
Neoadjuvant tepotinib in stage IIB N0 non-small cell lung carcinoma with MET exon 14 skipping mutation: a case report and review of the literature
Highlight box
Key findings
• Neoadjuvant use of tepotinib is safe and effective from clinical data from a single case report.
What is known and what is new?
• Perioperative treatment with chemotherapy and immunotherapy is effective and safe in resectable non-driver mutated non-small cell lung cancer (NSCLC).
• In mesenchymal epithelial transition exon 14 (MET Ex14) mutated NSCLC, selective kinase inhibitors are effective treatments compared to non-selective kinase inhibitors in the metastatic setting.
What is the implication, and what should change now?
• Large randomized controlled clinical trials evaluating the safety and efficacy of MET Ex14 targeted tyrosine kinase inhibitors in the perioperative setting are needed to further understand this topic.
Introduction
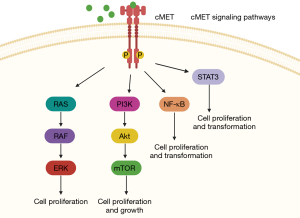
Lung cancer has the highest rate of mortality among malignancies world-wide (1). Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer diagnoses, which is further categorized by pathologic subtype, adenocarcinoma 78%, squamous 18% and others including adenosquamous and large cell carcinoma (2). Despite the high mortality rate, in the last two decades treatment options for NSCLC have greatly improved after the discovery of immune checkpoint inhibitors and a variety of targetable gene alterations (2,3). One of these above-mentioned driver alterations includes a mutation in the MET gene, found on chromosome 7, which was first discovered in 1984 and was later found to encode a receptor tyrosine kinase for hepatocyte growth factor (4). Additionally, MET is involved in several cell signaling pathways that promote cell proliferation and survival including mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), signal transducer and activator of transcription 3 (STAT3) and nuclear factor-κB (NF-kB) (5-8) (Figure 1).
After identification of MET gene amplification, MET gene sequencing and the use of small interfering RNAs (siRNAs) allowed for the discovery of the MET exon 14 mutation (MET Ex14), first in small cell lung cancer (SCLC) in 2003, followed by NSCLC in 2005 (9,10). A large analysis of tumor genomics followed, which found MET Ex14 mutations in 221 patients out of 38,028 patients with 126 distinct sequence variations (11). It should be noted that not all mutations in MET Ex14 mutations are alike, as only mutations affecting the splice site at exon 14 result in the “skipping mutation” and other mutations including point mutations outside this region are considered separate entities (12-14). Interestingly, the rate of the MET Ex14 skipping mutations varies across pathologic subtypes of lung cancer with higher rates reported in sarcomatoid and adenosquamous subtypes (2). Additionally, MET Ex14 skipping mutation is associated with a worse prognosis which may be in part due to the fact that the mutation in splice site of Ex14 results in the deletion of the juxtamembrane domain which is extensively involved signaling and receptor function leading to the accumulation of active ligand on the cell surface and therefore activation of other nearby downstream pathways (14). This has been the rationale for recent MET Ex14 targeted therapies. For the purpose of this case study regarding the use of MET Ex14 skipping mutation targeted therapy in the neoadjuvant setting, we will review the current treatment of NSCLC with a MET Ex 14 skipping mutation in the perioperative setting.
Current perioperative treatment of MET Ex14 mutated lung cancer
Neoadjuvant targeted therapy has been a focus of many investigators in the last few years. Several on-going umbrella trials are evaluating neoadjuvant treatment of multiple driver mutations, such as NAUTIKA1, PURPOSE and LEADER trials (15-17). The LEADER trial is a neoadjuvant screening trial designed to identify “driver mutations” defined as EGFR, BRAF p.V600E, MET Ex14, KRAS p.G12C, HER2, ALK, RET, NTRK, ROS1 as well as amplifications in MET and HER2 in patients with NSCLC stage IA2-III; once identified the patients will be able to enroll into independent neoadjuvant treatment trials (16) (NCT04712877). Alternatively, the PURPOSE and NAUTIKA1 trials include neoadjuvant treatment based on driver mutation, the former included ALK, MET, RET, HER2, EGFR (spilt into 3 groups based on mutation type) and the later included ALK, ROS1, NTRK, BRAF p.V600E, RET, KRAS p.G12C (15,17). Neoadjuvant and adjuvant use of capmatinib is currently under investigation in patients with resectable MET Ex14 NSCLC in the GEOMETRY-N trial (18). Additionally, there have been case reports of savolitinib’s success in the neoadjuvant setting (19). In a parallel phase II study of BRAF p.V600E and MET Ex14 mutated resectable NSCLC, targeted agents, dabrafenib plus trametinib or capmatinib are being evaluated in the perioperative setting with 8-week neoadjuvant treatment and 2-year adjuvant treatment (20) (NCT06054191). Due to limited data of immune check point inhibitors in the perioperative setting in driver mutated NSCLC, an going clinical trial is evaluating the efficacy of toripalimab with chemotherapy in localized NSCLC with rare driver mutations including RET fusions, BRAF p.V600E, ERBB2 exon20 insertion, MET amplification or exon 14 skipping (NCT05800340). We present this article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1197/rc).
Case presentation
A 77-year-old female never smoker with no significant past medical history developed diffuse generalized abdominal pain, prompting computed tomography (CT) of the abdomen and pelvis which was negative for acute abnormalities in the abdomen or pelvis but revealed ground glass opacity (GGO) in the left lower lobe in December 2022, the upper lung fields were not captured in the imaging (Figure 2). Her abdominal symptoms resolved and were thought to be related to gastroenteritis. A repeat CT of the chest 5 months later, revealed a new 5 cm × 3.5 cm × 3.2 cm mass in the right upper lobe, another 7 mm ground glass nodule in right apical region and stable appearance of the GGO in the left lower lobe. One month later, a fluorine-18 fluorodeoxyglucose (F18-FDG) positron emission tomography CT revealed intensely FDG avid mass measuring 4.6 cm in the right upper lobe and mildly FDG avid left hilar node. Additional CT chest a few weeks later showed a slight increase in the right upper lobe mass, now measuring 6.1 cm × 4.7 cm (Figure 3). Magnetic resonance imaging (MRI) of the brain revealed no metastatic disease. Due to potential nodal disease, she underwent a robotically assisted fine needle aspiration & transbronchial biopsy with endobronchial ultrasound (EBUS) of right upper lobe and mediastinal lymph nodes, pathology consistent with pulmonary adenocarcinoma and both station 4R and station 7 lymph were negative for malignant cells. Her disease was staged as a clinical stage IIB (T3N0M0). The patient’s case was discussed at thoracic tumor board, and there were concerns from the surgical team about proceeding directly to surgery without neoadjuvant treatment. Given the location of the tumor and potential involvement of the pulmonary artery, she would have required right upper and lower bilobectomy and possibly pneumonectomy. Additionally, she would have likely required an open surgical approach rather than a minimal invasive approach. Medical oncology was consulted for consideration of neoadjuvant therapy. Next generation sequencing (NGS) sent on the lung tissue from the initial biopsy revealed a MET mutation (c.3082+1G>A) causing a MET Ex14 deletion; programmed death-ligand 1 (PD-L1) expression/tumor proportion score was 2%. Among the detected variants, nine were classified as of unknown significance, one of which was a MET mutation (c.2638G>A; p.G880R).
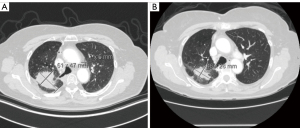
Given her advanced age, Eastern Cooperative Oncology Group (ECOG) performance status of 1, patient preference to avoid chemotherapy and concern for inability to tolerant neoadjuvant chemotherapy without delaying surgery, the decision was made to start the patient on neoadjuvant tepotinib at full dose 450 mg 3 months prior to surgery. As the patient was not an optimal chemotherapy candidate and declined chemotherapy as a personal preference but neoadjuvant treatment was recommended, she was an optimal candidate for targeted therapy. Of note, a similar rationale could be utilized with other targeted tyrosine kinase inhibitors (TKIs) in the less common driver mutations in the perioperative setting. Following two months of treatment, the patient exhibited a substantial response on interim CT of the chest, abdomen, and pelvis, with reduction of the right upper lobe mass to 4.6 cm × 2.8 cm (Figure 3). Due to the dramatic response with no side effects apart from mild lower extremity edema that self-resolved, she was continued on tepotinib up until 2 weeks prior to her surgery (total treatment 12 weeks). She was taken to the operating room for a robotic assisted right upper lobe lobectomy, en bloc right lower lobe superior segmentectomy and right middle lobe wedge resection. Pathology revealed residual poorly differentiated carcinoma consistent with adenosquamous invading through visceral pleura measuring 4 cm, 0/5 interlobar lymph nodes were positive for carcinoma. Lymph nodes at 10R, 12R, 11R, 7, 4R and 2R were all negative for carcinoma (at least 20 nodes sampled). Regarding the 4 cm re-moved tumor specimen, only 20% was viable tumor with 20% necrosis, 30% fibrosis, and 30% chronic inflammation. Therefore, the tumor size is calculated as 0.7 cm, downgrading staging to stage IA ypT1aN0M0. Repeat NGS testing on the resected tumor revealed again the presence of MET Ex14 deletion. Interestingly, the PD-L1 expression/tumor proportion score was 70%. The same variants of unknown significance were identified including the additional MET mutation with the addition of a RAD50 mutation. On follow up, the patient tolerated surgery without issue. She was started on adjuvant tepotinib at full dose 2 months after surgery, however required 50% dose reduction within 1 month of treatment and eventual cessation of treatment after 6 months due to intolerable side effects (lower extremity edema and fatigue). On post-surgical follow up imaging with CT chest 15 months after surgery the patient has no evidence of malignancy and remains in good health. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
NSCLC patients who are diagnosed with localized disease (stage I–II) are typically treated with surgical resection if candidates for surgery for a potential cure. The amount of residual tumor left after surgery greatly impacts survival as one study found 5-year survival rates of patients with R0, R1 and R2 resections of 73%, 36% and 28%, respectively (21). In localized disease stage beyond very early stage, stage IB–IIIA, perioperative platinum-based chemotherapy became a standard of care with improved survival outcomes (22,23). Despite the widespread success of chemo-immunotherapy in the perioperative setting, there is limited data on the efficacy of these regimens in more rare driver mutations, including MET Ex14. Therefore, additional clinical trials evaluating these non-ALK and non-EGFR mutations in the perioperative setting is required. Further, there is significant heterogeneity among MET Ex14 deletion mutations with varying rates of co-mutations and tumor mutational burden status which could contribute treatment response rates; additional studies could further clarify this point (24).
As mentioned previously, there are several on-going clinical trials evaluating neoadjuvant MET-targeting TKIs with major pathologic response (MPR) defined as <10% viable tumor at the time surgery as the primary end point in the majority of trials. In our case, the down-staging of the patient’s disease with only 20% viable tumor remaining further supports the use of neoadjuvant TKIs. In addition, the use of neoadjuvant chemotherapy with MET TKIs could be a strategy to increase the rate of MPR. In patients who do not achieve a MPR with neoadjuvant treatment, the question of which adjuvant treatment should be utilized remains. Should patients be continued on the neoadjuvant agent only if MPR or be continued regardless? Or in non-MPR patients should another agent be utilized instead.
The optimal timing to stop treatment with TKIs prior to surgery has not been established and there is some concern that potential side effects from neoadjuvant treatment could delay surgery or even preclude surgery. While rare, severe pneumonitis from TKIs is concerning and could be severe enough to have lasting effects. The most common toxicity of long-term MET TKI of total body fluid overload, anasarca, which is often seen in the metastatic setting; therefore a neoadjuvant strategy with a short course of treatment might be an alternative that can mitigate the toxicity (25). This concept was demonstrated in this patient case as the patient only developed slight lower extremity edema while on MET TKI treatment for a short period in the neoadjuvant setting but ultimately could not tolerate the longer duration of treatment in the adjuvant setting. However, given the short-term duration of follow-up of 15 months in our case study, long-term complications were not assessed including late toxicity and resistance. This along with lack of comparative analysis with no control group, and potential bias with optimal patient selection are limitations.
In addition to potential downstaging at the time of surgery, reduction in the rate of recurrence is one of the major rationales for perioperative treatment. In NSCLC, central nervous system (CNS) recurrence is not uncommon contributing to both morbidity and mortality in lung cancer with median survival of 12–15 months (26). As both tepotinib and capmatinib have high CNS penetration, this could potentially decrease the incidence of CNS metastasis, which would be greatly beneficial. From the VISION trial, MDR was 11.1 months but on long-term follow up MDR was 20.8 months, therefore it is important to determine the long-term efficacy of tepotinib in the perioperative setting with longer survival analysis and analysis of recurrence rates (25,27). Further, in the metastatic setting there was a slight difference in outcomes among patients who had liquid biopsies vs. tumor biopsies (25). As the patient was a surgical candidate, a tumor biopsy could easily be obtained in our case, but in cases where there is insufficient tumor tissue for analysis this difference should be noted. Additionally, there is a concern of contributing to early TKI resistance with neoadjuvant/adjuvant treatment, including in our patient presentation, but with limited follow up of 15 months post-op this could not be adequately assessed. Another consideration is the clinical implications of forgoing perioperative chemotherapy with immune checkpoint inhibitors in driver alterations, including MET Ex14 that are sensitive to immune check point inhibitors in the metastatic setting (28).
Conclusions
Recently, there has been great interest in the development of treatment strategies in the perioperative setting to further optimize patient care. Targeted therapy with kinase inhibitors is no exception. The MET pathway is complex with a heterogeneous group of targetable mutations, including MET gene amplification, MET Ex14 skipping mutations and other MET mutations. Our patient case is just one example of successful utilization of neoadjuvant MET Ex14 targeting. Further research is required to further stratify patients that would gain the greatest benefit from this treatment strategy. Additionally, identification of potential biomarkers capable of predicting prognosis could streamline this process.
Acknowledgments
We would like to thank the patient and her family for allowing the medical team to use her case for publication.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1197/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1197/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1197/coif). J.K. reports previously served on the advisory board for Astrazeneca and received honorarium from Intuitive Surgical, Inc. E.M. received Advisory Board fees and travel fees from Johnson & Johnson; Advisory Board fees from Sanofi, Astra Zeneca, Brystol Myers Squibb, Daiichi, Genentech and Data Safety Monitoring Board fees from Abbvie. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mattiuzzi C, Lippi G. Current Cancer Epidemiology. J Epidemiol Glob Health 2019;9:217-22. [Crossref] [PubMed]
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991;251:802-4. [Crossref] [PubMed]
- Zhang YW, Wang LM, Jove R, et al. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 2002;21:217-26. [Crossref] [PubMed]
- Van Der Steen N, Pauwels P, Gil-Bazo I, et al. cMET in NSCLC: Can We Cut off the Head of the Hydra? From the Pathway to the Resistance. Cancers (Basel) 2015;7:556-73. [Crossref] [PubMed]
- Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer 2018;18:341-58. [Crossref] [PubMed]
- Ariyawutyakorn W, Saichaemchan S, Varella-Garcia M. Understanding and Targeting MET Signaling in Solid Tumors - Are We There Yet? J Cancer 2016;7:633-49. [Crossref] [PubMed]
- Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005;65:1479-88. [Crossref] [PubMed]
- Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 2003;63:6272-81. [PubMed]
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Tong JH, Yeung SF, Chan AW, et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin Cancer Res 2016;22:3048-56. [Crossref] [PubMed]
- Cortot AB, Kherrouche Z, Descarpentries C, et al. Exon 14 Deleted MET Receptor as a New Biomarker and Target in Cancers. J Natl Cancer Inst 2017;109:djw262. [Crossref] [PubMed]
- Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89-103. [Crossref] [PubMed]
- Lee JM, Sepesi B, Toloza EM, et al. EP02.04-005 Phase II NAUTIKA1 Study of Targeted Therapies in Stage II-III NSCLC: Preliminary Data of Neoadjuvant Alectinib for ALK+ NSCLC. Journal of Thoracic Oncology 2022;17:S233-4. [Crossref]
- Sepesi B, Jones DR, Meyers BF, et al. LCMC LEADER neoadjuvant screening trial: LCMC4 evaluation of actionable drivers in early-stage lung cancers. J Clin Oncol 2022;40:TPS8596. [Crossref]
- Wang Y, Zhai H, Wang J, et al. Study protocol of an open-label prospective phase II umbrella study of precise neoadjuvant therapy for patients with stage II-IIIB resectable non-small cell lung cancer (PURPOSE). Front Oncol 2022;12:1052774. [Crossref] [PubMed]
- Lee JM, Awad MM, Saliba TR, et al. Neoadjuvant and adjuvant capmatinib in resectable non–small cell lung cancer with MET exon 14 skipping mutation or high MET amplification: GEOMETRY-N trial. J Clin Oncol 2022;40:TPS8590. [Crossref]
- Fu M, Feng CM, Xia DQ, et al. Neoadjuvant Savolitinib targeted therapy stage IIIA-N2 primary lung adenocarcinoma harboring MET Exon 14 skipping mutation: A case report. Front Oncol 2022;12:954886. [Crossref] [PubMed]
- Zhao S, Zhou H, Huang Y, et al. 128TiP A phase II, two parallel group study of neoadjuvant and adjuvant targeted treatment in NSCLC with BRAF V600 or MET exon 14 mutations. ESMO Open 2024;9:102921. [Crossref]
- Edwards JG, Chansky K, Van Schil P, et al. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for Residual Tumor Descriptors for Non-Small Cell Lung Cancer. J Thorac Oncol 2020;15:344-59. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Marks JA, Yin J, Halmos B, et al. Analysis of MET exon 14 skipping mutations in non–small cell lung cancer (NSCLC) by histology and specific mutation. J Clin Oncol 2022;40:9119. [Crossref]
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 2020;383:931-43. [Crossref] [PubMed]
- Sperduto PW, Mesko S, Li J, et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J Clin Oncol 2020;38:3773-84. [Crossref] [PubMed]
- Mazieres J, Paik PK, Garassino MC, et al. Tepotinib Treatment in Patients With MET Exon 14–Skipping Non–Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol 2023;9:1260-66. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-28. [Crossref] [PubMed]



