcinomas (previously described as bronchioloalveolar cancer or BAC) were far more prevalent in those with no COPD (45). Studies that have measured both COPD and volume doubling time (VDT) of the lung cancer nodules show that patients with lung cancer and underlying COPD have much shorter VDT suggesting more aggressive cancers (45,46). The big difference in the prevalence of BAC lung cancers occurs in those with normal lung function and is almost exclusively a feature of CT screening, infrequently identified in those undergoing CXR screening (10). We suggest that CT screening identifies more lung cancers than CXR in part by identifying indolent forms of adenocarcinomas (formerly BAC), almost exclusively in stage 1–2, creating an “histology shift” effect that represents possible over-diagnosis (10). This apparent beneficial stage shift (favouring early stage over late stage), generated in part from the histology shift (excess BACs), artificially improves survival rates but does not reduce lung cancer mortality per se (8). We conclude that in screening participants with airflow limitation, there is little if any overdiagnosis with CT screening (10,26,32).
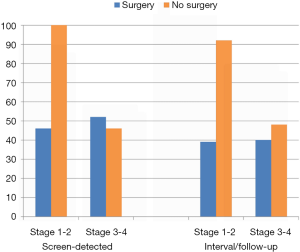
A second important feature of having COPD at the time lung cancer is identified during screening is its effect on operability (33). Lung cancer cases with significant comorbid disease are generally declined surgery. In the NLST-ACRIN substudy, we found COPD prevalence was 34% overall and 52% in lung cancer cases (GOLD 1 =10%, GOLD 2 =27% and GOLD 3–4 =14%) (10). It is noteworthy that the NLST protocol excluded patients who were oxygen-dependent. We found operability for stage 1–2 lung cancers in the NLST-ACRIN sub-study was consistent at about 90%, regardless of COPD grade (severity), for those cancers identified by the screening (defined as screen-detected lung cancers). The likelihood of operability was drastically reduced according to COPD status for those cancers identified during interval and follow-up periods (Figure 5). This indicates that operability is profoundly affected by the presence of COPD and that this may contribute to the reduced benefits of CT screening in this group. In this regard, when we stratified NLST-ACRIN screening participants according to the presence of airflow limitation, we found the % reduction in lung cancer mortality was 15% and 28% in those with and without COPD respectively (Table 4) (47). When we stratified COPD patients into those with undiagnosed COPD (N=2,209) and those with diagnosed COPD (N=1,213), we found that those with undiagnosed COPD had a two-fold greater reduction in lung cancer deaths compared to those with diagnosed COPD (32% vs. 17%, P<0.05) (Table 4). This finding and its implications will be described in more detail below.

Full table
Factors affecting outcomes of screening and reducing lung cancer deaths
We and others have emphasized that survival after lung cancer screening is not a reliable clinical endpoint from which to judge the success of screening (8). This is because survival after lung cancer screening not only suffers from lead-time bias but also completely ignores the important effects of over-diagnosis masquerading as a favourable stage shift (10,48). This is particularly the case in the prevalent (baseline) scan where over-diagnosis is greatest and high survival rates over-estimate the benefits of screening. Survival-based outcomes also fail to allow for overtreatment (41,42). Survival is greater in smokers who are at lower baseline risk of lung cancer due to younger age, lower pack years and less comorbid disease. This means that in single arm studies the net benefit of screening, where screen-detected lung cancers are followed without accounting for outcomes from non-screen detected lung cancer, may be misleading (8). This is the inherent benefit of randomised controlled trials where all lung cancers and their outcomes are considered.
As outlined above, the two outcomes best describing a benefit from CT screening come from lung cancer deaths averted per persons screened (absolute benefit) and percent reduction in lung cancer deaths (relative benefit) (8). From a cost- effective or efficiency perspective, the absolute reduction in lung cancer deaths averted per 1,000 persons screened provides a good basis for assessing outcomes (Table 2). Describing the number needed to screen to avert one lung cancer death provides another means of assessing the benefits of screening in the CT arm versus the CXR arm.
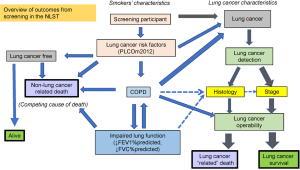
In a simple paradigm of lung cancer screening, it is too easy to view the success of CT-based screening as increasing the number (or proportion) of non-small cell lung cancers identified (i.e., excluding small cell), at an operable stage (stage 1 and 2), which will lead to improved survival by surgical removal. However, little consideration is given to factors undermining this such as co-morbid disease, inoperability and competing causes of death. This review has focused on these important features and shown how they relate to COPD and off-set the benefits of screening. In Figure 6, we show that there are many inter-related factors that can alter outcomes of CT screening that may serve to profoundly undermine the benefits of CT-based screening. We also suggest that the presence of COPD, based on spirometric assessment, is not only closely related to both inoperability and competing cause of death, but represents one of the most important comorbid conditions affecting outcomes from CT screening.
Several surprising findings have come from our analysis when we have stratified the screening population according to various COPD-related phenotypes (Figure 1 and Table 4). The first is the observation that a reduction in lung cancer mortality from CT is greatest in those with normal lung function and those with undiagnosed COPD (7) (Table 4). While there is a benefit in those with diagnosed COPD, where the severity of airflow limitation is marginally worse, the difference is two-fold better in the undiagnosed group and exceeds that seen in the whole group (Table 4). A second important finding is that screening those in the highest risk group is questionable because they have the highest rates of non-lung cancer deaths and a substantially reduced benefit from CT screening (Figures 3,4). We argue that those at highest risk of lung cancer also have the highest prevalence of COPD and deaths from cardiovascular disease, respiratory disease and other non-pulmonary cancers (Figure 3). In this regard it is interesting that with worsening airflow limitation, the increase in lung cancer related deaths is matched by an increase in cardiovascular deaths (Figure 3). Given comparable age and smoking exposure, this suggests that possible overlapping inflammatory pathways may be relevant in this context (49-51). In this setting it is of interest that in a recent clinical trial where interleukin 1β was inhibited, both cardiovascular events and lung cancer incidence were significantly reduced (52,53). This observation, together with numerous epidemiological studies showing elevated IL-6 levels are associated with COPD, lung cancer and cardiovascular deaths, points to overlapping pathways involving the innate immune system possibly underlying these complications of smoking (51).
Optimisation of screening outcomes by biomarker-based approach
Spirometry-based approach
Lung cancer screening is not like other forms of screening because, unlike screening for cancers of the breast, colon or cervix, the target population is at greater risk of premature death from common diseases whose risk is dramatically increased by smoking exposure. In this article, we show that the baseline prevalence of comorbid diseases such as COPD, cardiovascular disease and diabetes is high in participants eligible for lung cancer screening (Table 2). We also show that the presence of COPD in current or former smokers eligible for screening reflects a much greater propensity to die from cardiovascular disease, respiratory disease and non-pulmonary cancers (Figure 3). Using well validated risk models for lung cancer, it has been shown that among smokers eligible for CT screening in the USA, the risk varies by 15–30 folds (5,29). Moreover, we show these clinical-based risk models of lung cancer also predict the presence of COPD (Figure 2). Most importantly, we show that the benefits of screening with CT relative to CXR is strongly affected by the presence of COPD and related comorbid disease underlying premature death (Figure 4). Such an observation leads us to recommend routine use of spirometry, to assess the presence and severity of COPD, as part of the benefit to harm assessment, and shared-decision making, critical at the outset of screening. This is especially relevant as we show that those with undiagnosed COPD have a two-fold greater reduction in lung cancer deaths with CT compared to those with diagnosed COPD (Table 4). We conclude that the benefits from CT screening are not linearly related to the risk of developing lung cancer and that smokers at highest risk derive less benefit from screening than those in the intermediate level of risk. This is because as the risk of lung cancer increases, inoperability increases as does the risk of dying of a non-lung cancer death (Figure 2,6). The likelihood of surviving lung cancer in the context of screening involves a complex relationship between, the likelihood of identifying a lung cancer with annual CT screening amenable to curative surgery, rather than dying from an inoperable lung cancer or dying of another smoking-related complication. This is why 5- or 6-year lung cancer mortality reduction provides a useful measure of the success of screening. When considered in absolute terms, the maximum number of lung cancer deaths averted with CT screening is achieved in the intermediate risk group comprising those in the 2nd to 4th quintiles of clinical risk (Table 3). This indicates there exists a “sweet spot” for screening (54-56), where the balance between high risk of lung cancer is not offset by inoperability or competing causes of death.
Genetic-based approach
We have been able to further optimize the efficiency of screening by using a combination of genetic risk markers (single nucleotide polymorphisms) and clinical risk variables to better define both risk and outcomes from screening (57-59). These genetic markers are associated with both COPD and lung cancer related pathways reflecting their shared biology (56). Based on our analysis of the NLST-ACRIN sub-study, an overall 17% reduction in lung cancer deaths with CT screening was observed in the unstratified (untested) group equivalent to 3.4 lung cancer deaths averted/1,000 persons screened. This increases to a 44% reduction in lung cancer mortality (10.2/1,000 persons screened) in the middle 2nd–4th quintiles using a gene-based approach to risk and outperforms the PLCOm2012 clinical model (28% reduction in lung cancer deaths) (Table 3) (55). This increases further to a 55% reduction in lung cancer mortality (12.8/1,000 persons screened) for the middle tertile using our genetic approach (Table 3). Of note the all-cause mortality reduction is 21% in this gene-based middle tertile group, 3-fold greater than that reported for the whole study (6–7%) (1). We believe this enrichment effect using a gene-based approach occurs because the genetic markers better reflect lung cancer biology, and when combined with clinical variables of lung cancer (and COPD) risk, out-perform the clinical markers alone in regards to the outcomes from screening (Table 3). The use of biomarkers to enhance risk prediction (risk-based approach to selection) while useful, must also improve outcomes from screening (outcomes-based approach to selection) (59). This requires that any biomarker-based approach improves clinical decision making toward better outcomes for screening participants (more benefit and less harm) as illustrated by our gene-based approach (56,59). We therefore conclude that biomarkers, in combination with clinical variables, may have utility in assessing not only who is at greatest risk of developing lung cancer, but who gains the most from CT screening. This has important implications, not only in the decision on whom to screen, but who to defer screening (due to reduced benefit to harm ratio from low risk) and who to discontinue screening (due to reduced benefit from inoperability or competing mortality). Overtreatment is the unnecessary treatment of cancer where little benefit is achieved and in lung cancer screening, results from inoperability or competing cause of death where reduction in lung cancer mortality is sub-optimal. We suggest that a biomarker approach to screening requires validation in prospective screening studies such as the NLST, so that the benefits in the real-world setting can be shown (59).
Summary
The findings described above lead to us to number of conclusion about CT screening for lung cancer and how it might be improved in the future. First, the harms and benefits of screening should be considered in the context of which subgroups of eligible smokers benefit most from the screening process (outcomes based approach). The risk of lung cancer as measured by existing clinical models may help identify who is most likely to get lung cancer (risk- based approach), however this does not equate to who will get the most benefit from screening. We have shown that while the presence of airflow limitation is associated with greater risk of lung cancer, it also predicts a greater likelihood of more aggressive forms of lung cancer, greater lung cancer deaths, greater inoperability and greater likelihood of death by a competing cause. We believe there exists a “sweet spot” for screening where the lives saved by screening can be maximized and harms from overtreatment minimized. While identifying COPD through routine use of spirometry, or utilizing informative biomarkers, may increase the workload (and cost) of an already intensive screening programme, they add valuable knowledge about high risk smokers and their anticipated outcomes from CT screening. We conclude that COPD is a major contributor to the development, natural history, treatment and survival from lung cancer that has been all but ignored to date. We believe more judicious use of spirometry as part of a wider clinical assessment in CT-based screening for lung cancer, and biomarker-based clinical decision making, will make substantial improvements to future screening efforts for lung cancer. We suggest a biomarker-led outcomes-based approach may help to better define which eligible smokers could defer screening (low risk of lung cancer), discontinue screening (high risk of overtreatment with little benefit) or continue screening so that the likelihood of averting a lung cancer-related death is optimized.
Acknowledgements
We acknowledge the kind support of members of the American College of Radiology Imaging Network (ACRIN) for their contribution to our study, in particular Dr. Denise Aberle, Dr. Fenghai Duan and Dr. Caroline Chiles. We also acknowledge the support of Johnson and Johnson whose generous grant allowed us to undertake this study.
Footnote
Conflicts of Interest: RP Young is a founder, scientific advisor and shareholder of Synergenz BioScience Ltd that hold patents for gene-based risk testing for lung cancer and chronic obstructive pulmonary disease. RJ Hopkins has no conflicts of interest to declare.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung cancer mortality with low dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Int Med 2013;159:411-20. [Crossref] [PubMed]
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]
- Bach PB., Katten MW, Thornquist MD, et al. Variation in lung cancer risk among smokers. J Natl Cancer Inst 2003;95:470-8. [Crossref] [PubMed]
- Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung cancer death. N Engl J Med 2013;369:245-54. [Crossref] [PubMed]
- Hopkins RJ, Young RP, Duan F, et al. Lung Cancer Screening and the Effects of Competing Causes of Death in the ACRIN-NLST. Am J Respir Crit Care Med 2017;195:A5174.
- Hopkins RJ, Young RP, Duan F, et al. Airflow limitation and lung cancer risk in a large prospective screening study (NLST-ACRIN cohort analysis, N=18,714). Am J Respir Crit Care Med 2016;193:A6178.
- Young RP, Hopkins RJ. Measures of outcome in lung cancer screening: maximising the outcomes. J Thorac Dis 2016;8:E1317-20. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Baseline characteristics of participants in the randomized national lung screening trial. JNCI 2010;102:1771-9. [Crossref] [PubMed]
- Young RP, Duan F, Chiles C, et al. Airflow limitation and histology-shift in the National Lung Screening Trial: the NLST-ACRIN cohort study (N=18,714). Am J Respir Crit Care Med 2015;192:1060-7. [Crossref] [PubMed]
- Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ 2010;182:673-8. [Crossref] [PubMed]
- GOLD. Available online: www.goldcopd.org, last accessed November 15th, 2017.
- Vestbo J, Lange P. Can GOLD stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2002;166:329-32. [Crossref] [PubMed]
- Ekberg-Aronsson M, Pehrsson K, Nilsson JA, et al. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005;6:98. [Crossref] [PubMed]
- Wan ES, Hokanson JE, Murphy JR, et al. Clinical and radiographic predictors of GOLD-Unclassified smokers in the COPDGene study. Am J Respir Crit Care Med 2011;184:57-63. [Crossref] [PubMed]
- Maldonado F, Bartholmai BJ, Swensen SJ, et al. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest 2010;138:1295-302. [Crossref] [PubMed]
- Smith BM, Pinto L, Ezer N, et al. Emphysema detected on computed tomography and risk of lung cancer: A systematic review and meta-analysis. Lung Cancer 2012;77:58-63. [Crossref] [PubMed]
- Sanchez-Salcedo P, Wilson DO, de Torres JP, et al. Improving selection for lung cancer screening: the potential role of emphysema. Am J Respir Crit Care Med 2015;191:924-31. [Crossref] [PubMed]
- Hopkins RJ, Duan F, Chiles C, et al. Reduced Expiratory Flow Rates among Heavy Smokers Increases Lung Cancer Risk: Results from the National Lung Screening Trial-American College of Radiology Imaging Network Cohort. Ann Am Thorac Soc 2017;14:392-402. [Crossref] [PubMed]
- Burrows B, Knudson RJ, Cline MG, et al. Qualitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis 1977;115:195-205. [PubMed]
- Mannino DM, Aguayo SM, Petty TL, et al. Low Lung Function and Incident Lung Cancer in the United States. Data From the First National Health and Nutrition Examination Survey Follow-up. Arch Intern Med 2003;163:1475-80. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer independence of age, gender and smoking history. Eur Respir J 2009;34:380-6. [Crossref] [PubMed]
- Calabrò E, Randi G, La Vecchia C, et al. Lung function predicts lung cancer risk in smokers: a tool for targeting screening programmes. Eur Respir J 2010;35:146-51. [Crossref] [PubMed]
- de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low dose CT of the chest. Chest 2007;132:1932-8. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738-44. [Crossref] [PubMed]
- Young RP, Hopkins RJ. CT screening in COPD: Impact on lung cancer mortality. Respir Med 2014;108:813-4. [Crossref] [PubMed]
- Tammemagi MC, Lam SC, McWilliams AM, et al. Incremental Value of Pulmonary Function and Sputum DNA Image Cytometry in Lung Cancer Risk Prediction. Cancer Prev Res (Phila) 2011;4:552-61. [Crossref] [PubMed]
- Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers; screening rules applied to the PLCO and NLST cohorts. Plos Med 2014;11:e1001764. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Duan F, et al. The relationship between lung cancer risk according to the Brock PLCO2012 model and prevalence or presence of COPD in the NLST-ACRIN substudy (N=10,054). Am J Respir Crit Care Med 2018;197:A4411.
- Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med 2012;157:571-3. [Crossref] [PubMed]
- Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung Cancer Risk Prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Models and Validation. J Natl Cancer Inst 2011;103:1058-68. [Crossref] [PubMed]
- Young RP, Duan F, Hopkins RJ, et al. COPD-related risk factors in screen-detected lung cancer: preliminary results from a sub- analysis of the NLST. Am J Respir Crit Care Med 2013;187:A2345.
- Grose D, Devereux G, Brown L, et al. Variation in comorbidity and clinical management in patients newly diagnosed with lung cancer in four Scottish centres. J Thorac Oncol 2011;6:500-9. [Crossref] [PubMed]
- Prasad V, Lenzer J, Newman DH. Why screening has never been shown to ”save lives”- and what we can do about it. BMJ 2016;352:h6080. [Crossref] [PubMed]
- Mackenbach JP, Kunst AE, Lautenbach H, et al. Competing Causes of Death: A Death Certificate Study. J Clin Epidemiol 1997;50:1069-77. [Crossref] [PubMed]
- Mackenbach JP, Kunst AE, Lautenbach H, et al. Gains in life expectancy after elimination of major causes of death: revised estimates taking into account the effect of competing causes. J Epidemiol Community Health 1999;53:32-7. [Crossref] [PubMed]
- Janssen-Heijnen M. Comorbidity and competing causes of death in Lung cancer. IASLC Annual Meeting, 2013.
- Ording AG, Garne JP, Nystrom PMW, et al. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis – A Danish nationwide matched cohort study. Plos One 2013;8:e76013. [Crossref] [PubMed]
- Erichsen R, Horváth-Puhó E, Iversen LH, et al. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer 2013;109:2005-13. [Crossref] [PubMed]
- Lamerato LE, Marcus PM, Jacobsen G, et al. Recruitment in the Prostate, Long, Colorectal, and Ovarian (PLCO) Cancer Screening Trial: the first phase of recruitment at the Henry Ford Health System. Cancer Epidemiol Biomarkers Prev 2008;17:827-33. [Crossref] [PubMed]
- Ruano-Ravina A, Heleno B, Fernandez-Villar A. Lung cancer screening with low-dose CT (LDCT), or when a public health intervention is beyond the patient’s benefit. J Epidemiol Community Health 2015;69:99-100. [Crossref] [PubMed]
- Mazzone PJ. Obstacles to and Solutions for a successful Lung Cancer Screening program. Semin Respir Crit Care Med 2016;37:659-69. [Crossref] [PubMed]
- Papi A. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung cancer. Thorax 2004;59:679-81. [Crossref] [PubMed]
- Hopkins RJ, Ko J, Gamble GD, et al. Lung cancer surgery for early stage non-small cell lung cancer: systematic review of mortality according to the presence of COPD. Am J Respir Crit care Med 2017;195:A4887.
- Young RP, Hopkins RJ. Estimating Overdiagnosis of lung cancer. Ann Int Med 2013;158:635-6. [Crossref] [PubMed]
- Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: A cohort study. Ann Int Med 2012;157:776-84. [Crossref] [PubMed]
- Young RP, Duan F, Greco E, et al. Lung cancer-specific mortality reduction with CT screening: outcomes according to airflow limitation in the ACRIN- NLST study (N=18,475). Am J Respir Crit Care Med 2016;193:A6166.
- Black WC, Haggestrom DA, Welch HG. All-cause mortality in randomized trials of cancer screening. JNCI 2002;94:167-73. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Eaton TE. Forced expiratory volume in one second; not just a lung function test but a marker of premature death from all causes. Eur Respir J 2007;30:616-22. [Crossref] [PubMed]
- King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med 2015;4:68. [Crossref] [PubMed]
- Young RP, Hopkins RJ. The mevalonate pathway and innate immune hyper-responsiveness in the pathogenesis of COPD and lung cancer: potential for chemoprevention. Curr Mol Pharmacol 2017;10:46-59. [Crossref] [PubMed]
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-31. [Crossref] [PubMed]
- Ridker PM, MacFayden JG, Thuren T, et al. Effect of interleukin-1β inhibition with Canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833-42. [Crossref] [PubMed]
- Gould MK. Lung cancer screening in individuals with chronic obstructive pulmonary disease: Finding the sweet spot. Am J Respir Crit Care Med 2015;192:1027-8. [Crossref] [PubMed]
- Young RP, Duan F, Greco E, et al. SNP-based risk score out performs a clinical model for dying of lung cancer in the NLST-ACRIN sub-study (N=10,054). Am J Respir Crit Care Med 2017;195:A5127.
- Young RP, Hopkins RJ, Duan F, et al. Stratification of NLST-ACRIN screening participants identifies the “sweet spot” of screening by identifying early stage lung cancers most amenable to curative surgery (N=10,054). Am J Respir Crit Care Med 2017;195:A7656.
- Young RP, Hopkins RJ, Gamble GD. Clinical applications of gene-based risk prediction for lung cancer and the central role of chronic obstructive pulmonary disease. Front Genet 2012;3:210. [Crossref] [PubMed]
- Young RP, Hopkins RJ. Incorporating genomic data into multivariate risk models for lung cancer. Genet Med 2013;15:667-8. [Crossref] [PubMed]
- Mazzone PJ, Sears CR, Arenberg DA, et al. Evaluating molecular biomarkers for the early detection of lung cancer: When is a biomarker ready for clinical use? Am J Respir Crit Care Med 2017;196:e15-29. [Crossref] [PubMed]


