
Outcomes of chemotherapy with or without immunotherapy in older patients with non-small cell lung cancer and low PD-L1 expression
Highlight box
Key findings
• This study showed that combining immune checkpoint inhibitors (ICIs) with chemotherapy significantly improved progression-free survival (PFS) in older patients (aged ≥75 years) with non-small cell lung cancer (NSCLC) and low programmed death ligand 1 (PD-L1) expression. However, the improvement in overall survival was limited, and there was an increased risk of severe adverse events, such as pneumonitis.
What is known and what is new?
• While it is well known that ICI combined with chemotherapy improves outcomes in NSCLC patients with low PD-L1 expression, data on older patients have been limited.
• This study provides new insights into the benefits and risks of this combination therapy in the older population.
What is the implication, and what should change now?
• For older NSCLC patients, especially those with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1, the additional benefit of ICIs over chemotherapy alone appears to be minimal. Careful patient selection, considering ECOG PS and frailty, is essential to ensure that the benefits outweigh the risks for this population.
Introduction
Lung cancer remains one of the leading causes of cancer-related mortality worldwide. Non-small cell lung cancer (NSCLC) comprises approximately 80% of all lung cancer cases and is often diagnosed at an advanced, unresectable, or metastatic stage (1,2). Immune checkpoint inhibitors (ICIs), including antibodies targeting programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1), have shown remarkable efficacy in the treatment of advanced NSCLC (3-8). Moreover, regardless of the PD-L1 tumor proportion score (TPS), the combination of chemotherapy and ICIs has demonstrated superior efficacy compared to chemotherapy alone and is now recognized as the standard first-line treatment for this patient population (9-13). Additionally, several clinical trials have reported that chemotherapy in combination with ICI can prolong progression-free survival (PFS) and overall survival (OS) when compared with chemotherapy alone in patients with NSCLC and low PD-L1 expression (PD-L1 TPS of 1–49%) (9-13).
With the aging population, the proportion of older patients diagnosed with advanced-stage lung cancer has been steadily rising. However, older patients have been rarely included in several phase III clinical trials that investigated the efficacy of ICI plus chemotherapy (9-15). Furthermore, the KEYNOTE-189 trial, which evaluated pembrolizumab or placebo combined with pemetrexed and platinum for previously untreated metastatic non-squamous NSCLC, raised concerns about the efficacy of treatment in patients aged ≥75 years (15). Therefore, whether ICI plus chemotherapy is an optimal treatment strategy for this vulnerable population has not been fully explored. Meanwhile, previous phase III trials for older patients with advanced NSCLC revealed that carboplatin-based chemotherapy resulted in longer OS than docetaxel (16-18). Carboplatin plus chemotherapy has been established with robust clinical evidence as a standard treatment option for advanced older patients with NSCLC. Considering these findings, whether ICI plus chemotherapy is more effective than platinum-based chemotherapy remains unclear. In this study, we sought to identify an appropriate treatment option for older patients with advanced NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1236/rc).
Methods
Study design and patients
Our objective is to determine whether ICI combined with chemotherapy is more effective than platinum-based chemotherapy in older patients with advanced NSCLC and a PD-L1 TPS of 1–49%. We retrospectively included consecutive patients with advanced NSCLC (stage IIIB, IIIC, IVA, IVB, or postoperative recurrence, as defined by the American Joint Committee on Cancer Staging Manual, version 8) with a PD-L1 TPS of 1–49%. These patients received either platinum-based chemotherapy alone (Chemo) or in combination with ICIs (ICI/Chemo) as their initial treatment between March 2017 and July 2022 at 19 independent medical institutions across Japan, including university hospitals and designated cancer centers. Clinical data at the initiation of first-line treatment were obtained from electronic medical records. PD-L1 TPS was evaluated using PD-L1 immunohistochemistry with the 22C3 pharmDx antibody (clone 22C3; Dako North America, Inc., Carpinteria, CA, USA). PD-L1 expression was assessed at the time of initial diagnosis before treatment initiation. Patients were excluded if they had a sensitizing driver mutation (EGFR, ALK, ROS1, BRAF, KRAS, MET, RET, HER2, or NTRK alterations), an Eastern Cooperative Oncology Group performance status (ECOG PS) of 2–4, a history of prior ICI treatment, or had received concurrent radiotherapy with chemotherapy. These exclusions were based on the general ineligibility of patients with poor ECOG PS for several phase 3 clinical trials involving ICI/Chemo (9-13). Patients with recurrent disease were eligible if recurrence occurred more than 24 weeks after the last administration of perioperative chemotherapy. However, those who had received a combination of uracil and tegafur as perioperative chemotherapy were eligible regardless of the recurrence interval. According to the Japanese Lung Cancer Society Guideline, older patients were defined as those aged ≥75 years (19). This study was approved by the Ethics Review Board of Kyoto Prefectural University of Medicine (No. ERB-C-2934). The review board of each institution has been informed and approved the study protocol. A collective central review determined that informed consent was not required due to the retrospective nature of the study, and an opt-out method was included so that patients and families could opt out of participating in the study. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The data cut-off date for follow-up was January 5, 2024.
Efficacy and safety assessments
Treatment response was assessed based on the Response Evaluation Criteria in Solid Tumors version 1.1 (20). Adverse events (AEs) were evaluated according to the Common Terminology Criteria for Adverse Events version 5.0 (21). PFS was defined as the time from the initiation of first-line treatment to the first occurrence of lung cancer progression or death from any cause. OS was defined as the time from the initiation of first-line treatment to death from any cause.
Statistical analysis
Continuous variables were analyzed using the Wilcoxon rank-sum test, while dichotomous variables were evaluated using the Chi-squared test or Fisher’s exact test, as appropriate. Survival outcomes were estimated using the Kaplan-Meier method and compared with the log-rank test. To assess the association between patient characteristics and survival outcomes, Cox proportional hazard models were applied, with results expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). To compare treatment outcomes between the Chemo and ICI/Chemo groups, rigorous adjustments were made for significant baseline differences using propensity score matching (PSM). The matching process included the following variables: sex, smoking status, ECOG PS, histology, and disease stage. Nearest-neighbor matching was performed at a 1:1 ratio without replacement, with a caliper set at 0.2. All statistical analyses were conducted using JMP 14 software (SAS Institute, Cary, NC, USA). A two-tailed P value of <0.05 was considered statistically significant.
Results
Patient characteristics
In this study, we enrolled 613 consecutive patients with NSCLC and a PD-L1 TPS of 1–49%. Among them, 243 and 370 patients were treated with Chemo and ICI/Chemo as the first-line treatment, respectively (Figure S1). The median follow-up time was 14.2 months (95% CI: 12.8–15.4) in the Chemo group and 16.1 months (95% CI: 14.2–16.6) in the ICI/Chemo group, respectively. The baseline characteristics of the study subjects are summarized in Table 1. Compared with the ICI/Chemo group, the Chemo group had a higher proportion of patients with squamous cell carcinoma (101/243 vs. 127/370, P=0.07) and recurrence (58/243 vs. 66/370, P=0.07), although the differences were not statistically significant. In the ICI/Chemo group, the most commonly administered first-line treatment regimen included pembrolizumab as the ICI.
Table 1
| Patient characteristics | Chemotherapy group (n=243) | ICI plus chemotherapy group (n=370) | P value |
|---|---|---|---|
| Age (years), median [IQR] | 70 [64–75] | 70 [64–74] | |
| Age categorization, n [%] | 0.60 | ||
| <75 years | 180 [74] | 281 [76] | |
| ≥75 years | 63 [26] | 89 [24] | |
| Sex, n [%] | 0.60 | ||
| Male | 180 [74] | 281 [76] | |
| Female | 63 [26] | 89 [24] | |
| Smoking status, n [%] | 0.25 | ||
| Never-smoker | 28 [12] | 32 [9] | |
| Current or former smoker | 215 [88] | 338 [91] | |
| ECOG PS, n [%] | 0.15 | ||
| 0 | 77 [32] | 138 [37] | |
| 1 | 166 [68] | 232 [63] | |
| Histology, n [%] | 0.07 | ||
| Squamous cell carcinoma | 101 [42] | 127 [34] | |
| Adenocarcinoma | 120 [49] | 215 [58] | |
| Other | 22 [9] | 28 [8] | |
| Stage, n [%] | 0.07 | ||
| III | 22 [9] | 24 [6] | |
| IV | 163 [67] | 280 [76] | |
| Recurrence | 58 [24] | 66 [18] | |
| Liver metastasis, n [%] | 28 [12] | 33 [9] | 0.30 |
| Brain metastasis, n [%] | 37 [15] | 60 [16] | 0.74 |
| Treatment regimen, n [%] | – | ||
| Chemotherapy | 243 [100] | – | |
| Chemotherapy plus pembrolizumab | – | 269 [73] | |
| Chemotherapy plus atezolizumab | – | 50 [14] | |
| Chemotherapy plus ipilimumab and nivolumab | – | 51 [14] | |
| CBDCA based | 184 [76] | 328 [89] | |
| CDDP based | 59 [24] | 42 [11] | |
| Combination with taxane | 119 [49] | 184 [50] | |
| Combination with pemetrexed | 93 [38] | 186 [50] |
CBDCA, carboplatin; CDDP, cisplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; IQR, interquartile range.
Treatment outcomes
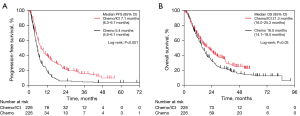
We compared treatment outcomes between the Chemo and ICI/Chemo groups. After applying PSM, each group included 226 patients. Baseline characteristics were well balanced between the two groups, with no significant differences observed (Table S1). Compared with the Chemo group, the ICI/Chemo group had significantly longer median PFS (5.4 vs. 7.1 months, P<0.001; Figure 1A) and median OS (16.0 vs. 21.3 months, P=0.03; Figure 1B). The median number of chemotherapy cycles received in each treatment arm and the detailed data of subsequent treatment after progression of Chemo or ICI/Chemo were summarized in Table S2. After adjusting the groups by PSM, the incidence of grade ≥3 AEs was higher in the ICI/Chemo group (48%) than in the Chemo group (36%). The frequency of pneumonitis (all grades) was also higher in the ICI/Chemo (19%) group than in the Chemo group (12%) (Table S3).
Treatment outcomes in older patients
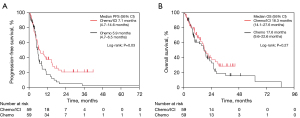
Among the enrolled patients, 152 were older patients, and among them, 63 and 89 patients were treated with Chemo and ICI/Chemo as the first-line treatment, respectively. After weighting by PSM, 59 patients were included in each group. No significant differences were observed in the baseline characteristics between the two groups (Table S4). The median PFS in the ICI/Chemo group (7.1 months) was significantly longer than in the Chemo group (5.9 months) (P=0.03; Figure 2A). However, no significant difference was observed in the median OS between the two groups (18.3 vs. 17.6 months, P=0.27; Figure 2B). The median number of chemotherapy cycles received in each treatment arm and the detailed data of subsequent treatment after progression of Chemo or ICI/Chemo were summarized in Table S5. In older patients adjusted by PSM, the incidence of grade ≥3 AEs was higher in the ICI/Chemo group (54%) than in the Chemo group (37%). The ICI/Chemo had a higher frequency (31%) of pneumonitis of all grades than the Chemo group (20%) (Table S6).
Multivariate analysis of ICI/Chemo patients
We also examined the association between PFS and OS and patient characteristics in the ICI/Chemo group (Table 2). Multivariate analysis using Cox proportional hazards models revealed that non-squamous histology (HR: 0.76, 95% CI: 0.58–0.99, P=0.05) and an ECOG PS of 0 (HR: 0.71, 95% CI: 0.55–0.93, P=0.01) were significantly associated with longer PFS. Additionally, an ECOG PS of 0 (HR: 0.65, 95% CI: 0.48–0.88, P=0.005) was significantly associated with longer OS. In contrast, older age was not significantly associated with either PFS (HR: 0.92, 95% CI: 0.69–1.23, P=0.57) or OS (HR: 0.86, 95% CI: 0.62–1.19, P=0.36).
Table 2
| Characteristics | PFS multivariate | OS multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age <75 years (vs. ≥75 years) | 0.92 (0.69–1.23) | 0.57 | 0.86 (0.62–1.19) | 0.36 | |
| Sex female (vs. male) | 0.79 (0.56–1.12) | 0.19 | 0.90 (0.61–1.32) | 0.59 | |
| Smoking status never (vs. current or former smoker) | 0.84 (0.52–1.36) | 0.49 | 0.80 (0.46–1.38) | 0.42 | |
| Histology non-squamous (vs. squamous) | 0.76 (0.58–0.99) | 0.05 | 0.75 (0.56–1.01) | 0.06 | |
| ECOG PS 0 (vs. 1) | 0.71 (0.55–0.93) | 0.01 | 0.65 (0.48–0.88) | 0.005 | |
| Stage recurrence (vs. non-recurrence) | 0.77 (0.55–1.07) | 0.12 | 0.69 (0.47–1.02) | 0.07 | |
| Liver metastasis no (vs. yes) | 0.86 (0.58–1.36) | 0.58 | 0.76 (0.49–1.18) | 0.22 | |
| Brain metastasis no (vs. yes) | 1.00 (0.71–1.39) | 0.98 | 0.80 (0.54–1.20) | 0.28 | |
Chemo, platinum-based chemotherapy; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression-free survival.
Treatment outcomes in older patients according to ECOG PS
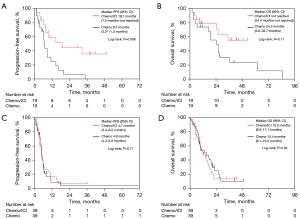
Next, we compared treatment outcomes between older patients in the Chemo and ICI/Chemo groups based on ECOG PS. After PSM weighting, each group included 19 patients with an ECOG PS of 0 and 39 patients with an ECOG PS of 1. Baseline characteristics were well balanced, with no significant differences between the two groups for patients with ECOG PS 0 and 1 (Tables S7,S8, respectively). Among older patients with an ECOG PS of 0, the median PFS was significantly longer in the ICI/Chemo group (19.1 months) compared to the Chemo group (6.5 months) (P=0.008; Figure 3A). Similarly, the median OS was longer in the ICI/Chemo group (not reached) than in the Chemo group (24.2 months, P=0.11) (Figure 3B), although the difference was not significant. The median number of chemotherapy cycles received in each treatment arm and the detailed data of subsequent treatment after progression of Chemo or ICI/Chemo were summarized in Table S9. In older patients with ECOG PS of 0 adjusted by PSM, the incidence of grade ≥3 AEs was higher in the ICI/Chemo group (58%) than in the Chemo group (42%). The Chemo group had a higher frequency (26%) of pneumonitis of all grades than the ICI/Chemo group (21%) (Table S10). In contrast, in older patients with ECOG PS of 1, the ICI/Chemo and Chemo groups showed comparable median PFS (4.7 vs. 4.9 months, P=0.77) (Figure 3C) and median OS (12.2 vs. 15.4 months, P=0.64) (Figure 3D). The median number of chemotherapy cycles received in each treatment arm and the detailed data of subsequent treatment after progression of Chemo or ICI/Chemo were summarized in Table S11. In older patients with ECOG PS of 1 adjusted by PSM, the incidence of grade ≥3 AEs was higher in the ICI/Chemo group (56%) than in the Chemo group (41%). The ICI/Chemo had a higher frequency (28%) of pneumonitis of all grades than the Chemo group (21%) (Table S12).
Discussion
ICIs enhance anti-cancer immunity by targeting immune checkpoints such as PD-1, its ligand PD-L1, and cytotoxic T lymphocyte-associated protein 4, thereby promoting cancer cell death. Chemotherapy complements ICIs by inducing immunogenic cell death, increasing CD8+ T cell infiltration, and suppressing immunosuppressive cells, suggesting that their combination may exert synergistic effects (22-24). However, aging introduces complexities in treatment due to various genetic and environmental factors that result in telomere shortening, genomic instability, and a decline in immune function, particularly through impaired T-cell diversity and thymic output (25). As the population continues to age, the susceptibility to lung cancer increases, raising concerns about age-related immune changes that may affect the efficacy of ICIs and chemotherapeutic agents in older patients.
Our study revealed that the combination of ICIs and chemotherapy could be effective in older patients with NSCLC and low PD-L1 expression. However, the benefit of adding ICIs to chemotherapy tends to be reduced in this population compared to the overall population, and the potential for increased toxicity is a concern. Particularly in patients with ECOG PS 1, the incremental benefit of adding ICI to chemotherapy over chemotherapy alone may be limited, suggesting that the combination may not be the most suitable option for this patient group. To the best of our knowledge, this is the first study to highlight these treatment considerations, which can guide the selection of optimal therapies for this clinical population.
Consistent with several previous phase III clinical trials, we found that in the total cohort, the combination of ICI and chemotherapy was associated with a significantly longer PFS and OS than the use of chemotherapy alone (9-12). However, among older patients aged ≥75 years, although PFS was significantly longer in the ICI/Chemo group, OS did not differ significantly between the two treatment groups. These differences suggest that while combining ICI and chemotherapy may delay disease progression, it may not translate into a survival benefit in older patients, possibly due to age-related vulnerabilities and competing risks such as comorbidities. In a previous report on older patients with cancer, including lung cancer, comorbidities such as hypertension, renal failure, and cognitive impairment were commonly observed, and a correlation between a higher burden of comorbidities and reduced OS was reported (26). Another study on patients with NSCLC receiving chemotherapy plus pembrolizumab found that older patients had significantly shorter PFS and OS than non-older patients (27). Considering these findings, combining ICI and chemotherapy may not be recommended for older patients with NSCLC. Clinical biomarkers that correlate with treatment outcomes are warranted to help the selection of appropriate patients who are more likely to benefit from the combination therapy.
In addition to efficacy, older patients in the ICI/Chemo group exhibited a relatively higher incidence of grade ≥3 AEs and any grade pneumonitis than the Chemo group, raising concerns regarding the safety of this regimen. In a pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies, older patients with NSCLC who were administered pembrolizumab monotherapy or chemotherapy had a higher incidence of any grade pneumonitis and grade ≥3 AEs than the non-elderly group (28). Additionally, a large retrospective study on older patients with NSCLC in Japan reported a significantly higher incidence of grade ≥3 immune-related AEs in patients treated with ICI plus chemotherapy than in those treated with ICI alone (29). Given the increased toxicity profile, clinicians must carefully weigh the potential benefits of combining ICI and chemotherapy against the risk of severe AEs in older patients.
We further evaluated the relationship between ECOG PS and treatment outcomes in older patients. The combination of ICI and chemotherapy was associated with longer PFS and OS in patients with ECOG PS 0, although the difference in OS was not statistically significant. Conversely, both combining ICI and chemotherapy and chemotherapy alone resulted in similar PFS and OS in patients with ECOG PS 1. These results suggest that the benefits of combining ICI and chemotherapy may be more pronounced in older patients with ECOG PS 0, highlighting the importance of baseline functional status in determining treatment efficacy. We have previously reported that ECOG PS may be a clinical factor that needs to be considered when deciding between ICI monotherapy or ICI plus chemotherapy in older patients with NSCLC with a PD-L1 TPS ≥50% (30). Our current findings on older patients with NSCLC and a PD-L1 TPS 1–49% confirm our previous results (30). Consequently, ECOG PS may be an important biomarker for appropriate treatment selection in older patients with NSCLC, irrespective of PD-L1 expression. Although the ECOG PS is a practical tool in clinical settings, it has limitations, such as variability in assessment, and focuses only on functional aspects. Therefore, comprehensive geriatric assessments, which encompass physical, social, and psychological dimensions, are being increasingly recommended, especially for older patients (31-33). Clinical tools, such as the G8 geriatric screening tool, allow for a more nuanced evaluation of frailty in older patients (34,35), allowing for the selection of more tailored treatment choices for older patients with cancer.
This study has several limitations. First, as a multicenter retrospective study, the possibility of selection bias cannot be entirely excluded. However, patients were consecutively enrolled, and PSM was performed to minimize selection bias. Nonetheless, as treatment decisions were based on each patient’s clinical fitness, some degree of selection bias may still be present. Second, the use of PSM to adjust for differences in patient background inevitably resulted in a smaller sample size compared to the overall study population. Third, we were unable to collect comprehensive geriatric clinical data, including comorbidity indices (Charlson comorbidity score), polypharmacy, nutritional status [body mass index (BMI) and albumin], and the G8 score, due to variability in medical record documentation across participating institutions. Future studies incorporating comprehensive geriatric assessments could help refine risk stratification and optimize treatment selection for older patients with NSCLC receiving ICI/Chemo. Fourth, this study did not collect data on the time from diagnosis to treatment initiation. We acknowledge that variations in treatment timing could potentially influence survival outcomes, and future studies should consider evaluating this factor. Finally, this study was limited to Japanese patients, and the sample size was relatively small, highlighting the need for larger global cohort studies to validate our findings. In this context, a retrospective study has been reported, and a phase 3 trial (NCT03977194) is currently underway (36).
Conclusions
In conclusion, a combination of ICI and chemotherapy may be a potentially effective treatment strategy for older patients with NSCLC and low PD-L1 expression. However, compared with the overall population, the increased efficacy of adding ICI to chemotherapy tends to decrease, and the risk of toxicity may increase, highlighting the need for appropriate patient selection. Particularly, in patients with ECOG PS 1, the additional benefit of ICI over chemotherapy was minimal in terms of efficacy, suggesting that ICI combined with chemotherapy may be better avoided in this group.
Acknowledgments
We are grateful to all the patients and investigators involved in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1236/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1236/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1236/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1236/coif). T.Y. serves as an unpaid editorial board member of Translational Lung Cancer Research from October 2023 to September 2025. H.K. received personal fees from Bristol-Myers Squibb, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan KK, and MSD KK, outside the purview of the submitted work. T.Y. received research grants from Ono Pharmaceutical, Janssen, AstraZeneca, and Takeda Pharmaceutical. He has also received speaking honoraria from Eli Lilly and Chugai-Roshe, outside the purview of the submitted work. S.W. received grants from Boehringer Ingelheim and Nippon Kayaku. He also received honoraria for speaker bureaus from Lilly, Novartis Pharma, Chugai Pharma Bristol-Myers, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Nippon Kayaku, Kyowa Kirin, Merck, Takeda Pharmaceutical, Celltrion, and AstraZeneca, outside the purview of the submitted work. H.T. received lecture fees from AstraZeneca and Chugai Pharma. T.F. received personal fees from AstraZeneca K.K., Boehringer-Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Nippon Kayaku Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., and Pfizer Japan Inc., outside the purview of the submitted work. K.K. has received a speaker honorarium from Ono Pharmaceutical Company, Chugai Pharmaceutical, Bristol-Myers Company, Boehringer Ingelheim, and AstraZeneca, and research grants from AstraZeneca. A.O. received personal fees from Chugai-Roshe, AstraZeneca, Boehringer Ingelheim, Eli Lilly Japan, Nippon Kayaku, and Bristol-Myers Squibb, outside the purview of the submitted work. T.K. received personal fees from Chugai Pharmaceutical Co. Ltd. and MSD KK, outside the purview of the submitted work. K.T. received research grants from Chugai Pharmaceutical Co. Ltd. and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai Pharmaceutical Co. Ltd., MSD-Merck, Eli Lilly, Boehringer Ingelheim, and Daiichi-Sankyo, outside the purview of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Review Board of Kyoto Prefectural University of Medicine (No. ERB-C-2934). The review board of each institution has been informed and approved the study protocol. A collective central review determined that informed consent was not required due to the retrospective nature of the study, and an opt-out method was included so that patients and families could opt out of participating in the study. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. Erratum in: CA Cancer J Clin 2024;74:203. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021;397:592-604. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Nishio M, Barlesi F, West H, et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J Thorac Oncol 2021;16:653-64. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Pallis AG, Gridelli C, Wedding U, et al. Management of elderly patients with NSCLC; updated expert's opinion paper: EORTC Elderly Task Force, Lung Cancer Group and International Society for Geriatric Oncology. Ann Oncol 2014;25:1270-83. [Crossref] [PubMed]
- Song S, Lam EW, Tchkonia T, et al. Senescent Cells: Emerging Targets for Human Aging and Age-Related Diseases. Trends Biochem Sci 2020;45:578-92. [Crossref] [PubMed]
- Okamoto I, Nokihara H, Nomura S, et al. Comparison of Carboplatin Plus Pemetrexed Followed by Maintenance Pemetrexed With Docetaxel Monotherapy in Elderly Patients With Advanced Nonsquamous Non-Small Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:e196828. [Crossref] [PubMed]
- Kogure Y, Iwasawa S, Saka H, et al. Efficacy and safety of carboplatin with nab-paclitaxel versus docetaxel in older patients with squamous non-small-cell lung cancer (CAPITAL): a randomised, multicentre, open-label, phase 3 trial. Lancet Healthy Longev 2021;2:e791-800. [Crossref] [PubMed]
- Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079-88. [Crossref] [PubMed]
- Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol 2019;24:731-70. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
- Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90-2. [Crossref] [PubMed]
- Lee L, Gupta M, Sahasranaman S. Immune Checkpoint inhibitors: An introduction to the next-generation cancer immunotherapy. J Clin Pharmacol 2016;56:157-69. [Crossref] [PubMed]
- Li JY, Chen YP, Li YQ, et al. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol Cancer 2021;20:27. [Crossref] [PubMed]
- Mellman I, Chen DS, Powles T, et al. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 2023;56:2188-205. [Crossref] [PubMed]
- Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol 2005;174:7446-52. [Crossref] [PubMed]
- Benderra MA, Serrano AG, Paillaud E, et al. Prognostic value of comorbidities in older patients with cancer: the ELCAPA cohort study. ESMO Open 2023;8:101831. [Crossref] [PubMed]
- Morimoto K, Yamada T, Yokoi T, et al. Clinical impact of pembrolizumab combined with chemotherapy in elderly patients with advanced non-small-cell lung cancer. Lung Cancer 2021;161:26-33. [Crossref] [PubMed]
- Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019;135:188-95. [Crossref] [PubMed]
- Tsukita Y, Tozuka T, Kushiro K, et al. Immunotherapy or Chemoimmunotherapy in Older Adults With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2024;10:439-47. [Crossref] [PubMed]
- Takei S, Kawachi H, Yamada T, et al. Prognostic impact of clinical factors for immune checkpoint inhibitor with or without chemotherapy in older patients with non-small cell lung cancer and PD-L1 TPS ≥ 50. Front Immunol 2024;15:1348034. [Crossref] [PubMed]
- Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326-47. [Crossref] [PubMed]
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595-603. [Crossref] [PubMed]
- Puts MT, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst 2012;104:1133-63. [Crossref] [PubMed]
- Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 2012;23:2166-72. [Crossref] [PubMed]
- Kenis C, Decoster L, Van Puyvelde K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol 2014;32:19-26. [Crossref] [PubMed]
- Thomas QD, Chaabouni M, Al Herk A, et al. Exploring the Efficacy of Pembrolizumab in Combination with Carboplatin and Weekly Paclitaxel for Frail Patients with Advanced Non-Small-Cell Lung Cancer: A Key Investigative Study. Cancers (Basel) 2024;16:992. [Crossref] [PubMed]


