
One-year mortality risk prediction model for patients with interstitial lung disease and lung cancer
Highlight box
Key findings
• Lactate dehydrogenase (LDH) and albumin (Alb) levels were independently associated with the 1-year mortality of patients with interstitial lung disease (ILD) and lung cancer (LC). The prediction model incorporating age, sex, neutrophil count, and LDH and Alb levels demonstrated good discriminative ability, consistency, and clinical applicability.
What is known and what is new?
• The association of ILD with LC has garnered increased research attention in recent years, but developing effective treatment for patients with both of these conditions remains challenging. This is because both of these lethal diseases have high mortality rate and the two mutually accelerate the disease courses.
• The study developed a 1-year mortality risk prediction model for patients with ILD and LC.
What is the implication, and what should change now?
• The prediction model could effectively identify high risk populations among patients with concurrent ILD and LC for precise and regular management.
• Multicenter, large-sample studies are needed to externally validate the findings from this study.
Introduction
Interstitial lung disease (ILD) comprises a broad spectrum of diffuse lung disorders characterized by different degrees of parenchymal inflammation or fibrosis (1). In recent years, the association of ILD with lung cancer (LC) has attracted increased research interest. First, epidemiological evidence suggests that patients with ILD have a 3.5- to 7.3-time increased risk of developing LC compared to the general population. The cumulative incidence of LC increases over the course of ILD and reaches 31.3% at 10 years in patients with idiopathic pulmonary fibrosis (IPF) (2,3). Furthermore, over 70% of LC are located within or adjacent to the fibrotic when LC occurs concurrently with IPF (2,4). Shared genetic mutants and epigenetic alterations are common mechanisms linking IPF and LC (5). For example, tobacco and exposure to occupational hazards are strongly associated with a heightened incidence of LC and pulmonary fibrosis (6,7). Additionally, the secretion of transforming growth factor-β (TGF-β) and interleukins activating the proliferation of fibroblasts and cancer-associated fibroblasts can contribute to the pathogenesis pulmonary fibrosis concurrent with LC (5,7,8).
ILD is typically assessed using various clinical and laboratory markers to evaluate disease activity and severity. Serum biomarkers such as Krebs von den Lungen-6 (KL-6) and surfactant proteins A and D (SP-A, SP-D) are widely used to assess alveolar epithelial injury and pulmonary fibrosis, often showing elevated levels in active or progressive ILD (9,10). Inflammatory markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are valuable indicators of inflammation and are commonly elevated during disease exacerbations (10-12). These markers, together with clinical assessment, provide crucial information on disease progression and potential complications, and should be considered as part of the disease prognostic evaluation when ILD coexists with LC. However, KL-6, SP-A and SP-D were not available in our study, so we included only inflammatory markers in the analysis.
Developing effective treatment for patients with concurrent ILD and LC is challenging. Implementing therapies for LC such as surgical resection, immunotherapy, chemotherapy, and radiotherapy may lead to the acute exacerbation of ILD (AE-ILD) (13-15). Additionally, preoperative glucocorticoid used is an independent risk factor for the occurrence of postoperative AE-ILD (16). Due to the complex interaction between cancer progression and ILD exacerbation, managing these patient groups remains challenging. While previous research has focused on individual prognostic factors, no comprehensive predictive tool has been developed. In this study, we aimed to identify the clinical characteristics and determine the prognostic factors and overall survival of patients with concurrent ILD and LC. Additionally, a 1-year mortality prediction model was developed to identify patients at higher risk of mortality. This novel model may assist clinicians in decision-making, risk communication, and personalized care. We present this article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-235/rc).
Methods
Patients
From May 2017 to January 2022, consecutive patients who diagnosed with both ILD and LC at the Department of Respiratory and Critical Care Medicine in Nanjing Drum Tower Hospital were enrolled, irrespective of diagnostic sequence. Although the temporal relationship between ILD and LC diagnoses may reflect etiological heterogeneity, this study focused on developing a clinically generalizable prediction model for the overall population with coexisting diseases. The diagnosis of ILD was based on a combination of clinical manifestations, physical examination, and chest high-resolution computed tomography (HRCT) outlined by the American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines (17). LC was diagnosed through histological examination, with the histological type and staging determined according to international guidelines and recommendations (18). The staging of non-small cell lung cancer (NSCLC) was determined according to the 8th edition of the tumor-node-metastasis (TNM) classification system. For small-cell lung cancer (SCLC), staging followed the Veterans Administration Lung Cancer Study Group (VALG) classification, distinguishing between limited-stage and extensive-stage disease. Staging was determined through a comprehensive assessment, including chest computed tomography (CT), positron emission tomography-computed tomography (PET-CT), and pathological findings, to ensure an accurate evaluation of the disease extent. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University (protocol No. 2022-067-02; March 28, 2022). The requirement for written informed consent was waived due to the retrospective nature of the analysis.
Clinical data
The medical records of the patients were reviewed. Demographic data including age of onset, sex, and smoking history were collected. Laboratory examination findings including white blood cell (WBC) count, lymphocyte count (L) and percentage (L%), neutrophil count (N) and percentage (N%), platelet count (PLT) and hemoglobin (Hb) level, ESR, lactate dehydrogenase (LDH), C-reactive protein (CRP), albumin (Alb), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and cytokeratin 19 (cyfra21-1) were recorded. Certain markers that are part of the extended ILD assessment, such as Krebs von den Lungen-6 (KL-6) and surfactant protein D (SP-D), were not available in our dataset and thus were not included in the analysis. Genetic alterations and immunohistochemical markers (PD-L1 TPS and TTF-1) were collected when available, but due to incomplete testing across the cohort, they were summarized descriptively and not included in the main analysis (Table S1). Therapeutic information comprised antifibrotic therapy, immunosuppressive therapy and corticosteroid therapy for ILD; and surgery, radiotherapy, chemotherapy, antiangiogenic therapy, immunotherapy, targeted therapy, and palliative care for LC.
Radiological analysis
All patients underwent chest HRCT during hospitalization for initial disease evaluation, with serial follow-up scans conducted throughout the treatment period. We acquired imaging at the time of the diagnosis of both ILD and LC for subsequent evaluation. Images were reviewed independently by two experienced radiologists blinded to the clinical information. HRCT findings of ILD were described as nonspecific interstitial pneumonia (NSIP) pattern, organizing pneumonia (OP) pattern, NSIP-OP overlap pattern, and usual interstitial pneumonia (UIP) pattern based on the 2013 ATS classification of idiopathic interstitial pneumonia guidelines (15). The relative anatomical locations of the tumor and the ILD lesions on HRCT were recorded.
Follow-up data
The vital status of patients was determined by reviewing medical records and via telephone communication. Survival time was defined as the period from the diagnosis of both ILD and LC to death, loss to follow-up or last follow-up. Follow-up data were collected until May 2024. Due to the retrospective nature of the study, specific causes of death were not consistently available, and therefore, all-cause mortality was used for survival analysis.
Construction and validation of a 1-year mortality risk prediction model
Given the high early mortality observed in this population (41.3% at 1 year), we selected 1-year mortality as the primary endpoint for risk prediction. This time point balances clinical relevance with feasibility for model development, as longer-term survival was limited in our cohort. After excluding 46 patients with incomplete survival data, we enrolled 160 patients and divided them into a training set and validation set at a ratio of 7:3 (Figure 1). First, univariate logistic regression was used to assess the association between indicators and the 1-year mortality of patients in the training set. In addition, multivariate logistic regression was further applied to assess independent predictors, and variables with P<0.05, along with factors reported in literature, were incorporated into a nomogram for predicting 1-year mortality. Each variable included in the model was assigned a regression coefficient (β), reflecting its relative contribution to the outcome. In the nomogram, individual variable values were converted into corresponding points according to the magnitude of their coefficients, and the total risk score was calculated as the sum of all individual points. Then, we evaluated the prediction model in terms of discrimination, calibration, and clinical applicability. The receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) were analyzed to evaluate the ability of the nomogram to distinguish outcome events. We performed bootstrapping with 1,000 replicates and drew calibration curves to assess the agreement between the actual observations and nomogram predictions. Clinical applicability was assessed through conducting decision curve analysis (DCA). Based on the risk scores in the training set, we divided patients into high-, medium-, and low-risk groups. Survival analysis was used to establish the 1-year survival probability in the three groups. Finally, an internal validation cohort was used to further assess the stability of the nomogram via AUC, calibration, DCA, and survival curves.
Statistical analysis
Qualitative data were presented as numbers and percentages, while quantitative data were expressed as the mean and standard deviation (SD) or median and interquartile range (IQR). The Cox proportional hazards model was used for univariate and multivariate survival analyses to calculate the hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs). Univariable and multivariable regressions were used to select the variables associated with 1-year mortality. Statistical analyses were performed using R software (version 4.3.1). Missing data were addressed using multiple imputation with the R package “mice” to reduce bias and loss of statistical power associated with complete-case analysis. P<0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of patients
A total of 206 patients with a diagnosis of ILD and LC were enrolled. There were 27 (13.1%) females and 179 (86.9%) males, with a mean age of 67.28±8.84 years. Among the patients, 127 (61.7%) had a smoking history. As shown in Figure 1, there were 67 patients with pre-existing ILD diagnosed with LC, 107 patients diagnosed with concurrent ILD and LC, and 32 patients with pre-existing LC diagnosed with ILD. Regarding the classification of ILD, 33 patients had connective tissue disease-associated ILD (CTD-ILD), 21 had IPF, and 139 had unclassified ILD according to clinical diagnosis (Figure 2A).
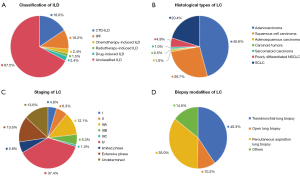
The pattern of ILD on HRCT was mainly UIP (102/206, 49.5%), followed by NSIP (85/206, 41.3%) and OP (10/206, 4.9%). Among the tumors, 43.7% (90/206) were located inside the ILD lesion (Table 1). Regarding the LC subtype, 164 patients had NSCLC. The distribution of LC histological types was as follows: 94 (45.6%) patients with adenocarcinoma, 55 (26.7%) with squamous cell carcinoma, 42 (20.4%) with SCLC, and 10 (4.9%) with poorly differentiated NSCLC. Of the patients with NSCLC, 48 were diagnosed with early-stage disease (≤ stage IIIA) and 90 with advanced-stage disease (> stage IIIA). Among the patients with SCLC, 28 had extensive-stage disease and 12 had limited-stage disease. All patients were diagnosed through histological examination, with 83 patients undergoing transbronchial lung biopsy, 72 undergoing percutaneous aspiration lung biopsy, and 21 diagnosed through pathological examination following surgical resection (Figure 2B-2D).
Table 1
| Variable | Overall (N=206) | NSCLC (N=164) | SCLC (N=42) | P value |
|---|---|---|---|---|
| Age (years) | 67.28±8.84 | 66.84±9.15 | 69.00±7.37 | 0.16 |
| Male | 179 (86.9) | 137 (83.5) | 42 (100.0) | 0.005 |
| Smoking history | 127 (61.7) | 96 (58.5) | 31 (73.8) | 0.07 |
| HRCT pattern of ILD | 0.21 | |||
| UIP | 102 (49.5) | 78 (47.6) | 24 (57.1) | |
| NSIP | 85 (41.3) | 70 (42.7) | 15 (35.7) | |
| OP | 10 (4.9) | 10 (6.1) | 0 (0.0) | |
| Non | 9 (4.4) | 6 (3.7) | 3 (7.1) | |
| Location of tumor inside ILD lesion | 90 (43.7) | 71 (43.3) | 19 (45.2) | 0.82 |
| Laboratory examination | ||||
| WBC (109/L) | 7.73±4.23 | 7.96±4.58 | 6.85±2.28 | 0.03 |
| Neutrophil percentage | 66.96±11.29 | 67.47±11.70 | 64.99±9.47 | 0.21 |
| Lymphocyte percentage | 22.46±9.16 | 22.01±9.26 | 24.18±8.67 | 0.17 |
| Platelets (109/L) | 214.61±84.53 | 217.75±88.62 | 202.50±65.97 | 0.30 |
| Hemoglobin (g/L) | 127.43±19.64 | 126.28±20.22 | 131.83±16.68 | 0.10 |
| ESR (mm/h) | 36.26±27.01 | 36.62±27.45 | 34.65±25.54 | 0.77 |
| CRP (mg/dL) | 24.37±34.22 | 26.89±36.87 | 14.85±18.92 | 0.005 |
| LDH (U/L) | 289.97±174.32 | 272.68±131.53 | 355.05±275.22 | 0.07 |
| Albumin (g/L) | 37.57±4.71 | 37.04±4.08 | 39.57±6.24 | 0.002 |
| CEA (ng/mL) | 3.50 (1.70–8.18) | 3.66 (1.57–8.15) | 3.07 (2.03–11.22) | 0.90 |
| NSE (ng/mL) | 15.95 (13.36–23.37) | 15.40 (12.70–19.96) | 31.53 (16.61–78.54) | <0.001 |
| Cyfra21-1 (ng/mL) | 4.78 (3.28–8.07) | 4.89 (3.42–8.77) | 4.37 (3.01–5.65) | 0.055 |
| ILD therapy | ||||
| Antifibrotic drugs | 70 (34.0) | 54 (32.9) | 16 (38.1) | 0.53 |
| Glucocorticoids | 62 (30.1) | 57 (34.8) | 5 (11.9) | 0.004 |
| Immunosuppressants | 20 (9.7) | 18 (11.0) | 2 (4.8) | 0.38 |
| None | 102 (49.5) | 77 (47.0) | 25 (59.5) | 0.15 |
| LC therapy | ||||
| Surgery | 38 (18.4) | 33 (20.1) | 5 (11.9) | 0.22 |
| Radiotherapy | 17 (8.3) | 13 (7.9) | 4 (9.5) | 0.76 |
| Chemotherapy | 130 (63.1) | 96 (58.5) | 34 (81.0) | 0.007 |
| Antiangiogenic therapy | 24 (11.7) | 23 (14.0) | 1 (2.4) | 0.03 |
| Immunotherapy | 27 (13.1) | 23 (14.0) | 4 (9.5) | 0.44 |
| Targeted therapy | 5 (2.4) | 5 (3.0) | 0 (0.0) | 0.59 |
| Palliative care | 53 (25.7) | 46 (28.0) | 7 (16.7) | 0.13 |
| 1-year mortality | 66/160 (41.3) | 48/131 (36.7) | 18/29 (62.1) | 0.02 |
Data are presented as mean ± SD, n (%) or median (interquartile range). CEA, carcinoembryonic antigen; CRP, C-reactive protein; Cyfra21-1, cytokeratin 21-1; ESR, erythrocyte sedimentation rate; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; LC, lung cancer; LDH, lactate dehydrogenase; NSCLC, non-small cell lung cancer; NSE, neuron-specific enolase; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; SCLC, small-cell lung cancer; SD, standard deviation; UIP, usual interstitial pneumonia; WBC, white blood cell.
A comparison of the patients with NSCLC and those with SCLC revealed that males were more common among those with SCLC than among those with NSCLC (100.0% vs. 83.5%; P=0.005; Table 1). No statistically significant difference was found in terms of age, smoking history, HRCT pattern, nor location of tumor between the two groups of patients. As summarized in Table 1, patients with SCLC had significantly lower CRP levels (P=0.005) and WBC (P=0.03) than did those with NSCLC, while the values of Alb (P=0.002) and NSE (P<0.001) were significantly higher in patients with SCLC.
Among the entire cohort, genetic testing and programmed death-ligand 1 (PD-L1) assessment were available for a limited number of patients (16 out of 206, 7.8%). Epidermal growth factor receptor (EGFR) mutations were the most frequently detected genetic alteration. PD-L1 TPS results were available in a few cases, with variable expression levels. TTF-1 expression was assessed in selected adenocarcinoma samples. Detailed molecular and immunohistochemical data are presented in Table S1.
We observed a significant difference in 1-year mortality between patients with advanced and early-stage NSCLC. Specifically, patients with advanced NSCLC exhibited a higher 1-year mortality compared to those with stage IIIA or lower NSCLC (50.0% vs. 26.2%, P=0.02). As shown in the Table S2, no significant differences were observed in LDH and Alb levels across LC stages.
Treatment information
As shown in Table 1, 50.5% (104/206) patients had received therapy of ILD, of whom 34.0% (70/206) had received antifibrotic treatment, 30.1% (62/206) had received glucocorticoid therapy, and 9.7% (20/206) had received immunosuppressants. For the treatment of LC, 130 patients received chemotherapy, 38 with surgery, 27 with immunotherapy, 24 with antiangiogenic therapy, and 17 with radiotherapy. Glucocorticoid therapy was more prevalent in the NSCLC group than in the SCLC group (34.8% vs. 11.9%; P=0.004), while chemotherapy therapy was more prevalent in the SCLC group than in the NSCLC group (81.0% vs. 58.5%; P=0.007); meanwhile, antiangiogenic therapy was more prevalent in the NSCLC group (14.0% vs. 2.4%; P=0.03).
Mortality and prognostic factors in the entire cohort
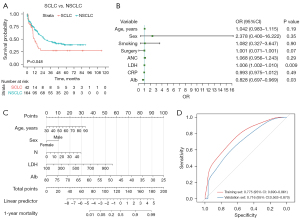
The mean follow-up time was 21.08±1.08 months. The all-cause 1-year mortality for patients was 41.3% (66/160) (Table 1). Patients with SCLC had a significantly higher 1-year mortality rate compared to those with NSCLC (62.1% vs. 36.7%; P=0.02). As shown in Figure 3A, patients with SCLC had a poorer overall survival than did those with NSCLC (P=0.048).
The Cox proportional hazards model was used to identify prognostic factors (Table 2). According to univariate analysis, absolute neutrophil count (ANC), ESR, LDH, CRP, and NSE were significantly associated with an increased risk of death (HR >1; P<0.05), whereas surgery, hemoglobin (Hb), and Alb were protective factors (HR <1; P<0.05). Targeted therapy for NSCLC and anti-fibrosis for ILD in patients were further analyzed, but no significant correlation with prognosis was found (P>0.05). HRCT-based classification into fibrotic versus non-fibrotic ILD patterns revealed no significant survival difference (P>0.05).
Table 2
| Variable | Univariate analysis | Multivariate analysis† | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.017 (0.995–1.040) | 0.13 | 1.021 (0.984–1.059) | 0.28 | |
| Male sex | 1.098 (0.644–1.871) | 0.73 | 0.708 (0.293–1.713) | 0.44 | |
| Smoking history | 0.958 (0.651–1.408) | 0.83 | 0.890 (0.436–1.818) | 0.75 | |
| Type of LC (NSCLC vs. SCLC) | 0.665 (0.418–1.058) | 0.08 | |||
| Order of occurrence of ILD and LC | |||||
| ILD-LC vs. LC-ILD | 1.106 (0.644–1.899) | 0.72 | |||
| Duration from the onset of ILD to LC development | 0.999 (0.999–1.000) | 0.47 | |||
| HRCT pattern of ILD | |||||
| Fibrotic pattern vs. non-fibrotic pattern | 0.955 (0.649–1.405) | 0.82 | |||
| Tumor located inside ILD lesion vs. outside ILD lesion | 1.117 (0.760–1.643) | 0.57 | |||
| NSCLC stage | |||||
| > Stage IIIA vs. ≤ stage IIIA | 1.469 (0.929–2.324) | 0.10 | |||
| Biopsy modalities of LC | |||||
| Transbronchial lung biopsy vs. other biopsies | 0.869 (0.581–1.301) | 0.50 | |||
| Therapy of ILD | |||||
| Antifibrotic drugs | 1.297 (0.874–1.924) | 0.20 | |||
| Antifibrotic drugs for FILD | 1.160 (0.651–2.060) | 0.62 | |||
| Glucocorticoids | 1.148 (0.769–1.716) | 0.50 | |||
| Immunosuppressants | 1.006 (0.539–1.878) | 0.98 | |||
| LC therapy | |||||
| Surgery | 0.553 (0.329–0.931) | 0.03 | 0.407 (0.139-1.190) | 0.10 | |
| Radiotherapy | 1.347 (0.737–2.461) | 0.33 | |||
| Chemotherapy | 0.858 (0.582–1.266) | 0.44 | |||
| Antiangiogenic therapy | 1.079 (0.592–1.967) | 0.81 | |||
| Immunotherapy | 0.925 (0.527–1.625) | 0.79 | |||
| Targeted therapy | 0.794 (0.196–3.221) | 0.75 | |||
| Targeted therapy for NSCLC | 0.168 (0.023–1.24) | 0.08 | |||
| Palliative care | 1.493 (0.987–2.259) | 0.058 | |||
| Laboratory examination | |||||
| WBC | 1.094 (1.056–1.134) | <0.001 | |||
| Neutrophil count | 1.100 (1.060–1.142) | <0.001 | 1.081 (0.985–1.186) | 0.099 | |
| Lymphocyte count | 1.051 (0.789–1.400) | 0.73 | |||
| Neutrophil percentage | 1.026 (1.008–1.044) | 0.004 | |||
| Lymphocyte percentage | 0.969 (0.948–0.991) | 0.005 | |||
| Platelet count | 0.999 (0.998–1.002) | 0.86 | |||
| Hemoglobin level | 0.991 (0.982–0.999) | 0.04 | 0.992 (0.969–1.017) | 0.54 | |
| ESR | 1.011 (1.002–1.021) | 0.02 | 0.999 (0.983–1.015) | 0.88 | |
| CRP level | 1.007 (1.002–1.012) | 0.01 | 1.004 (0.993–1.016) | 0.46 | |
| LDH level | 1.002 (1.001–1.003) | <0.001 | 1.002 (0.999–1.004) | 0.25 | |
| Albumin level | 0.921 (0.879–0.964) | <0.001 | 1.006 (0.893–1.133) | 0.93 | |
| CEA level | 1.005 (0.998–1.012) | 0.20 | |||
| NSE level | 1.005 (1.001–1.009) | 0.03 | 0.998 (0.983–1.013) | 0.78 | |
| Cyfra21-1 level | 1.002 (0.997–1.007) | 0.43 | |||
†, age, sex, smoking history, surgery, neutrophil count, hemoglobin, ESR, CRP, LDH, albumin, and NSE were included in the multivariate Cox analysis. CEA, carcinoembryonic antigen; CI, confidence interval; CRP, C-reactive protein; Cyfra21-1, cytokeratin 21-1; ESR, erythrocyte sedimentation rate; FILD, fibrotic interstitial lung disease; HR, hazard ratio; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; LC, lung cancer; LDH, lactate dehydrogenase; NSCLC, non-small cell lung cancer; NSE, neuron-specific enolase; SCLC, small-cell lung cancer; WBC, white blood cell.
Construction and validation of a 1-year mortality risk prediction model
As shown in Table 3, 115 patients were placed in a training set and 45 patients in a validation set. The baseline characteristics of the two sets showed no significant differences. In the univariable logistic regression analysis of the training set, age (P=0.04), neutrophil count (P=0.03), LDH (P<0.001), Alb (P<0.001), CRP (P=0.04), and surgery (P=0.02) exhibited significantly statistical differences (Table 4). We included these significant factors, sex, and smoking history into multivariable logistic regression analysis, which established LDH (OR =1.006; 95% CI: 1.002–1.010; P=0.009) and Alb (OR =0.828; 95% CI: 0.697–0.969; P=0.03) as being independently associated with the 1-year mortality of patients (Table 4 and Figure 3B).
Table 3
| Variables | Overall (N=160) | Training set (N=115) | Validation set (N=45) | P value |
|---|---|---|---|---|
| Age (years) | 67.10±8.98 | 66.90±8.67 | 67.60±9.79 | 0.66 |
| Male | 137 (85.62) | 101 (87.83) | 36 (80.00) | 0.31 |
| Smoking history | 98 (61.25) | 75 (65.22) | 23 (51.11) | 0.14 |
| HRCT pattern of ILD | 0.75 | |||
| UIP | 71 (44.38) | 50 (43.48) | 21 (46.67) | |
| NSIP/OP | 84 (52.50) | 62 (53.91) | 22 (48.89) | |
| None | 5 (3.12) | 3 (2.61) | 2 (4.44) | |
| Location of tumor inside ILD lesion | 68 (42.50) | 49 (42.61) | 19 (42.22) | >0.99 |
| Laboratory examination | ||||
| WBC (109/L) | 8.01±4.62 | 8.06±5.00 | 7.88±3.49 | 0.83 |
| Neutrophil percentage | 67.36±11.42 | 68.11±11.79 | 65.46±10.31 | 0.19 |
| Lymphocyte percentage | 22.17±9.33 | 21.58±9.39 | 23.66±9.11 | 0.21 |
| Platelet (109/L) | 217.24±86.85 | 219.05±90.18 | 212.62±78.46 | 0.68 |
| Hemoglobin (g/L) | 126.66±20.48 | 126.43±21.63 | 127.24±17.42 | 0.82 |
| ESR (mm/h) | 37.54±30.03 | 38.87±30.15 | 34.13±29.80 | 0.37 |
| CRP (mg/dL) | 22.51±33.30 | 24.58±35.03 | 17.22±28.07 | 0.21 |
| LDH (U/L) | 287.73±151.36 | 283.26±138.46 | 299.16±181.46 | 0.55 |
| Albumin (g/L) | 37.57±4.95 | 37.67±5.15 | 37.30±4.44 | 0.67 |
| CEA (ng/mL) | 3.08 (1.37, 6.64) | 3.33 (1.37, 7.56) | 2.25 (1.55, 6.03) | 0.44 |
| NSE (ng/mL) | 15.84 (13.09, 21.89) | 15.90 (12.81, 21.98) | 15.40 (13.11, 21.70) | 0.92 |
| Cyfra21-1 (ng/mL) | 4.81 (3.14, 7.96) | 4.90 (3.13, 8.23) | 4.53 (3.17, 6.94) | 0.44 |
| ILD therapy | ||||
| Antifibrotic drugs | 54 (33.75) | 40 (34.78) | 14 (31.11) | 0.80 |
| Glucocorticoids | 50 (31.25) | 37 (32.17) | 13 (28.89) | 0.83 |
| Immunosuppressants | 16 (10.00) | 12 (10.43) | 4 (8.89) | >0.99 |
| None | 102 (49.5) | 77 (47.00) | 25 (59.5) | 0.15 |
| LC therapy | ||||
| Surgery | 32 (20.00) | 25 (21.74) | 7 (15.56) | 0.51 |
| Radiotherapy | 14 (8.75) | 11 (9.57) | 3 (6.67) | 0.79 |
| Chemotherapy | 98 (61.25) | 72 (62.61) | 26 (57.78) | 0.70 |
| Antiangiogenic therapy | 18 (11.25) | 12 (10.43) | 6 (13.33) | 0.81 |
| Immunotherapy | 22 (13.75) | 17 (14.78) | 5 (11.11) | 0.73 |
| Targeted therapy | 2 (1.25) | 2 (1.74) | 0 (0.00) | 0.92 |
| Palliative care | 43 (26.88) | 30 (26.09) | 13 (28.89) | 0.87 |
Data are presented as mean ± SD, n (%) or median (interquartile range). CEA, carcinoembryonic antigen; CRP, C-reactive protein; Cyfra21-1, cytokeratin 21-1; ESR, erythrocyte sedimentation rate; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; LC, lung cancer; LDH, lactate dehydrogenase; NSIP, nonspecific interstitial pneumonia; NSE, neuron-specific enolase; OP, organizing pneumonia; SD, standard deviation; UIP, usual interstitial pneumonia; WB, white blood cell.
Table 4
| Variable | Univariate analysis | Multivariate analysis† | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | 1.056 (1.007–1.116) | 0.04 | 1.042 (0.983–1.115) | 0.19 | |
| Male sex | 1.930 (0.601–1.871) | 0.29 | 2.378 (0.40–16.222) | 0.35 | |
| Smoking history | 0.953 (0.439–2.090) | 0.90 | 1.082 (0.326–3.647) | 0.90 | |
| Type of LC (NSCLC vs. SCLC) | 0.418 (0.157–1.067) | 0.07 | |||
| Tumor located inside ILD lesion | 1.451 (0.686–3.086) | 0.33 | |||
| Staging of NSCLC | |||||
| > Stage IIIA vs. ≤ stage IIIA | 2.317 (0.958–5.894) | 0.07 | |||
| Biopsy modalities of LC | |||||
| Transbronchial lung biopsy vs. other biopsies | 0.760 (0.337–1.701) | 0.51 | |||
| ILD therapy | |||||
| Antifibrotic drugs | 1.227 (0.561–2.669) | 0.65 | |||
| Glucocorticoids | 0.665 (0.291–1.479) | 0.32 | |||
| Immunosuppressants | 1.452 (0.427–4.941) | 0.54 | |||
| LC therapy | |||||
| Surgery | 0.273 (0.085–0.742) | 0.02 | 0.297 (0.071–1.001) | 0.07 | |
| Radiotherapy | 0.492 (0.103–1.808) | 0.31 | |||
| Chemotherapy | 0.628 (0.291–1.351) | 0.23 | |||
| Antiangiogenic therapy | 0.997 (0.279–3.331) | > 0.99 | |||
| Immunotherapy | 0.973 (0.329–2.747) | 0.96 | |||
| Targeted therapy | 0.535 (0.329–3.271) | 0.99 | |||
| Palliative care | 2.278 (0.983–5.397) | 0.057 | |||
| Laboratory examination | 1.079 (0.592–1.967) | 0.81 | |||
| WBC | 1.117 (1.015–1.259) | 0.047 | |||
| Neutrophil count | 1.159 (1.033–1.333) | 0.03 | 1.068 (0.956–1.243) | 0.29 | |
| Lymphocytes count | 0.710 (0.382–1.288) | 0.27 | |||
| Neutrophil percentage | 1.044 (1.010–1.081) | 0.01 | |||
| Lymphocytes percentage | 0.942 (0.900–0.982) | 0.007 | |||
| Platelet count | 0.999 (0.995–1.003) | 0.67 | |||
| Hemoglobin level | 0.986 (0.968–1.003) | 0.12 | |||
| ESR | 1.011 (0.999–1.024) | 0.08 | |||
| CRP level | 1.012 (1.001–1.025) | 0.04 | 0.993 (0.974–1.012) | 0.49 | |
| LDH level | 1.007 (1.003–1.011) | <0.001 | 1.006 (1.002–1.010) | 0.009 | |
| Albumin level | 0.821 (0.726–0.915) | <0.001 | 0.828 (0.697–0.969) | 0.03 | |
| CEA level | 1.006 (0.987–1.025) | 0.54 | |||
| NSE level | 1.021 (1.003–1.048) | 0.07 | |||
| Cyfra21-1 level | 0.998 (0.983–1.010) | 0.79 | |||
†, age, sex, smoking history, surgery, neutrophil count, CRP, LDH, and albumin were included in multivariate logistic regression analysis. CEA, carcinoembryonic antigen; CI, confidence interval; CRP, C-reactive protein; Cyfra21-1, cytokeratin 21-1; ESR, erythrocyte sedimentation rate; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; LC, lung cancer; LDH, lactate dehydrogenase; NSCLC, non-small cell lung cancer; NSE, neuron-specific enolase; OR, odds ratio; SCLC, small-cell lung cancer; WBC, white blood cell.
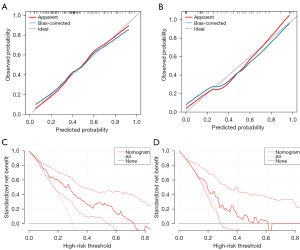
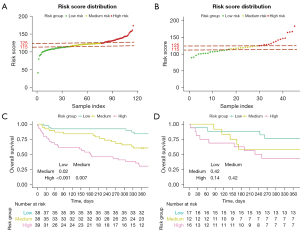
Considering clinical values, we incorporated sex, age, neutrophil count, LDH, and Alb into the model. We then created a visual nomogram to represent the model (Figure 3C). The AUC values in the training set and the internal validation set were 0.775 (95% CI: 0.690–0.861) and 0.716 (95% CI: 0.563–0.870), respectively (Figure 3D). In the calibration curve, the bias-corrected lines for the training and the internal validation sets closely overlapped with the ideal lines (Figure 4A,4B). DCA provided additional insights into the clinical applicability of the model across various thresholds. The results indicated that within a wide range of clinical decision thresholds, the net benefit of using our model was superior to intervening on all patients or not intervening on any patients (Figure 4C,4D). Patients were divided into high-, medium-, and low-risk groups based on total risk scores of the five factors in the nomogram according to various individual’s parameters (115 and 125) (Figure 5A,5B). In the training set, the 1-year survival probabilities varied significantly (high vs. medium vs. low: 30.7% vs. 60.5% vs. 84.2%) among the three groups (Figure 5C). However, in the internal validation cohort, the survival curves did not show statistical significance (high vs. medium vs. low: 43.8% vs. 58.3% vs. 76.5%) (Figure 5D).
Discussion
This retrospective study found that the 1-year mortality in patients with concurrent ILD and LC was 41.3%, which aligns with existing clinical observation that 53.5% of these patients succumb within the first year after diagnosis (19). The mean follow-up time was 21.08±1.08 months in this cohort, reflecting the characteristically poor prognosis observed in patients with concurrent ILD and LC. Therefore, we developed a 1-year mortality prediction model incorporating some easily accessible clinical parameters including age, sex, neutrophil count, and LDH concentration and Alb levels. The risk prediction model demonstrated consistency and good utility in the training and the validation sets. The risk stratification capability of the prediction model can assist in early identification of high-risk patients and enable them benefit from intensified monitoring, early referral to palliative care, or more conservative treatment approaches.
ILD and LC share several pathogenic mechanisms, including common epigenetic and gene expression. A genome-wide methylation analysis revealed that 402 CpG islands overlap between IPF and LC (20). Moreover, mutations in telomerase reverse transcriptase (TERT) and telomerase reverse transcriptase (TERC) genes and telomere shortening are found in familial IPF (21), which are also observed to be associated with the increased risk in LC (22). Furthermore, the abnormal activation of Wnt/β-catenin signaling pathway, phosphatidylinositol 3-kinase (PI3K)/Akt pathway, and tyrosine kinases has been reported to be present in both diseases (8). Interestingly, a majority of patients with LC have a predilection for the subpleural region which is the typical distribution of UIP (23).
LC and IPF are more prevalent among men, which can be attributed to a greater tendency to consume tobacco and higher occupational exposure (24,25). Elevated testosterone is also associated with increased risk of LC (26), and men have a worse prognosis in ILD and lower transplant-free survival (27). Neutrophils may accelerate the onset and development of fibrotic ILD by forming neutrophil extracellular traps (NETs), which critically contribute to the fibrotic process by causing proinflammatory effects. Moreover, neutrophils forming NETs can promote cancer metastasis by activating the pathways mediating tumor cell proliferation (28,29). Increased neutrophil count in bronchoalveolar lavage fluid (BALF) has been correlated with the elevated mortality of patients with IPF, microscopic polyangiitis (MPA), and rheumatoid arthritis (RA)-associated ILD (30-32). Similarly, elevated neutrophil levels may also contribute to an increased risk of mortality in patients with NSCLC (33). One study found that circulating neutrophils are positively correlated with increased tumor burden, and surgical tumor resection is followed by a subsequent reduction in peripheral neutrophil count (34). Another study reported that neutrophils promote resistance to radiation therapy (35). In our study, neutrophil counts were marginally significantly associated with overall survival with a P value between 0.05 and 0.1, and associated with an increased risk of 1-year mortality.
Elevated LDH levels are associated with increased 3-year mortality in progressive fibrosing ILD (PFILD) and anti-MDA5-positive-dermatomyositis (DM)-associated rapidly progressive interstitial lung disease (RP-ILD) (36-38). In one study on NSCLC, an increased level of LDH was associated with poorer prognosis and radioresistance (39). In another study on patients with limited-stage SCLC, the 1-year overall survival in the high-LDH group was 33.1%, while it was 60.2% in the normal groups (40). In other research, patients with NSCLC who were treated with immune checkpoint inhibitors (ICIs) demonstrated an elevated LDH level, which was correlated with significantly shorter progression-free survival (PFS) (41). Similarly, patients with advanced-stage NSCLC and LDH <240 U/L were found to have a favorable response to programmed cell death protein 1 (PD-1) inhibitors (42).
A lower serum Alb level has been observed in progressive pulmonary fibrosis (PPF) and linked to increased mortality among patients with CTD-ILD (43). Moreover, poorer survival in patients with polymyositis- or dermatomyositis-associated ILD has been correlated with a lower serum Alb level (44). Additionally, Alb has been associated with the development of ILD among patients with NSCLC with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) (45). A large-scale retrospective study evaluated preoperative serum Alb levels in surgically resected patients with NSCLC and found that patients with Alb <4.2 g/dL had significantly poorer prognosis; moreover, the serum Alb levels were significantly lower in the pulmonary fibrosis group or emphysema group than in the control group (46).
Serum CRP levels were significantly higher in patients with NSCLC compared to those with SCLC in our study. Previous research has demonstrated a positive correlation between CRP levels and maximum tumor diameter in NSCLC (47). Male smokers with squamous cell carcinoma were predominantly observed with having elevated CRP levels, which may be attributed to the tendency of squamous tumors to undergo necrosis and provoke obstructive inflammation, thereby promoting CRP elevation (48,49). Therefore, we hypothesized that the significant difference of CRP levels may reflect the marked histological and biological differences between NSCLC and SCLC. NSCLC typically exhibits slower growth and larger tumor burden, which can induce a sustained local inflammatory response. In contrast, SCLC is characterized by rapid proliferation and high aggressiveness, but its swift progression may not provoke a notable inflammatory response in the early stages, resulting in relatively lower CRP levels (50).
The subtype and severity of ILD play a significant role in determining patient prognosis. Fibrotic ILD, particularly IPF, is associated with a poorer prognosis and more rapid progression, whereas non-fibrotic forms of ILD, such as hypersensitivity pneumonitis or some autoimmune-related ILDs, exhibit more variable courses, with some patients remaining stable or even improving (51). Additionally, the severity of ILD, as assessed by pulmonary function tests, imaging findings, and clinical symptoms, directly correlates with survival outcomes. Reduced forced vital capacity (FVC) and the presence of honeycombing on CT scans are indicative of more advanced fibrosis and are associated with worse prognosis (52).
The treatment of patients with concurrent ILD and LC is a considerable challenge. Surgical resection is the preferred treatment for early-stage NSCLC. However, ILD is an independent predictor for higher mortality and incidence of acute respiratory distress syndrome (ARDS) in patients treated with surgery, even in those with normal pulmonary function (53,54). A large cohort study in Japan reported that 35.6% of patients with ILD-LC died of AE-ILD within 30 days of surgery (16). Furthermore, individuals with pre-existing ILD are at a higher risk of radiation-related pulmonary toxicity (55). Evidence suggests that chemotherapy can exacerbate radiation-related pneumonitis and increase the risk of AE-ILD (56,57). Similarly, EGFR-TKIs and ICI-associated pneumonitis (CIP) may cause drug-related lung injury (58), with pre-existing ILD increasing the risk of acute exacerbation (AE) of ILD, characterized by a rapid decline in pulmonary function and worsening of clinical manifestations (59). Therefore, it is necessary to carefully balance the potential benefit and treatment-associated risks.
Nintedanib, a TKI, can slow fibrotic progression and reduce the incidence of AE (60,61). A phase I study demonstrated that nintedanib (200 mg bid) plus cisplatin/gemcitabine for squamous NSCLC (sqNSCLC) has a good safety profile (62). One case report described a patient with NSCLC complicated by IPF who achieved HRCT partial remission without exacerbation of IPF after treatment with nintedanib. Moreover, nintedanib in combination with carboplatin plus nab-paclitaxel can improve overall survival in patients with advanced NSCLC and IPF (63). Additionally, the use of pirfenidone postoperatively can reduce perioperative mortality and the incidence of AE-IPF (64). Our results indicated that antifibrotic treatment exerted no impact on prognosis, probably due to the small sample of patients receiving antifibrotic therapy. Further studies are required to clarify the effect of antifibrotic therapy on the prognosis of patients with ILD and LC.
This study involved several limitations which should be addressed. First, we employed a retrospective, single-center design with a small sample size, and thus selection bias was inevitable. Therefore, external validation using independent cohorts from multiple centers is essential to assess the model’s robustness and to refine its applicability in varied clinical environments. Future studies should focus on validating the model in external cohorts to confirm its generalizability and utility. Moreover, some data were missing, and no external validation was performed in other populations, restricting the generalizability of our prediction model. Second, we did not analyze the indicators of pulmonary function due to patients’ low tolerance, precluding accurate classification of ILD severity. Data on percutaneous ablation were also not available in our cohort. Given the potential therapeutic option for selected patients with LC and the unique risk profile of ILD patients, further studies are warranted to evaluate the safety and efficacy of ablation in this population. ILD-specific biomarkers, KL-6 and SP-D, were not included due to limited availability in routine clinical practice. Future models may benefit from incorporating such biomarkers to improve risk stratification in patients with IPF. Third, due to the nature of the retrospective study, the treatment data of many patients from other or our hospital were not accurate, which may have some impact on the statistical analysis. In addition, survival analyses in the internal validation set indicated no significant differences among the high-, medium-, and low-risk groups stratified based on training set, potentially due to the small sample size (n=45). Multicenter, large-sample studies are needed to further validate our results. Lastly, the event per variable (EPV) was approximately 13.2 in our study, which is slightly above the commonly recommended threshold of 10 for multivariate logistic regression models. We evaluated the model’s robustness using (cross-validation/bootstrap) techniques, and no significant instability was observed. Nonetheless, the estimated regression coefficients should be interpreted carefully. Future studies with larger cohorts or external validation are needed to further assess and strengthen the model’s generalizability and reliability.
Conclusions
We developed a prediction model, which incorporated age, sex, ANC, LDH, and Alb. The model may be useful for predicting the 1-year mortality of patients with concurrent ILD and LC. Multicenter studies are needed to externally validate our findings.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-235/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-235/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-235/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-235/coif). L.M. serves as an unpaid editorial board member of Translational Lung Cancer Research from July 2024 to July 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University (protocol No. 2022-067-02; March 28, 2022). The requirement for written informed consent was waived due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet 2022;400:769-86. [Crossref] [PubMed]
- Yoo H, Jeong BH, Chung MJ, et al. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med 2019;19:149. [Crossref] [PubMed]
- Choi WI, Park SH, Park BJ, et al. Interstitial Lung Disease and Lung Cancer Development: A 5-Year Nationwide Population-Based Study. Cancer Res Treat 2018;50:374-81. [Crossref] [PubMed]
- Kawasaki H, Nagai K, Yokose T, et al. Clinicopathological characteristics of surgically resected lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 2001;76:53-7. [Crossref] [PubMed]
- Kinoshita T, Goto T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int J Mol Sci 2019;20:1461. [Crossref] [PubMed]
- Krabbe J, Steffens KM, Drießen S, et al. Lung cancer risk and occupational pulmonary fibrosis: systematic review and meta-analysis. Eur Respir Rev 2024;33:230224. [Crossref] [PubMed]
- Gosens R, Giangreco A, Sahai E, et al. Mechanistic overlap between chronic lung injury and cancer: ERS Lung Science Conference 2017 report. Eur Respir Rev 2017;26:170060. [Crossref] [PubMed]
- Tzouvelekis A, Gomatou G, Bouros E, et al. Common Pathogenic Mechanisms Between Idiopathic Pulmonary Fibrosis and Lung Cancer. Chest 2019;156:383-91. [Crossref] [PubMed]
- Lee JS, Lee EY, Ha YJ, et al. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 2019;21:58. [Crossref] [PubMed]
- Parker MJS, Jee AS, Hansen D, et al. Multiple serum biomarkers associate with mortality and interstitial lung disease progression in systemic sclerosis. Rheumatology (Oxford) 2024;63:2981-8. [Crossref] [PubMed]
- Ghuman A, Khanna D, Lin CJF, et al. Prognostic and predictive markers of systemic sclerosis-associated interstitial lung disease in a clinical trial and long-term observational cohort. Rheumatology (Oxford) 2024;63:472-81. [Crossref] [PubMed]
- Otsuka J, Yoshizawa S, Ikematsu Y, et al. Acute exacerbation in antineutrophil cytoplasmic antibody-associated interstitial lung disease: Clinical features and risk factors. Respir Med 2022;203:106992. [Crossref] [PubMed]
- Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018;9:847-55. [Crossref] [PubMed]
- Karampitsakos T, Tzilas V, Tringidou R, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 2017;45:1-10. [Crossref] [PubMed]
- Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol 2015;25:100-9. [Crossref] [PubMed]
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-1611.e3. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Kato E, Takayanagi N, Takaku Y, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ Open Res 2018;4:00111-2016. [Crossref] [PubMed]
- Rabinovich EI, Kapetanaki MG, Steinfeld I, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One 2012;7:e33770. [Crossref] [PubMed]
- Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007;356:1317-26. [Crossref] [PubMed]
- de-Torres JP, Sanchez-Salcedo P, Bastarrika G, et al. Telomere length, COPD and emphysema as risk factors for lung cancer. Eur Respir J 2017;49:1601521. [Crossref] [PubMed]
- Watanabe Y, Kawabata Y, Koyama N, et al. A clinicopathological study of surgically resected lung cancer in patients with usual interstitial pneumonia. Respir Med 2017;129:158-63. [Crossref] [PubMed]
- May L, Shows K, Nana-Sinkam P, et al. Sex Differences in Lung Cancer. Cancers (Basel) 2023;15:3111. [Crossref] [PubMed]
- Koudstaal T, Wijsenbeek MS. Idiopathic pulmonary fibrosis. Presse Med 2023;52:104166. [Crossref] [PubMed]
- Chan YX, Alfonso H, Chubb SA, et al. Higher Dihydrotestosterone Is Associated with the Incidence of Lung Cancer in Older Men. Horm Cancer 2017;8:119-26. [Crossref] [PubMed]
- Zaman T, Moua T, Vittinghoff E, et al. Differences in Clinical Characteristics and Outcomes Between Men and Women With Idiopathic Pulmonary Fibrosis: A Multicenter Retrospective Cohort Study. Chest 2020;158:245-51. [Crossref] [PubMed]
- Yan S, Li M, Liu B, et al. Neutrophil extracellular traps and pulmonary fibrosis: an update. J Inflamm (Lond) 2023;20:2. [Crossref] [PubMed]
- Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 2016;8:361ra138. [Crossref] [PubMed]
- Kinder BW, Brown KK, Schwarz MI, et al. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 2008;133:226-32. [Crossref] [PubMed]
- Kim MJ, Lee D, Choe J, et al. Long-term clinical course and outcomes of patients with microscopic polyangiitis-associated interstitial lung disease. Front Pharmacol 2023;14:1064307. [Crossref] [PubMed]
- Qin Y, Wang Y, Meng F, et al. Identification of biomarkers by machine learning classifiers to assist diagnose rheumatoid arthritis-associated interstitial lung disease. Arthritis Res Ther 2022;24:115. [Crossref] [PubMed]
- Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 2009;45:1950-8. [Crossref] [PubMed]
- Mitchell KG, Diao L, Karpinets T, et al. Neutrophil expansion defines an immunoinhibitory peripheral and intratumoral inflammatory milieu in resected non-small cell lung cancer: a descriptive analysis of a prospectively immunoprofiled cohort. J Immunother Cancer 2020;8:e000405. [Crossref] [PubMed]
- Wisdom AJ, Hong CS, Lin AJ, et al. Neutrophils promote tumor resistance to radiation therapy. Proc Natl Acad Sci U S A 2019;116:18584-9. [Crossref] [PubMed]
- Li M, Zhao X, Liu B, et al. Predictors of rapidly progressive interstitial lung disease and prognosis in Chinese patients with anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Front Immunol 2023;14:1209282. [Crossref] [PubMed]
- Wang H, Chen X, Du Y, et al. Mortality risk in patients with anti-MDA5 dermatomyositis is related to rapidly progressive interstitial lung disease and anti-Ro52 antibody. Arthritis Res Ther 2023;25:127. [Crossref] [PubMed]
- Watase M, Mochimaru T, Kawase H, et al. Diagnostic and prognostic biomarkers for progressive fibrosing interstitial lung disease. PLoS One 2023;18:e0283288. [Crossref] [PubMed]
- Yang Y, Chong Y, Chen M, et al. Targeting lactate dehydrogenase a improves radiotherapy efficacy in non-small cell lung cancer: from bedside to bench. J Transl Med 2021;19:170. [Crossref] [PubMed]
- Tas F, Aydiner A, Demir C, et al. Serum lactate dehydrogenase levels at presentation predict outcome of patients with limited-stage small-cell lung cancer. Am J Clin Oncol 2001;24:376-8. [Crossref] [PubMed]
- Zhang Z, Li Y, Yan X, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med 2019;8:1467-73. [Crossref] [PubMed]
- Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother 2020;69:1813-22. [Crossref] [PubMed]
- Lee JK, Ahn Y, Noh HN, et al. Clinical effect of progressive pulmonary fibrosis on patients with connective tissue disease-associated interstitial lung disease: a single center retrospective cohort study. Clin Exp Med 2023;23:4797-807. [Crossref] [PubMed]
- Ji SY, Zeng FQ, Guo Q, et al. Predictive factors and unfavourable prognostic factors of interstitial lung disease in patients with polymyositis or dermatomyositis: a retrospective study. Chin Med J (Engl) 2010;123:517-22. [Crossref] [PubMed]
- Fukuda Y, Uchida Y, Ando K, et al. Risk factors for interstitial lung disease in patients with non-small cell lung cancer with epidermal growth factor receptor-tyrosine kinase inhibitors: A systematic review and meta-analysis. Respir Investig 2024;62:481-7. [Crossref] [PubMed]
- Miura K, Hamanaka K, Koizumi T, et al. Clinical significance of preoperative serum albumin level for prognosis in surgically resected patients with non-small cell lung cancer: Comparative study of normal lung, emphysema, and pulmonary fibrosis. Lung Cancer 2017;111:88-95. [Crossref] [PubMed]
- Xu Y, Li Z, Shen-Tu Y. The prognostic value of detection of serum C-reactive protein in the patients with stage I lung cancer. Zhongguo Fei Ai Za Zhi 2011;14:400-3. [PubMed]
- Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol 2006;24:5216-22. [Crossref] [PubMed]
- Ji M, Du L, Ma Z, et al. Circulating C-reactive protein increases lung cancer risk: Results from a prospective cohort of UK Biobank. Int J Cancer 2022;150:47-55. [Crossref] [PubMed]
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Takei R, Brown KK, Yamano Y, et al. Prevalence and prognosis of chronic fibrosing interstitial lung diseases with a progressive phenotype. Respirology 2022;27:333-40. [Crossref] [PubMed]
- Macaluso C, Boccabella C, Kokosi M, et al. Short-term lung function changes predict mortality in patients with fibrotic hypersensitivity pneumonitis. Respirology 2022;27:202-8. [Crossref] [PubMed]
- Axtell AL, David EA, Block MI, et al. Association Between Interstitial Lung Disease and Outcomes After Lung Cancer Resection. Ann Thorac Surg 2023;116:533-41. [Crossref] [PubMed]
- Lee T, Park JY, Lee HY, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med 2014;108:1549-55. [Crossref] [PubMed]
- Chen H, Senan S, Nossent EJ, et al. Treatment-Related Toxicity in Patients With Early-Stage Non-Small Cell Lung Cancer and Coexisting Interstitial Lung Disease: A Systematic Review. Int J Radiat Oncol Biol Phys 2017;98:622-31. [Crossref] [PubMed]
- Parashar B, Edwards A, Mehta R, et al. Chemotherapy significantly increases the risk of radiation pneumonitis in radiation therapy of advanced lung cancer. Am J Clin Oncol 2011;34:160-4. [Crossref] [PubMed]
- Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011;6:1242-6. [Crossref] [PubMed]
- Wang H, Guo X, Zhou J, et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer 2020;11:191-7. [Crossref] [PubMed]
- Shi L, Tang J, Tong L, et al. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer 2014;83:231-9. [Crossref] [PubMed]
- Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020;8:453-60. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial. Eur Respir J 2022;59:2004538. [Crossref] [PubMed]
- Forster M, Hackshaw A, De Pas T, et al. A phase I study of nintedanib combined with cisplatin/gemcitabine as first-line therapy for advanced squamous non-small cell lung cancer (LUME-Lung 3). Lung Cancer 2018;120:27-33. [Crossref] [PubMed]
- Otsubo K, Kishimoto J, Ando M, et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: a randomised phase 3 trial. Eur Respir J 2022;60:2200380. [Crossref] [PubMed]
- Iwata T, Yoshida S, Fujiwara T, et al. Effect of Perioperative Pirfenidone Treatment in Lung Cancer Patients With Idiopathic Pulmonary Fibrosis. Ann Thorac Surg 2016;102:1905-10. [Crossref] [PubMed]
(English Language Editor: J. Gray)



