
Molecular testing strategies in non-small cell lung cancer: optimizing the diagnostic journey
Introduction
Lung cancer continues to be the leading cause of cancer death in the United States (US), with 209,703 new cases predicted in 2020 (1,2). Lung cancer is categorized as either small-cell lung cancer in approximately 10% to 15% of cases or non-small cell lung cancer (NSCLC) in approximately 80% to 85% of cases (2). Lung cancer is diagnosed at an advanced stage in 72% to 76% of patients in the UK and 57% in the US, precluding curative treatment, and is associated with poor prognosis (3,4).
Primary care providers, emergency medicine physicians, and pulmonologists are often the first point of contact for patients. From initial presentation, patients have a long journey that includes referral, clinical work-up, biopsy, molecular testing, formal diagnosis and treatments. There is an unmet need for strategies to improve efficiency in this process. One step in disease diagnosis where improvements can be made is molecular testing, used to identify specific molecular characteristics treatable with targeted therapies (5). Without appropriate molecular testing and corresponding results, empiric therapy will likely be initiated, which may be inappropriate and detrimental to patients with targetable mutations (6-8). Herein, we review the role of molecular testing in advanced NSCLC, along with current guidelines and recommended methodologies, and propose three strategies to optimize current practices.
NSCLC histology and the role of molecular testing
Among the three main histologic subtypes of NSCLC, lung adenocarcinoma (LUAD) is the most common, followed by lung squamous cell carcinoma (LUSC) and large cell carcinoma (9). Besides having different histologic bases, these subtypes have disparate clinical presentations and unique genetic profiles (9). Common genetic drivers in LUAD involve alterations in the epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) genes (9). Over 90% of the known activating EGFR mutations (EGFRm) are either short, in-frame deletions in exon 19 or an L858R point mutation in exon 21 (10). The pooled prevalence of EGFRm in exons 18, 19, 20, or 21 in patients with NSCLC (all subtypes) is 23.9% (95% CI: 21.3–26.5%) in the US (11). Approximately 5% of patients display a rearrangement in ALK, commonly presenting as an echinoderm microtubule-associated protein-like 4-ALK (EML4-ALK) fusion, resulting in the aberrant activation of downstream signaling targets (9,12,13). ROS proto-oncogene 1 (ROS1) rearrangements, leading to aberrant activation of downstream signaling, are observed in approximately 1% to 2% of patients with LUAD (9,14). B-Raf proto-oncogene (BRAF) mutations, the most common of which is BRAF V600E, leading to activation of the mitogen-activated protein kinase signaling pathway, are observed in approximately 2–4% of patients with LUAD (9).
The treatment paradigm for advanced NSCLC has evolved, and targeted therapy is now recommended if tumors contain certain molecular mutations (5,15,16). This precision oncology approach utilizes targeted therapies, including EGFR tyrosine kinase inhibitors (EGFR-TKIs), ALK inhibitors, ROS1 inhibitors, BRAF inhibitors, and immunotherapy [e.g., programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) inhibitors], over chemotherapy in the first-line setting.
Evidence from Phase III clinical trials supports the use of EGFR-TKIs for first-line treatment of advanced NSCLC in patients harboring EGFRm (17-25) (Table 1). First-line treatment with the first-generation TKI crizotinib significantly prolonged median progression-free survival (PFS) compared with platinum chemotherapy (10.9 vs. 7 months, respectively) in patients with ALK rearrangement-positive (ALK+) advanced NSCLC (26). Crizotinib is approved by the US Food and Drug Administration (FDA) for the treatment of ROS1-positive or ALK+ advanced NSCLC, with a median PFS of 9.1 to 19.2 months in Phase I and II trials (27,28). Additionally, the second-generation ALK inhibitors ceritinib, alectinib, and brigatinib are associated with improved outcomes compared with crizotinib or platinum-based chemotherapy, and are approved by the US FDA for first- and later-line treatment of ALK+ metastatic NSCLC (29-34). In November 2018, the third-generation ALK inhibitor lorlatinib was granted US FDA accelerated approval for patients with ALK+ metastatic NSCLC whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic NSCLC, or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic NSCLC (35,36). In treatment-naïve and pre-treated patients with BRAF V600E-mutant metastatic NSCLC, the combination of dabrafenib plus trametinib has shown overall response rates of 64% and 63.2%, respectively, and is the first treatment regimen approved by the US FDA for these patients (37-40).

Full table
In patients with advanced NSCLC and ≥50% tumor PD-L1 expression, first-line pembrolizumab monotherapy is more effective compared with platinum chemotherapy, with a median PFS of 10.3 vs. 6.0 months, respectively (41,42). However, the efficacy of first-line immunotherapy in patients with EGFRm or ALK+ NSCLC is not well understood, as patients with targetable mutations have historically been excluded from first-line immunotherapy trials. However, recently, a Phase II clinical trial of pembrolizumab monotherapy in TKI-naïve patients with EGFRm advanced NSCLC with tumor PD-L1 expression ≥1% was terminated early due to lack of efficacy, suggesting that pembrolizumab is not an appropriate therapy for this patient population (8). Moreover, evidence from the second- and third-line settings suggests that single-agent immunotherapy may not be the optimal treatment strategy for patients with EGFRm or ALK rearrangements, as they exhibit a lower objective response rate to PD-1/PD-L1 inhibitor treatment compared with patients with EGFRm-negative or ALK-negative/unknown NSCLC (3.6% vs. 23.3%, respectively) (43). Additionally, a meta-analysis reported no overall survival advantage with immunotherapy (nivolumab, pembrolizumab, or atezolizumab) vs. docetaxel in patients with EGFRm tumors (44).
The early identification of tumor genotype at the time of NSCLC diagnosis is critical so that the most efficacious therapy can be prescribed before considering other treatments. US FDA-approved companion diagnostic assays are available for targeted agents to enable the identification of relevant mutations prior to initiating therapy (45,46) (Table 2).
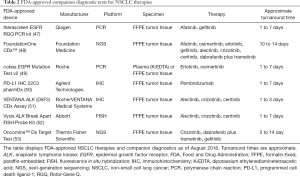
Full table
Molecular testing recommendations in advanced NSCLC
The College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP) state that mutation testing should be ordered at the time of diagnosis for patients with advanced NSCLC (5). Physicians can use molecular biomarker testing in LUAD; tumors with a non-squamous, non-small cell histology; or any non-small cell histology when clinical features indicate a higher probability of a targetable oncogenic driver [e.g., young age (<50 years) and light or absent tobacco exposure] (5,16). CAP/IASLC/AMP recommend that the “must-test” genes are EGFR, ALK, and ROS1, and, if adequate tissue is available, a second group of genes should be included in any expanded panel (Figure 1) (5). Pathology departments need to ensure that specimens with a final histopathologic diagnosis are sent to external molecular pathology laboratories within 3 working days of receiving requests, and within 24 hours to internal molecular pathology laboratories; it has been previously recommended that results should be available within 10 business days of receiving the specimen in the testing laboratory (5). Subsequently, the American Society of Clinical Oncology (ASCO) reaffirmed these recommendations but stated that stand-alone BRAF testing should be performed on all patients with advanced LUAD, irrespective of clinical characteristics (16). The National Comprehensive Cancer Network (NCCN®) recommends that all patients with advanced LUAD should be screened for EGFR, ALK, ROS1, and BRAF mutations, along with PD-L1 expression level (15). Additionally, NCCN strongly advises broader molecular profiling, with the goal of identifying rare driver mutations that include high-level MET proto-oncogene (MET) amplification or MET exon 14 skipping mutation, RET proto-oncogene (RET) rearrangements, and Erb-B2 receptor tyrosine kinase 2 (ERBB2) mutations for which effective drugs may already be available, or to appropriately counsel patients regarding the availability of clinical trials. They also included determination of tumor mutational burden (TMB) as an emerging biomarker that may be helpful for the selection of patients for immunotherapy; however, there currently remains no consensus on how to measure TMB (15).

The patient journey in real-world clinical practice
Real-world research published over the last decade suggests an urgent need to improve best practice in molecular testing in advanced NSCLC. In a single-institution study, only 21% of patients with advanced NSCLC referred from April 2010 to March 2013 had molecular testing results available at their initial oncology consultation, and 19% of patients received chemotherapy before molecular test results were available (6). In a study of 15 community oncology practices in New Jersey and Maryland (n=814) from January 2013 to December 2015, 41% of patients with advanced NSCLC were not tested for EGFRm and ALK, and only 8% of patients were tested for all gene mutations recommended by NCCN (7,15). In patients tested for EGFRm and ALK, the median turnaround time was 23 days instead of the previously recommended 10 days (7). In patients not tested for EGFRm and ALK, 52% received chemotherapy, and there was no documented reason for not testing these patients (7). Notably, median overall survival was lower for patients treated with chemotherapy, including those not tested for EGFRm and ALK, compared with patients treated with targeted therapy: 12.7 vs. 31.8 months, respectively (7). In a real-world analysis of 166 US community oncology practices from January 2014 to August 2015, only 41% and 65% of patients with EGFRm and ALK+ advanced NSCLC, respectively, were treated with targeted therapy when tested after initiation of first-line therapy, compared with 79% and 94% of patients, respectively, treated with targeted therapy when tested before initiation of first-line therapy (54).
Reflections on our clinical experience help put these research findings into context. A patient’s diagnostic journey often begins with a visit to a primary care provider who, after discovering an imaging abnormality, may make a referral to a thoracic surgeon, interventional radiologist, or pulmonologist. After biopsy establishes a histologic diagnosis of non-squamous NSCLC based on a basic hematoxylin and eosin (H&E) test with or without immunohistochemistry (IHC) results, the patient is referred to, and finally sees, an oncologist 1 to 4 weeks later. Only then is molecular testing ordered, the results of which may not be available for an additional 2 to 3 weeks. PD-L1 IHC results may be available 1 to 2 days after the biopsy, whereas molecular results arrive individually over several days using single-gene testing, or 10 to 14 days (or even longer) using comprehensive molecular profiling [e.g., next-generation sequencing (NGS)]. Inadequate staging at the time of referral to an oncologist can delay treatment planning, and, if molecular testing cannot be performed due to insufficient tissue, re-biopsy and re-testing cause further delays.
When counseling an anxious patient with NSCLC and incomplete pathologic data, the oncologist must either convince the patient to wait for further biopsy and/or testing to formulate the best treatment plan or embark on a treatment plan based on incomplete information, potentially subjecting a patient harboring genetic mutations to unnecessary treatment-related adverse events (AEs) and/or less-effective treatment (7,8,43,44). From our clinical experience, oncologists sometimes start empiric platinum-based chemotherapy pre-emptively while waiting for molecular test results, particularly if the patient is young, has a high symptom burden, and/or is psychologically distressed. Moreover, when oncologists receive PD-L1 IHC results earlier than molecular test results, immunotherapy is commenced with or without platinum-based chemotherapy. If molecular testing results do become available, patients are usually switched to the targeted treatment regimen, such as an EGFR-TKI, or, if there is a clinical response on the existing treatment, patients may continue to receive 4 to 6 cycles of platinum-based chemotherapy with or without immunotherapy.
Strategies for optimizing molecular testing in advanced NSCLC
We believe that molecular test results are needed much earlier during the diagnostic journey so that clinicians can deliver personalized care and maximize outcomes for patients with advanced NSCLC harboring targetable mutations. We offer three new strategies to help achieve this goal (Figure 2).

First, multidisciplinary team members, such as pulmonologists, interventional radiologists, and thoracic surgeons, could order molecular testing on tissue specimens as soon as there is a strong clinical suspicion of advanced NSCLC containing a LUAD component. This would need a standardized workflow to be successful, with education of the relevant clinicians, potentially through multidisciplinary molecular tumor boards (55). A similar strategy has been implemented with surgeon-initiated molecular testing in breast cancer, which resulted in the time between surgery and Oncotype DX® ordering, and the time between surgery and receipt of results, reduced by 7.3 and 6.3 days, respectively (56). Additionally, the mean number of days between surgery and initiation of chemotherapy was reduced by 6.4 days (56). In advanced NSCLC, this testing strategy, directed by the pulmonologist, interventional radiologist, or surgeon, could potentially reduce the time to receipt of molecular results, and, in many cases, these results would be available at the initial visit with the medical oncologist such that the treatment plan could be initiated at this first visit. Additionally, this strategy could mitigate the need for re-biopsy when LUAD is confirmed, thereby reducing the amount of tissue needed from the patient, saving time and resources.
Second, liquid biopsies, used to detect circulating tumor DNA (ctDNA), could be conducted early in the diagnostic pathway. Although tissue specimens remain the gold standard for tumor genotyping, ctDNA is detected in body fluids, including peripheral blood, making liquid biopsy a new option for molecular testing in advanced NSCLC (55). CAP/IASLC/AMP and ASCO recommend that when tissue is limited and/or insufficient for molecular testing, a ctDNA assay can be used to identify EGFRm (5,16). Additionally, NCCN recommend that a ctDNA assay can be used if the patient is medically unfit for invasive tissue sampling, although ctDNA testing should not be used in lieu of a tissue diagnosis (15). In the initial diagnostic setting, a ctDNA assay can be used if there is insufficient tissue for molecular testing following a pathological confirmation of an NSCLC diagnosis, only if follow-up tissue-based analysis is planned for all patients in which an oncogenic driver is not identified (15). A recent IASLC statement paper recommends that liquid biopsy can be considered at the time of initial diagnosis in all patients with advanced NSCLC who need tumor molecular profiling, particularly when tumor tissue is scarce, unavailable, or for patients in whom invasive procedures may be risky or contraindicated (55). It is also recommended that liquid biopsy be conducted at the time of initial diagnosis if the turnaround time for tissue biopsy is anticipated to be longer than 2 weeks (55). Following the CAP/IASLC/AMP recommendations and the recent IASLC statement paper on liquid biopsy, there is now more familiarity and clinical interest in liquid biopsy for NSCLC. Two commercial NGS-based liquid biopsies, FoundationOne™ Liquid (Foundation Medicine, MA, USA) and PGDx elio™ (Personal Genome Diagnostics, MD, US), were granted US FDA breakthrough device designation in April 2018 and July 2018, respectively (57,58). A third commercial NGS-based liquid biopsy, Guardant360® assay (Guardant Health, CA, USA), was granted US FDA expedited access pathway designation in February 2018 (59). All three of these assays include the genes recommended by NCCN, ASCO, and CAP/IASLC/AMP (5,15,16). However, it is important to note that NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) do not endorse specific testing modalities or techniques for biomarker tests (15).
The following case study from our institution demonstrates the utility of liquid biopsy. A 42-year-old Chinese male, never smoker, presented with a 2-month history of progressive dry cough, shortness of breath, and hemoptysis. He felt weak, had lost approximately 10 kg of weight over 6 months, and could not work. Physical examination showed supraclavicular lymphadenopathy. A computed tomography (CT) scan of the chest showed multiple lung masses, extensive lymphadenopathy, liver masses, and possible pancreatic head mass. The patient was referred from a family care physician to an oncologist; differential diagnosis included lymphoma, pancreatic or lung cancer. A positron emission tomography/CT scan showed extensive metabolic tumor burden, including multiple lung lesions; right hilar and mediastinal mass; pleural nodules; neck, left axillary, mediastinal, and retroperitoneal adenopathy; and multiple liver lesions. Brain magnetic resonance imaging did not show any metastatic disease. At the first oncology consultation, liquid biopsy and a fine-needle aspiration (FNA) of the supraclavicular lymph node were ordered. FNA was performed on day 3 with 5 passes of a 21-gauge needle, with 3 passes used for the tissue block. On day 7, FNA results showed metastatic adenocarcinoma of a lung origin, and the PD-L1 tumor proportion score was 20% to 25%. Molecular testing could not be performed, as the tissue block had been exhausted of tissue following extensive IHC staining to determine the tumor of origin and to rule out lymphoma. The patient had a rapid decline in his functional status, elevated liver function tests, anemia, and presented with sudden onset of bilateral lower leg pain and swelling. He was found to have acute deep vein thrombosis in bilateral lower extremities and was started with low molecular weight heparin. Owing to the acuity of the patient, combination therapy with carboplatin, pemetrexed, and pembrolizumab was planned. On day 9, a second FNA of the same supraclavicular lymph node for tumor genomic profiling was performed with 5 passes, with 4 passes used for the tissue block. On day 12, liquid biopsy results indicated an EML4-ALK fusion (variant 3a/b). The planned combination therapy was cancelled, and alectinib therapy commenced on day 15. Two weeks later, a tissue biopsy was received by an external laboratory for molecular testing, which confirmed the liquid biopsy results. The use of liquid biopsy at the time of the initial oncology consultation reduced the time to molecular test results, prevented treatment with inappropriate therapy, and allowed for targeted therapy to be commenced within 15 days of diagnosis. The patient had immediate symptomatic improvement, with normalized liver function and resolution of anemia within the first 2 weeks. He remains in near-complete remission at 15 months.
Advantages of liquid biopsy compared with tissue biopsy include being minimally invasive, having a shorter turnaround time for test results, and potentially lower overall health care costs (55). For liquid biopsy to be successful, it should have acceptable concordance with tissue biopsy, and rates of approximately 70% to 97% between ctDNA and tissue have been reported (55,60). Disadvantages include differences in sensitivity across testing platforms and variability of ctDNA in the plasma (55,61). Owing to tumor biology factors, such as absence of shedding into the plasma, treatment-naïve patients with slow-growing tumors may be at a heightened risk of false-negative ctDNA results, and a false-negative range of 3% to 57% has been reported (55,62-64). Moreover, in a recent study, we observed a significant association between tumor metabolic burden and the ability of an NGS-based liquid biopsy to detect gene mutations in patients with advanced solid tumors, suggesting that sufficient plasma ctDNA shed from metabolically active tumors is required for the successful detection of gene mutations in plasma ctDNA (65). Therefore, negative ctDNA results should be considered inconclusive and followed-up with tissue biopsy (5,15,16,55,63).
The final approach is that pathologists automatically order molecular testing immediately after histological diagnosis of LUAD in patients with advanced NSCLC, referred to as reflex testing. Unfortunately, however, patient consent is usually required for molecular/genetic testing, and there is the problem of who consents the patient in such a case. CAP/IASLC/AMP recommend that pathologist-directed reflex testing is reasonable, if establishment of a reflex testing program is an institutional decision and includes close communication between pathologists and oncologists (5). Reflex testing is standard practice with other solid tumor types: ASCO/CAP breast cancer guidelines state that reflex testing must be conducted when human EGFR 2 (HER2) IHC results are equivocal (66). Real-world advantages of reflex testing include an increase in the number of patients being tested for molecular targets such as EGFRm, reduction in turnaround time to treatment due to early availability of test results, and increased quality of biomarker testing, with fewer unsuccessful tests (67,68). It is therefore likely that widespread implementation of reflex testing in the NSCLC setting would ensure molecular results are available early, allowing for selection of the most appropriate therapy.
Sample collection and stewardship
When introducing molecular testing early in the diagnostic pathway, clinicians need to be aware of recommendations for optimal sample collection and stewardship. Clinical findings, imaging studies, the patient’s history, and H&E histologic testing are recommended [along with thyroid transcription factor-1 (TTF1), p40, and other IHC tests as needed] for diagnosis in patients with suspected lung cancer (15). However, an adequate amount of high-quality tissue needs to be available following pathological diagnosis to facilitate testing for the recommended genetic alterations (5,15,16). Further, it is important to accurately track samples from collection through testing, to ensure that there are no delays or errors in the diagnostic process that would negatively impact patient treatment (69).
When selecting the biopsy site to determine EGFR and ALK status for initial treatment, primary tumor tissue can be used as a surrogate for profiling of metastatic lesions (70). Cytology samples with adequate cellularity and preservation are appropriate for accurate diagnosis as well as for molecular testing (5,15,70). Biopsies of metastatic bone lesions are discouraged and, in many institutions, prohibited for molecular testing as certain decalcifying agents containing strong acids do not yield adequate DNA for molecular testing (15,70).
For tissue biopsy, multiple methods are used to collect tumor samples for diagnostic and biomarker testing (Table 3). CT-guided core needle biopsy is the preferred methodology for peripheral solitary pulmonary lesions. In our clinical practice, a 20- or 22-gauge cutting needle has been used, which can produce ≤15 unstained slides (≥4-µm cut slides). However, we currently recommend an 18-gauge cutting needle; an adequate core with this needle can produce ≤35 unstained slides (4-µm cut slides). The use of an 18-gauge cutting needle with two or more passes from a lesion is sufficient for molecular testing in >96% of diagnosed tissue samples, and while the use of a larger needle may cause more bleeding, the gauge of the needle and the number of cores are not associated with pneumothorax (71,72). Additional cores are preferred, and many institutions require ≥2 cores at the minimum, with ≥3 preferred. In cases with <5 cores, we recommend placing each core in its own cassette, and if >5 cores, place 2 cores in each cassette. This approach enables each core to be assessed independently and allows for tissue samples to be reserved for molecular testing or additional testing for clinical trials.
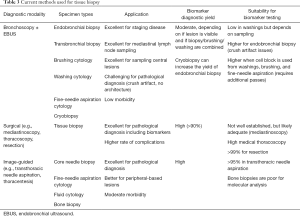
Full table
Peripheral pulmonary lesions, particularly when there is an air bronchogram present on chest CT, can reliably be diagnosed by navigation bronchoscopy with a yield of approximately 75%, and an adequacy for molecular analysis of 80% (73,74). In advanced disease, where there is often a large, central, endobronchial tumor visible on standard bronchoscopy, the yield of endobronchial biopsy with a cryoprobe is 95% to 100% (75). As large, undistorted specimens are obtained, the yield of molecular analysis is expected to be high; however, empirical research is needed to test this hypothesis.
In advanced NSCLC, mediastinal and hilar lymph node involvement is expected. In a recent meta-analysis, the pooled probability of endobronchial ultrasound-guided trans-bronchial needle aspiration (EBUS-TBNA) obtaining a sample sufficient for EGFRm and ALK rearrangement testing was 94.5% and 94.9%, respectively (76). Moreover, NGS was successful in 95.3% of EBUS-TBNA samples (77). While 2 to 3 EBUS-TBNA samples are normally obtained for molecular testing, a single EBUS-TBNA pass yields DNA of high quantity, quality, and accuracy for molecular profiling (78). True core biopsy samples cannot be obtained with standard EBUS-TBNA needles; however, using a three-point 22-gauge needle designed for gastrointestinal endoscopic ultrasound (EUS) is feasible, and does not increase the rate of procedure-related complications (79). While an EUS needle can be used with a standard EBUS bronchoscope, a 22-gauge core needle specifically designed for the EBUS bronchoscope would meet the demand for core lymph node biopsy specimens.
Rapid on-site evaluation (ROSE) is a methodology to assess the adequacy of the biopsy. With ROSE, biopsy material is evaluated immediately following sample collection for feedback regarding specimen adequacy for biomarker testing and potential diagnosis (80). Advantages of incorporating ROSE into the workflow include improved diagnostic yield, along with reductions in additional procedures, number of biopsy sites, and complication rates (81,82). However, the application of ROSE is institution dependent; it requires the availability of experienced on-site professionals, optimal clinician-pathologist communication, and optimal staining quality (83).
Following sample collection, pre-fixation time should be minimized, and formalin fixation is recommended for the biopsy specimen (15,70). The routinely used fixative is 10% neutral-buffered formalin, with recommended fixation times of 6 to 12 hours and 8 to 19 hours for small and large biopsy specimens, respectively (70). Pathologists should use formalin-fixed, paraffin-embedded specimens (FFPE) or fresh, frozen, or alcohol-fixed specimens for polymerase chain reaction (PCR)-based EGFRm testing; most ancillary tests are now validated and optimized for use on FFPE samples (70). For hybrid capture (HC)-based NGS, we recommend 10 unstained slides, 4- to 5-µm thick, with a surface area between 5 and 25 mm2, and ≥20% tumor nuclei. Macro- or micro-dissection is recommended to maximize tumor DNA content and reach the ≥20% threshold (70).
Malignant pleural effusion (MPE) is a unique source that is currently underutilized for molecular diagnosis. Currently, NGS is optimized for cases with ≥20% of the tumor cells present in the cell block from malignant pleural fluid (5,84). However, because there are often abundant inflammatory and mesothelial cells in MPE, the ≥20% threshold may not be achieved. Based on our experience, the yield of NGS on known MPE is ≤80%. Processing larger volumes of fluid or obtaining a second sample is unlikely to significantly improve yield; we argue that methods for enriching the percent of tumor cells and improving this yield should be explored.
Good judgment should be exercised in the use of IHC during pathological diagnosis to help preserve tissue that can be tested for the recommended genetic alterations (5,15,16). IHC may not be needed if samples are large enough for routine H&E; however, when used, IHC can provide a considerable amount of diagnostic information (15). In small specimens, IHC with one LUAD marker (e.g., TTF1 or napsin A) and one LUSC marker (e.g., p40 or p63) will suffice (15). TTF1 is expressed in 77% of patients with LUAD and is not expressed in LUSC, while p40 is highly expressed in LUSC and minimally expressed in LUAD (15,85).
For the collection of liquid biopsy samples, plasma should be used over serum as it has greater sensitivity (55,86,87). Following blood draw, plasma is obtained by centrifugation of the sample at 1,200 to 1,600 ×g for 10 minutes after which the supernatant is harvested. Following this, a second centrifugation at 3,000 to 16,000 ×g for 10 minutes prior to freezing is recommended (55,86,88). To prevent cellular degradation, ethylenediaminetetraacetic acid (EDTA) tubes should only be used if the sample can be processed within 1 to 2 hours from collection (55). If longer, stabilization tubes [e.g., Cell-Free DNA BCT® (Streck, NE, USA), the Cell-Free DNA Collection Tube (Roche, Basel, Switzerland), and the PAXgene® Blood ccfDNA tube (Qiagen, Venlo, The Netherlands)], which use unique preservatives to prevent the release of genomic DNA, can stabilize blood at room temperature for >7 days, after which mutations can still be detected (55,86,88,89). Stabilization tubes allow for greater flexibility in processing time and reduce the risk of degradation and contamination (55). Fresh plasma should be stored at −20 or −80 °C (on dry ice for shipping), with long-term stability of DNA in plasma best demonstrated at −80 °C (86,88). Specifically designed methods for DNA extraction are essential, and several kits are now commercially available (55,86,90).
Molecular testing methods in advanced NSCLC
Real-time PCR, Sanger sequencing, and NGS are common methodologies used for molecular testing in advanced NSCLC (5,15,86). In cases where single-gene testing is utilized, the amount of tissue required for each test is less than the specimen required for HC-based NGS, but to analyze all recommended genes can require ≥10 slides, and, in many cases, there is inadequate tissue to complete testing for all recommended genes. Therefore, where available, multiplexed genetic sequencing panels are preferred over multiple single-gene tests to identify treatment options beyond EGFR, ALK, BRAF, and ROS1, including selecting patients for clinical trials (5,15,16). TMB is an emerging biomarker that may be used for the selection of patients suitable for immunotherapy (15). NGS panels that are of sufficient size (>0.8 megabase) can be used to determine patients with high TMB who may be more likely to benefit from immunotherapy (91). However, TMB is yet to be approved by the US FDA as a biomarker for immunotherapy. The US FDA has granted accelerated approval to pembrolizumab for the treatment of adult and pediatric patients with unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) solid tumors that have progressed following prior treatment, and who have no satisfactory alternative treatment options; and MSI-H or dMMR colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan (42). MSI-H is found in 0.53% of LUAD, and some NGS panels have the ability to determine MSI-H status across cancer types, including NSCLC (92,93). Given that MSI-H lung cancers are extremely rare (0.6%), and up to 75% of MSI-H NSCLC tumors will exhibit PD-L1 expression, MSI testing/MMR IHC is not considered as routine in lung cancer (93).
NGS-based tests can require less tissue compared with individual testing of ≥4 genes [approximately 10 slides (4 µm each)] and have a higher throughput than traditional methods (94). However, in real-world clinical practice, delays in NGS turnaround time arising from insurance authorization and logistical factors have been observed (95). Moreover, the success rate of NGS can be lower in real-world clinical practice compared with experimental settings, suggesting that other tests, such as liquid biopsy, are needed when NGS is unusable due to insufficient quantity of tissue (55,95).
Several analytical methods are available for ctDNA analysis (55,86). Mutant enriched-PCR, Scorpion Amplified Refractory Mutation System (SARMS), and peptide nucleic acid clamping provide greater sensitivity compared with traditional sequencing methods (86,96). Digital droplet PCR and BEAMing (beads, emulsions, amplification, and magnetics) also demonstrate greater sensitivity and are now routinely used (55,86,97). For molecular analysis of ctDNA from treatment-naïve patients with advanced NSCLC, the IASLC recommends NGS where available, and that NGS panels employ error-proofing technologies with sufficient technical sensitivity and specificity for ctDNA applications (55).
Costs
Targeted cancer therapy can improve survival without increasing health care costs (98). AEs occur in approximately 19% of patients undergoing tissue biopsy, and the mean biopsy cost with an AE increases approximately 4-fold compared with an AE-free biopsy ($37,745 vs. $8,869, respectively) (99). Therefore, alternative methodologies that can provide genomic information with low AE rates, such as liquid biopsy, can reduce the total cost of care for patients with NSCLC; nevertheless, it should be recognized that tissue biopsy is still needed at important points in the diagnostic process (100). For testing modalities, the use of NGS could save Centers for Medicare and Medicaid Services (CMS) payers approximately $1.5 million vs. sequential single-gene testing (101). In real-world practice, it is likely that educational efforts to maximize the use of tissue acquired for histology for molecular testing will reduce costs arising from repeat tissue biopsy at disease progression. Further, in cases where tissue is not sufficient for molecular analysis, liquid biopsy is a cost-effective option over repeat biopsy (55).
According to the date of service (DOS) or the “14 Day Rule” set by the CMS, any laboratory tests, including molecular testing for advanced NSCLC, ordered within 14 days of patient discharge were considered to overlap with the claim submitted by the hospital or hospital-owned facility and were, therefore, considered part of the payment for inpatients (102). Consequently, some laboratories and oncologists did not order testing until after 14 days, causing delays in molecular results. As of January 2018, the CMS revised the Medicare Hospital Outpatient Prospective Payment System (OPPS) and the laboratory DOS policy. As a result, laboratories can now bill Medicare directly under the Clinical Laboratory Fee Schedule for molecular pathology tests and advanced diagnostic laboratory tests that are excluded from OPPS packaging rules and ordered within 14 days after a patient’s outpatient procedure (102). These revisions will allow laboratories to order molecular tests more quickly, thus expediting treatment of advanced NSCLC in the US, recognizing that the 14-day rule still applies in the inpatient setting. In March 2018, the CMS widened their Medicare coverage to include US FDA-approved NGS tests for recurrent, metastatic, relapsed, refractory, or Stage III or IV cancer with a companion diagnostic claim (103).
Conclusions
Treatment of advanced NSCLC requires tumors to be tested for a range of biomarkers that predict response to available therapies. Conducting molecular testing early in the diagnostic journey has numerous benefits for patients and the health care system. These include the selection of appropriate first-line targeted therapy, which reduces the side effects and costs of suboptimal therapies. Implementation of strategies that change how and when molecular testing occurs in the diagnostic journey will allow treating clinicians to have all molecular results available as close to the time of diagnosis as possible. The approaches outlined in this review could maximize use of treatment options, prevent use of suboptimal treatment, and ultimately improve patient outcomes.
Acknowledgments
Medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines, was provided by Liam Gillies, PhD, of Cactus Communications. This work was supported by AstraZeneca, who reviewed all drafts of the manuscript and approved the final manuscript for publication. This work was supported by AstraZeneca, who reviewed all drafts of the manuscript and approved the final manuscript for publication.
Footnote
Conflicts of Interest: JP Gregg has acted as an advisor and consultant for AstraZeneca, Foundation Medicine, BMS, and Roche. T Li has received research grants from Foundation Medicine and Pfizer. KY Yoneda has no conflicts of interest to declare.
References
- Weir HK, Thompson TD, Soman A, et al. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 2015;121:1827-37. [Crossref] [PubMed]
- American Cancer Society (2018). About non-small cell lung cancer. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8703.00.pdf
- Cancer Research UK. Lung cancer incidence statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#heading-Three
- American Cancer Society (2018). Non-small cell lung cancer survival rates, by stage. Available online: https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018;142:321-46. [Crossref] [PubMed]
- Lim C, Tsao MS, Le LW, et al. Biomarker testing and time to treatment decision in patients with advanced non-small cell lung cancer. Ann Oncol 2015;26:1415-21. [Crossref] [PubMed]
- Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer 2017;18:651-9. [Crossref] [PubMed]
- Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol 2018;13:1138-45. [Crossref] [PubMed]
- Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer:a systematic review and meta-analysis. Oncotarget 2016;7:78985-93. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 2007;110:2259-67. [Crossref] [PubMed]
- Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49. [Crossref] [PubMed]
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.2.2019. © National Comprehensive Cancer Network, Inc 2018. All rights reserved. Accessed November 26, 2018. Available online: www.NCCN.org
- Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice guideline update. J Clin Oncol 2018;36:911-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): a open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): an multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): an multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6):an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- XALKORI® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202570s023lbl.pdf
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4):a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- ZYKADIA® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205755s009lbl.pdf
- ALECENSA® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf
- ALUNBRIG® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208772lbl.pdf
- LORBRENA® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- MEKINIST® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204114s009lbl.pdf
- TAFINLAR® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202806s010lbl.pdf
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- KEYTRUDA® prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125514s034lbl.pdf
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- US Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm
- US Food and Drug Administration. Nucleic Acid Based Tests. Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm
- US Food and Drug Administration. Therascreen® EGFR RGQ PCR kit. Summary of safety and effectiveness data. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf12/P120022b.pdf
- US Food and Drug Administration. FoundationOne CDx™. Summary of safety and effectiveness data. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019B.pdf
- US Food and Drug Administration. cobas® EGFR Mutation Test v2. Summary of safety and effectiveness data. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf12/P120019S007B.pdf
- US Food and Drug Administration. PD-L1 IHC 22C3 pharmDx. Summary of safety and effectiveness data. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150013S006b.pdf
- US Food and Drug Administration. VENTANA ALK (D5F3) CDx Assay. Summary of safety and effectiveness data. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf14/p140025b.pdf
- US Food and Drug Administration. Vysis ALK Break Apart FISH Probe Kit. Summary of safety and effectiveness data. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf11/p110012b.pdf
- US Food and Drug Administration. Oncomine™ Dx Target Test. Summary of safety and effectiveness data. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160045B.pdf
- Ruggiero JE, Rughani J, Neiman J, et al. Real-world concordance of clinical practice with ASCO and NCCN Guidelines for EGFR/ALK testing in a NSCLC. J Clin Oncol 2017;35:abstr 212.
- Rolfo C, Mack PC, Scagliotti GV, et al. IASLC statement paper: liquid biopsy for advanced non-small cell lung cancer (NSCLC). J Thorac Oncol 2018;13:1248-68. [Crossref] [PubMed]
- Losk K, Freedman RA, Lin NU, et al. Implementation of surgeon-initiated gene expression profile testing (Onco type DX) among patients with early-stage breast cancer to reduce delays in chemotherapy initiation. J Oncol Pract 2017;13:e815-20. [Crossref] [PubMed]
- Foundation Medicine Press Release. Foundation Medicine’s new liquid biopsy assay granted Breakthrough Device Designation by U.S. Food and Drug Administration. April 26, 2018. Available online: http://investors.foundationmedicine.com/news-releases/news-release-details/foundation-medicines-new-liquid-biopsy-assay-granted
- Personal Genome Diagnostics Press Release. Personal Genome Diagnostics’ PGDx elio™ plasma resolve Receives Breakthrough Device Designation from FDA. July 2018. Available online: http://www.personalgenome.com/wp-content/uploads/2018/07/Personal-Genome-Diagnostics-PGDx-elio-plasma-resolve-Receives-Breakthrough-Device-Designation-from-FDA.pdf
- Guardant Health Press Release. The Guardant360® Assay Receives Expedited Access Pathway Designation for Breakthrough Devices from FDA. February 15, 2018. Available online: https://www.prnewswire.com/news-releases/the-guardant360-assay-receives-expedited-access-pathway-designation-for-breakthrough-devices-from-fda-300599629.html
- Clark TA, Chung JH, Kennedy M, et al. Analytical validation of a hybrid capture-based next-generation sequencing clinical assay for genomic profiling of cell-free circulating tumor DNA. J Mol Diagn 2018;20:686-702. [Crossref] [PubMed]
- Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017;17:223-38. [Crossref] [PubMed]
- Veldore VH, Choughule A, Routhu T, et al. Validation of liquid biopsy: plasma cell-free DNA testing in clinical management of advanced non-small cell lung cancer. Lung Cancer (Auckl) 2018;9:1-11. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol 2012;7:115-21. [Crossref] [PubMed]
- Zhou C, Yuan Z, Ma W, et al. Clinical utility of tumor genomic profiling in patients with high plasma circulating tumor DNA burden or metabolically active tumors. J Hematol Oncol 2018;11:129. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 2018;142:1364-82. [Crossref] [PubMed]
- Cheema PK, Raphael S, El-Maraghi R, et al. Rate of EGFR mutation testing for patients with nonsquamous non-small-cell lung cancer with implementation of reflex testing by pathologists. Curr Oncol 2017;24:16-22. [Crossref] [PubMed]
- Cheema PK, Menjak IB, Winterton-Perks Z, et al. Impact of reflex EGFR/ALK testing on time to treatment of patients with advanced nonsquamous non-small-cell lung cancer. J Oncol Pract 2017;13:e130-8. [Crossref] [PubMed]
- Vaught JB, Henderson MK. Biological sample collection, processing, storage and information management. IARC Sci Publ 2011;163:23-42. [PubMed]
- Kim L, Tsao MS. Tumour tissue sampling for lung cancer management in the era of personalized therapy: what is good enough for molecular testing? Eur Respir J 2014;44:1011-22. [Crossref] [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6:S99-107. [PubMed]
- Tian P, Wang Y, Li L, et al. CT-guided transthoracic core needle biopsy for small pulmonary lesions: diagnostic performance and adequacy for molecular testing. J Thorac Dis 2017;9:333-43. [Crossref] [PubMed]
- Ali MS, Sethi J, Taneja A, et al. Computed tomography bronchus sign and the diagnostic yield of guided bronchoscopy for peripheral pulmonary lesions. A systematic review and meta-analysis. Ann Am Thorac Soc 2018;15:978-87. [Crossref] [PubMed]
- Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med 2017;17:59. [Crossref] [PubMed]
- Lin CY, Chung FT. Central airway tumors: interventional bronchoscopy in diagnosis and management. J Thorac Dis 2016;8:E1168-76. [Crossref] [PubMed]
- Labarca G, Folch E, Jantz M, et al. Adequacy of samples obtained by endobronchial ultrasound with transbronchial needle aspiration for molecular analysis in patients with non-small cell lung cancer. Systematic review and meta-analysis. Ann Am Thorac Soc 2018;15:1205-16. [Crossref] [PubMed]
- Stoy SP, Segal JP, Mueller J, et al. Feasibility of endobronchial ultrasound-guided transbronchial needle aspiration cytology specimens for next generation sequencing in non-small-cell lung cancer. Clin Lung Cancer 2018;19:230-8.e2. [Crossref] [PubMed]
- Leong TL, Christie M, Kranz S, et al. Evaluating the genomic yield of a single endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer: meeting the challenge of doing more with less. Clin Lung Cancer 2017;18:e467-72. [Crossref] [PubMed]
- Iravani A, Ansari S, Reddy C, et al. Feasibility and safety of obtaining mediastinal and hilar lymph node core biopsy using three point 22 gauge (G) endoscopic ultrasound (EUS) needle. Am J Respir Crit Care Med 2018;197:A7316.
- Bonifazi M, Sediari M, Ferretti M, et al. The role of the pulmonologist in rapid on-site cytologic evaluation of transbronchial needle aspiration: a prospective study. Chest 2014;145:60-5. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest 2011;139:395-401. [Crossref] [PubMed]
- Jain D, Allen TC, Aisner DL, et al. Rapid on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspirations for the diagnosis of lung cancer: a perspective from members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2018;142:253-62. [Crossref] [PubMed]
- da Cunha Santos G, Ko HM, Saieg MA, et al. "The petals and thorns" of ROSE (rapid on-site evaluation). Cancer Cytopathol 2013;121:4-8. [Crossref] [PubMed]
- Roy-Chowdhuri S, Stewart J. Preanalytic variables in cytology: lessons learned from next-generation sequencing-the MD Anderson experience. Arch Pathol Lab Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Nobre AR, Albergaria A, Schmitt F. p40:a p63 isoform useful for lung cancer diagnosis - a review of the physiological and pathological role of p63. Acta Cytol 2013;57:1-8. [Crossref] [PubMed]
- Normanno N, Denis MG, Thress KS, et al. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget 2017;8:12501-16. [Crossref] [PubMed]
- Vallée A, Marcq M, Bizieux A, et al. Plasma is a better source of tumor-derived circulating cell-free DNA than serum for the detection of EGFR alterations in lung tumor patients. Lung Cancer 2013;82:373-4. [Crossref] [PubMed]
- El Messaoudi S, Rolet F, Mouliere F, et al. Circulating cell free DNA: preanalytical considerations. Clin Chim Acta 2013;424:222-30. [Crossref] [PubMed]
- Toro PV, Erlanger B, Beaver JA, et al. Comparison of cell stabilizing blood collection tubes for circulating plasma tumor DNA. Clin Biochem 2015;48:993-8. [Crossref] [PubMed]
- Warton K, Graham LJ, Yuwono N, et al. Comparison of 4 commercial kits for the extraction of circulating DNA from plasma. Cancer Genet 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017. doi:. [Crossref]
- Vanderwalde A, Spetzler D, Xiao N, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med 2018;7:746-56. [Crossref] [PubMed]
- Lu YQ, Lu KH. Advancements in next-generation sequencing for diagnosis and treatment of non-small-cell lung cancer. Chronic Dis Transl Med 2017;3:1-7. [Crossref] [PubMed]
- Hagemann IS, Devarakonda S, Lockwood CM, et al. Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer 2015;121:631-9. [Crossref] [PubMed]
- Asano H, Toyooka S, Tokumo M, et al. Detection of EGFR gene mutation in lung cancer by mutant-enriched polymerase chain reaction assay. Clin Cancer Res 2006;12:43-8. [Crossref] [PubMed]
- Dressman D, Yan H, Traverso G, et al. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci USA 2003;100:8817-22. [Crossref] [PubMed]
- Haslem DS, Van Norman SB, Fulde G, et al. A retrospective analysis of precision medicine outcomes in patients with advanced cancer reveals improved progression-free survival without increased health care costs. J Oncol Pract 2017;13:e108-19. [Crossref] [PubMed]
- Lokhandwala T, Bittoni MA, Dann RA, et al. Costs of diagnostic assessment for lung cancer: a Medicare claims analysis. Clin Lung Cancer 2017;18:e27-34. [Crossref] [PubMed]
- Arnaud A. Costs and outcomes comparison of tissue and blood based biopsies for the purpose of biomarker testing. Value Health 2016;19:A143-4. [Crossref]
- Pennell NA, Mutebi A, Zhou, ZY, et al. Economic impact of next generation sequencing vs sequential single-gene testing modalities to detect genomic alterations in metastatic non-small cell lung cancer using a decision analytic model. J Clin Oncol 2018;36:abstr 9031.
- Centers for Medicare and Medicaid Services. Medicare program: hospital outpatient prospective payment and ambulatory surgical center payment systems and quality reporting programs (CMS-1678-FC). Available online: https://www.gpo.gov/fdsys/pkg/FR-2017-12-14/pdf/R1-2017-23932.pdf
- Centers for Medicare and Medicaid Services. Proposed Decision Memo for Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer (CAG-00450N). Available online: https://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=290&bc=AAAAAAAAACAA&


