
Absent preoperative non-small cell lung cancer confirmation and relevant stage migration in the era before neoadjuvant chemoimmunotherapy: implications for treatment decisions in resectable non-small cell lung cancer
Highlight box
Key findings
• Two fundamental aspects of the work-up of resectable non-small cell lung cancer (NSCLC) were suboptimal in a real-world population prior to the introduction of neoadjuvant chemoimmunotherapy.
• First, preoperative confirmation of NSCLC was missing in one-third of patients; thirty percent of whom had clinical stage II or III disease.
• Second, relevant clinicopathological stage migration was observed in one-fifth of the total study cohort and in one-fifth of the confirmed upfront NSCLC diagnosis cohort.
What is known and what is new?
• It is known that in almost half of patients with resectable NSCLC, stage migration occurs.
• This study investigated relevant stage migration—clinical stage I towards pathological stage II–III and vice versa—in the era before the introduction of neoadjuvant chemoimmunotherapy.
What is the implication, and what should change now?
• Optimizing diagnostic and staging procedures is needed to adequately administer neoadjuvant treatment.
Introduction
Missing upfront pathological confirmation and suboptimal staging in potentially resectable non-small cell lung cancer (NSCLC) may reduce the number of patients appropriately receiving neo-adjuvant chemoimmunotherapy.
Background
In patients with early-stage NSCLC limited to hilar nodal disease, surgery is the cornerstone treatment. Current adjuvant treatment strategies include radiotherapy, chemotherapy and more recently, immunotherapy and targeted therapy (1-4). For many years, neoadjuvant treatment regimens included platinum-based chemotherapy with or without radiotherapy, administered in patients with resectable stage III NSCLC without bulky N2 disease (5). In recent trials, however, neoadjuvant and perioperative treatment strategies including immune checkpoint inhibitors (ICIs) were investigated in patients with resectable NSCLC (6-9). These studies showed an event-free survival (EFS) benefit in patients receiving neoadjuvant chemoimmunotherapy versus chemotherapy alone. Based on the European Medicines Agency (EMA) recommendations, the European Commission (EC) recently approved neoadjuvant nivolumab for patients with stage II–IIIA NSCLC* and a programmed death-ligand 1 (PD-L1) expression of at least 1% and perioperative pembrolizumab for patients with stage II–IIIB NSCLC. In addition, durvalumab has recently been approved by the Food and Drugs Administration (FDA) for stage II–IIIB NSCLC. Consequently, these treatment strategies are currently being implemented in leading national and international guidelines (10-12).
Rationale and knowledge gap
Confirmative upfront diagnosing and adequate clinical staging of NSCLC can be challenging (13). Obtaining a preoperative diagnosis of NSCLC before curative resection is recommended in the majority of relevant guidelines (10-12). In clinical practice, a substantial part of patients with NSCLC undergo surgery without preoperative pathological confirmation. For example, a recent Dutch retrospective cohort study showed that surgical resection of the tumor without an upfront pathological diagnosis occurred in 35% of cases (14). In 38% of patients without lymph node involvement, no preoperative diagnosis was established, versus 22% of patients with at least N1 disease. Consequently, administration of induction treatment in this subgroup will be impeded, as pathological confirmation is recommended before the administration of systemic treatment (15).
Adequate clinical staging of resectable NSCLC is crucial in order to optimize treatment decision-making. Mediastinal and hilar staging by endoscopic ultrasound (EUS) endobronchial ultrasound (EBUS) are widely recommended in case of a central tumor, a tumor size exceeding 3 cm or signs of lymph node involvement on computed tomography (CT) or positron emission tomography (PET) (10-12). Examples of tissue sampling techniques are transbronchial needle aspiration (TBNA), cryobiopsy or intranodal forceps biopsy (IFB) (16). However, different quality standards and guidelines exist on how to perform EBUS systematically in NSCLC diagnostics and staging procedures (17). In addition, marked variation has been noticed in EBUS frequency performed in clinical practice and widespread evaluation reports are regularly inadequate or even missing (17,18). As a result, discrepancies between initial clinical staging versus pathological staging after resection are common (19). In the emerging era of neoadjuvant and perioperative treatment, suboptimal staging might lead to under- and overtreatment, resulting in suboptimal patient care and avertible expenditures. To the best of our knowledge, stage migration has not been investigated comprehensively in the context of the multimodal treatment regimens in resectable NSCLC.
Objective
In this study, we examined absence of preoperative pathological NSCLC confirmation with focus on clinical stage II and III disease in an era before the introduction of neoadjuvant chemoimmunotherapy. In addition, we assessed the degree of relevant stage migration in patients with a preoperative NSCLC diagnosis. We hypothesized that these factors are substantially present in the study population with resectable NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1120/rc).
Methods
A retrospective observational cohort study was performed to assess the prevalence of cases where resection occurred without upfront confirmation of NSCLC. In addition, relevant stage migration was investigated with focus on patients with an adequate preoperative NSCLC diagnosis.
Study population
Patients above 18 years of age diagnosed between 2015 and 2019 with resectable NSCLC were included. Patients were diagnosed in hospitals within two lung cancer networks located in the South-East of the Netherlands†. All included patients were registered in the Dutch Lung Cancer Audit (DLCA). Patients with either small cell lung cancer (SCLC), lung metastasis from another primary tumor after resection or participation in an interventional trial were excluded. Moreover, patients with neoadjuvant treatment and unknown clinical or pathological stage were excluded, as clinical to pathological staging comparisons could not be studied in these cases. Missing data were traced and restored by inspection of local electronic patient files. Regarding our key variables, no missing data were noticed after careful patient selection.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the review board of the Dutch Clinical Research Foundation (DCRF) (MK-3475/NIS101592). The investigators refrained from asking for consent because an exception rule had been applied: asking for permission could not reasonably be required because very large numbers of patients were involved. All participating hospitals were informed and agreed the study.
Definitions and scope
Clinical and pathological staging were based on the AJCC/IUCC/IASLC tumor-node-metastasis (TNM) classification, 8th edition (20). Stage migration was defined as any stage difference occurring between the initial clinical and confirmative pathological stage after resection without any neoadjuvant treatment. Relevant upstaging was described as clinical stage I increasing to histopathological stage IIA–IIIB disease. Relevant downstaging was defined as clinical stage IIA–IIIB decreasing to histopathological stage I disease. These definitions are based on conventional borders of perioperative treatment administration in recently published phase III randomized clinical trials and the implementation of perioperative pembrolizumab in a national guideline (7-10,21). Equal staging was described as clinical and final pathological stage both being categorized as either stage I, II or III. Date of diagnosis was determined by date of the most recent multidisciplinary tumor board (MTB) before surgical resection of NSCLC was performed. Follow-up duration was calculated as time between date of resection and date of death, loss to follow-up or most recent visit at the respiratory oncologist if patients were still alive. Patients with a recent visit at the respiratory oncologist and patients lost to follow-up were censored in the survival analysis. Time-to-treatment was defined as date of diagnosis until the resection date. Alternatively, we calculated time-to-treatment from date of first outpatient visit at the pulmonologist until resection date, to maximize potential disparities in the stage migration subgroups. Disease-free survival (DFS) was defined as time from surgery until recurrence or death from any cause. Relapse was confirmed through imaging or by pathological confirmation. Overall survival (OS) was defined as time from surgery until date of death. At least a period of four years of follow-up was pursued: patients were diagnosed from January 2015 until December 2019 and follow-up was realized until December 2023.
For each included patient, tumor and patient characteristics, demographic information, diagnostic approach, type of surgical procedure and the Charlson Comorbidity Index (CCI) were documented (22). Outcomes of interest included clinical stage, histopathological stage after resection, time-to-treatment, DFS and OS. The first main objective was to investigate the absolute and relative number of patients without preoperative pathological confirmation with focus on clinical stage II and III disease. The second main objective was to analyse the prevalence of clinicopathological stage migration in resectable NSCLC. Stage migration was addressed in the context of upfront pathological confirmation by forming two cohorts divided by the presence or absence of a preoperative NSCLC diagnosis. Relevant stage migration was compared across these two cohorts. Relevant up- and downstaging were carefully analyzed on etiological factors: different tumor (T) or nodal (N) stage, thoracic wall invasion, parietal pleura invasion or the presence of at least one satellite lesion. Additionally, relevant stage migration rates were analyzed in a subgroup of patients with an indication for invasive staging according to leading national and international guidelines. Finally, we studied survival rates in the total study population and across up- and downstaging cohorts to provide insights regarding implications of clinicopathological up- or downstaging.
Statistical analysis
Baseline characteristics were documented for the entire study population and stratified for the presence and absence of a preoperative pathological diagnosis of NSCLC. For continuous variables, means, standard deviations and medians were calculated. Statistical significance was evaluated using Chi-squared tests for categorical variables and Student’s t-tests or analysis of variance (ANOVA) tests for continuous variables. DFS and OS across the different stage migration subgroups were assessed. Kaplan-Meier curves were generated to display DFS and OS rates and survival outcomes were compared by log-rank tests. Estimates in Kaplan-Meier graphics derived from Cox regression were presented as hazard ratio (HR) and 95% confidence interval (CI). Adjusted survival analysis using the Cox proportional hazard regression model was used to investigate potential confounders in the migration subgroups. Hazard ratios were adjusted for age at diagnosis, gender, smoking status, Eastern Cooperative Oncology Group performance score (ECOG PS) and the CCI. Statistical analyses were performed using SPSS version 29.0 and GraphPad 10.2.2. P values of <0.05 were considered statistically significant, measured two-sided when relevant.
Results
In total, a number of 882 cases with resected NSCLC were selected of which 817 cases did not receive any neoadjuvant treatment. Finally, we selected 809 individual patients in our final database by excluding eight cases with metachronous NSCLC (Figure 1).

Baseline characteristics
Baseline characteristics are summarized in Table 1 and Table S1. Regarding ECOG PS at diagnosis, smoking history and morphology after resection, missing values were noticed in 10 (1%), 16 (2%) and 1 (~0%) patients, respectively. Age, gender, ECOG PS, smoking status, morphology after resection and CCI were well balanced in the subgroups categorized by stage migration status. Across each relevant stage migration subgroup, most patients had an adenocarcinoma (n=65; 68% in the relevant upstaging cohort and n=35; 56% in the relevant downstaging cohort). Regarding operative procedures, the vast majority underwent lobectomy across the relevant up- and downstaging cohort (n=82; 86%, n=59; 93%). In addition, mild (n=27; 28%, n=14; 23%), moderate (n=31; 32%, n=28; 45%) and severe (n=36; 38%, n=20; 32%).
Table 1
| Variable | Total cases (n=809) |
Upfront NSCLC confirmation (n=532) |
No upfront NSCLC confirmation (n=277) | P value |
|---|---|---|---|---|
| Age at diagnosis (years) | 0.78 | |||
| Mean ± SD | 66±8.9 | 66±9.2 | 66±8.5 | |
| Median [range] | 67 [18–88] | 67 [18–88] | 67 [33–82] | |
| Gender | 0.01 | |||
| Male | 403 [50] | 282 [53] | 121 [44] | |
| Female | 406 [50] | 250 [47] | 156 [56] | |
| ECOG PS | 0.004 | |||
| 0 | 533 [66] | 344 [65] | 189 [68] | |
| 1 | 232 [29] | 168 [31] | 64 [23] | |
| 2 or more | 34 [4] | 16 [3] | 18 [7] | |
| Unknown | 10 [1] | 4 [1] | 6 [2] | |
| Smoking status | 0.01 | |||
| Current | 290 [36] | 183 [34] | 107 [39] | |
| Former | 435 [54] | 296 [56] | 139 [50] | |
| Never | 68 [8] | 48 [9] | 20 [7] | |
| Unknown | 16 [2] | 5 [1] | 11 [4] | |
| Morphology after resection | 0.003 | |||
| Adenocarcinoma | 461 [57] | 284 [53] | 177 [64] | |
| Squamous cell carcinoma | 253 [31] | 191 [36] | 62 [22] | |
| Large cell carcinoma | 19 [2] | 10 [2] | 9 [3] | |
| NSCLC NOS | 20 [3] | 12 [2] | 8 [3] | |
| Other or unknown* | 56 [7] | 35 [7] | 21 [8] | |
| Clinical stage at baseline | <0.001 | |||
| I | 466 [58] | 272 [51] | 194 [70] | |
| II | 224 [28] | 165 [31] | 59 [21] | |
| III | 119 [14] | 95 [18] | 24 [9] | |
| Pulmonary function test—FEV1 (%) | 0.83 | |||
| Mean ± SD | 85±20.0 | 85±20.8 | 85±18.6 | |
| Median [range] | 85 [27–150] | 84 [27–150] | 85 [28–143] | |
| Surgical entrance | <0.001 | |||
| VATS | 535 [66] | 318 [60] | 217 [78] | |
| VATS converted to thoracotomy | 86 [11] | 59 [11] | 27 [10] | |
| Thoracotomy | 166 [20] | 142 [27] | 24 [9] | |
| Other or unknown | 22 [3] | 13 [2] | 9 [3] | |
| Operative procedure | <0.001 | |||
| Wedge or segmental resection | 48 [6] | 10 [2] | 38 [14] | |
| Lobectomy | 670 [83] | 448 [84] | 222 [80] | |
| Bilobectomy | 34 [4] | 24 [5] | 10 [3] | |
| Pneumonectomy | 55 [7] | 50 [9] | 5 [2] | |
| Other | 2 [~0] | 0 [0] | 2 [1] | |
| Radicality of resection | 0.88 | |||
| R0 | 758 [94] | 498 [94] | 260 [94] | |
| R1 or R2 | 28 [3] | 18 [3] | 10 [4] | |
| Unknown | 23 [3] | 16 [3] | 7 [2] | |
| Adjuvant treatment | 0.005 | |||
| Adjuvant | 199 [25] | 147 [28] | 52 [19] | |
| No adjuvant treatment | 610 [75] | 385 [72] | 225 [81] | |
| Type of adjuvant treatment | 0.34 | |||
| Chemotherapy | 157 [79] | 113 [77] | 44 [84] | |
| Chemo- and radiotherapy | 26 [13] | 23 [16] | 3 [6] | |
| Radiotherapy | 13 [7] | 9 [6] | 4 [8] | |
| Other | 3 [1] | 2 [1] | 1 [2] | |
| Charlson comorbidity index | 0.80 | |||
| No comorbidity (0) | 18 [2] | 12 [2] | 6 [2] | |
| Mild (1–2) | 191 [24] | 129 [24] | 62 [22] | |
| Moderate (3–4) | 326 [40] | 217 [41] | 109 [40] | |
| Severe (≥5) | 274 [34] | 174 [33] | 100 [36] |
Data are presented as n [%] unless otherwise specified. *, morphology after resection was only missing in 1 case (~0%). Baseline characteristics were categorized by the presence of preoperative NSCLC confirmation. P values were evaluated using Chi-squared tests for categorical variables and a Student’s t-test for continuous variables. ECOG PS, Eastern Cooperative Oncology Group performance score; FEV1, forced expiratory volume in 1 second; NSCLC NOS, non-small cell lung cancer not otherwise specified; SD, standard deviation; VATS, video-assisted thoracoscopy.
Mean time-to-treatment was 20.7±10.7 days in the relevant upstaging cohort and 21.2±13.7 days in the relevant downstaging cohort (P=0.83). When time-to-treatment was defined as first visit at the pulmonologist until resection, again no relevant difference was measured (mean 44.0±21.0 days in the relevant upstaging cohort versus 48.0±26.2 days in the relevant downstaging cohort (P=0.29).
Diagnostic work-up of resectable NSCLC
CT imaging was conducted in 806 patients (99.6%). PET imaging was performed in 804 patients (99.4%). EBUS-TBNA was conducted in 321 patients (39.7%). EUS fine needle aspiration (EUS-FNA) was carried out in 70 patients; in 51 patients this procedure was combined with EBUS during the diagnostic work-up of NSCLC. Invasive lymph node staging by mediastinoscopy was performed in 188 patients. EBUS-TBNA followed by a mediastinoscopy procedure was executed in 131 patients.
Absent preoperative pathological NSCLC confirmation
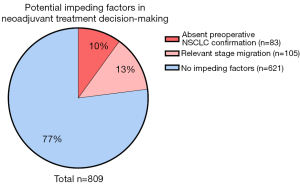
In 532 patients (65.8%) preoperative pathological confirmation of NSCLC was noticed, including 260 patients with clinical stage II or III disease (49%) (Figure 2A). In 277 patients (34.2%) no preoperative pathological confirmation of NSCLC was established, harbouring 83 patients with clinical stage II or III disease (30%) (Figure 2B).

Absent preoperative confirmation was detected in 97 patients (35.0%) with at least one invasive staging procedure (EBUS-TBNA, EUS-FNA or mediastinoscopy) and in 53 patients (63.9%) with stage II–III disease.
Stage migration
Clinicopathological stage comparisons resulted in upstaging in 250 patients (30.9%) with relevant upstaging in 96 patients (11.9%). Downstaging was observed in 129 patients (15.9%) of which 62 showed relevant downstaging (7.9%). Equal staging was established in 430 patients (53.2%) (Table S2).
In the total study population, relevant upstaging was found in 96 patients (11.9%) and relevant downstaging in 62 patients (7.7%) (Table 2).
Table 2
| Clinical stage before resection | Pathological stage after resection | Total | ||||
|---|---|---|---|---|---|---|
| 0/occult | I | II | III | IV | ||
| I | 7 | 359§ | 75† | 21† | 4 | 466 |
| II | 0 | 53‡ | 123§ | 47 | 1 | 224 |
| III | 0 | 9‡ | 27 | 81§ | 2 | 119 |
| Total | 7 | 421 | 225 | 149 | 7 | 809 |
Migration status was simplified on the level of the total study cohort (n=809). Simplified staging was applied, meaning no subcategories (for example IA or IB) were plotted. †, values represent relevant upstaging; ‡, values represent relevant downstaging; §, values represent equal staging.
In 532 patients with an upfront pathological diagnosis (65.8%) relevant upstaging occurred in 65 patients (12%). Relevant downstaging was observed in 40 patients (8%) (Figure 3A and Table 3). Relevant stage migration in this subpopulation was observed in 105 patients (19.7%).

Table 3
| Clinical stage before resection | Pathological stage after resection | Total | ||||
|---|---|---|---|---|---|---|
| 0/occult | I | II | III | IV | ||
| I | 3 | 201§ | 51† | 14† | 3 | 272 |
| II | 0 | 33‡ | 91§ | 40 | 1 | 165 |
| III | 0 | 7‡ | 22 | 66§ | 0 | 95 |
| Total | 3 | 241 | 164 | 120 | 4 | 532 |
Migration status was simplified on the level of the subgroup with upfront pathological confirmation (n=532). Simplified staging was applied, meaning no subcategories (for example IA or IB) were plotted. †, values represent relevant upstaging; ‡, values represent relevant downstaging; §, values represent equal staging.
In 277 patients without an upfront pathological diagnosis relevant up- and downstaging were noticed in 31 (11.2%) and 22 cases (8%), respectively (Figure 3B and Table 4). When compared to the cohort with upfront pathological diagnosis, no significant differences regarding relevant stage migration were present (P=0.91).
Table 4
| Clinical stage before resection | Pathological stage after resection | Total | ||||
|---|---|---|---|---|---|---|
| 0/occult | I | II | III | IV | ||
| I | 4 | 160§ | 24† | 7† | 1 | 196 |
| II | 0 | 20‡ | 30§ | 7 | 0 | 57 |
| III | 0 | 2‡ | 5 | 15§ | 2 | 24 |
| Total | 4 | 182 | 59 | 29 | 3 | 277 |
Migration status was simplified on the level of the subgroup without upfront pathological confirmation (n=277). Simplified staging was applied, meaning no subcategories (for example IA or IB) were plotted. †, values represent relevant upstaging; ‡, values represent relevant downstaging; §, values represent equal staging.
Two impeding factors in total study population
We considered absence of preoperative NSCLC confirmation to be relevant in stage II–III resectable NSCLC, as neoadjuvant chemoimmunotherapy has become standard of care in current guidelines. In 83 patients with stage II–III disease, no preoperative NSCLC diagnosis was established (10% of total study population). In addition, relevant stage migration was observed in 105 patients with preoperative NSCLC diagnosis (13% on total study population). Therefore, in 23.3% of patients one of these limitations was observed (Figure 4).
Relevant upstaging was caused by lymph node involvement in 37 patients (56.9%) and an increasing tumor size in 15 patients (23.1%). In 4 patients (6.2%), a combination of these two factors was observed. Furthermore, in 3 patients (4.6%), at least one satellite lesion was found in the resection preparate, leading to pT3 disease. In another 3 patients (4.6%), parietal pleura or chest wall invasion led to pT3 disease. In 3 patients (4.6%), a combination of these three factors was observed.
Relevant downstaging was caused by unexpected absence of lymph node involvement in 12 patients (30.0%) and a different tumor size in 11 patients (27.5%) in the resection specimens. Interestingly, in 13 patients (32.5%), unexpected lack of satellite lesions was noticed as underlying reason for relevant downstaging. Finally, in 4 patients (10.0%), a combination of etiological factors was detected (see also Table S3 for more details).
Notably, 453 study patients were diagnosed with at least clinical stage IB disease, for which invasive staging was indicated according to national and international guidelines (10,11). However, only patients underwent at least one invasive staging procedure (EBUS-TBNA, EUS-FNA or cervical mediastinoscopy) (64.0%).
Stage migration was significantly more noticed in patients without an invasive staging procedure before surgery versus patients who underwent a present invasive procedure (31.3% vs. 19.7%, P=0.005). Relevant stage migration was found in 108 patients with at least clinical stage IB disease. In 42 patients relevant stage migration was only caused by lymph node discrepancies (38.9%). However, only 27 patients (64.3%) underwent an invasive staging procedure (EBUS-TBNA, EUS-FNA or cervical mediastinoscopy), although indicated according to leading national and international guidelines.
Stage migration and survival
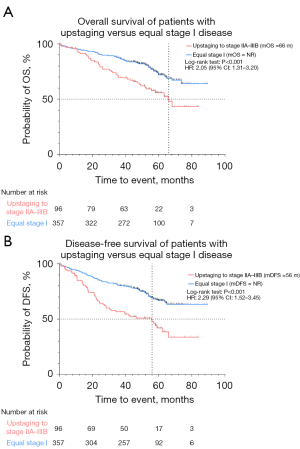
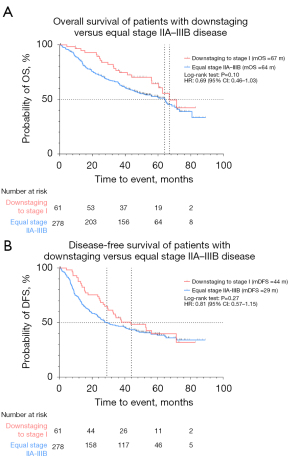
We compared survival outcomes in patients with up- and downstaging and equal clinical stage. The median follow-up time for the total study population was 49.4 months. OS was not reached in the equal stage I cohort and 66 months in the relevant upstaging cohort [hazard ratio (HR) 2.05; 95% confidence interval (CI): 1.31 to 3.20; P<0.001]. Furthermore, median DFS (mDFS) was not reached in the equal stage I cohort and 56 months in the relevant upstaging cohort (HR: 2.29; 95% CI: 1.52 to 3.45; P<0.001) (Figure 5). Relevant upstaging remained associated with worse OS after correction for age at diagnosis, gender, smoking status, ECOG PS and CCI (HR: 1.85; 95% CI: 1.24 to 2.77; P=0.003). In addition, median OS (mOS) was 67 months in the downstaging cohort and 64 months in the equal stage IIA–IIIB cohort (HR 0.69; 95% CI: 0.46 to 1.03; P=0.10), while mDFS was 44 months in the downstaging cohort versus 29 months in the equal stage IIA–IIIB cohort (HR 0.81; 95% CI: 0.57 to 1.15; P=0.27) (Figure 6).
In addition, we compared survival outcomes in patients with up- and downstaging and equal pathological stage; mOS was 66 months in the relevant upstaging cohort and 64 months in the equal stage IIA–IIIB cohort (HR 0.82; 95% CI: 0.59 to 1.14; P=0.27). Interestingly, mOS was not reached in the equal stage I cohort versus 67 months in the relevant downstaging cohort (HR 1.71; 95% CI: 0.98 to 3.01; P=0.02) (Figure S1).
Discussion
Key findings
In this multicenter retrospective cohort study, we studied two potential barriers in the work-up of resectable NSCLC: obtaining a preoperative diagnosis and accurate clinical staging. Absent preoperative NSCLC confirmation and relevant clinicopathological stage migration were observed in nearly a quarter of potential candidates for neoadjuvant chemoimmunotherapy administration.
Strengths and limitations
Our study includes several strengths. First, this topic is of major relevance in the new era of neoadjuvant (chemo)therapy in resectable NSCLC. To our knowledge, the combination of barriers in treatment decision-making investigated in this study have not been studied in previous literature. Moreover, we were able to build a comprehensive real-world database enriched with various patient, tumor and treatment characteristics. Patients were diagnosed between 2015 and 2019 and follow-up data were sufficient for survival analyses. This period encompasses the treatment landscape before neoadjuvant ICI administration was introduced in resectable NSCLC. Consequently, our study population was considered suitable to meet our research objectives. It would be interesting to compare our cohort with a future cohort diagnosed in the neoadjuvant ICI treatment era to assess whether the rate of pathologically confirmed NSCLC has increased upon the growing importance of accurate staging in neo-adjuvant treatment decision-making.
There are also limitations to our study. First, data regarding preoperative biopsy attempts for example by bronchoscopy or transthoracic puncture could not be provided. Second, we were not able to estimate the impact of tumor growth within the upstaging and relevant upstaging cohorts. Although we studied etiological factors such as diameter growth of the primary tumor and lymph node involvement, it was impossible to distinguish tumor development and mismatching due to suboptimal staging regarding these study cohorts. Third, detailed information on invasive staging procedures is missing. For example, we could not obtain data on accuracy of systematic mediastinal and hilar lymph node detection during EBUS-TBNA procedures and were not able to ascertain whether representative tissue was obtained. Fourth, we lack information on CT or PET images produced after the latest tumor board. As a consequence, clinicopathological staging could be altered by new information about tumor size and lymph node involvement before resection was executed.
Comparison with similar research
In our cohort, one-third of patients had no preoperative NSCLC confirmation. This ratio is consistent with an earlier study performed in the Dutch population, in which 35% of patients diagnosed with either adenocarcinoma or squamous cell carcinoma between 2010 and 2015 lacked an upfront pathological diagnosis (14). This study did not provide data stratified to clinical stage. In our study, 83 patients without preoperative diagnosis were diagnosed with clinical stage II or III disease (10% of the total study population). As a result, neoadjuvant treatment would be contra-indicated in this patient subgroup. Additional diagnostics including PD-L1 staining and molecular profiling are not feasible in this context. Consequently, relative contra-indications regarding neoadjuvant ICI administration will be unnoticed. New relevant diagnostic strategies have been developed over recent years to counter this issue. For example, tremendous advancements in PET imaging have been made in the areas of imaging technology, contrast development and data quality (23). Moreover, navigation bronchoscopy techniques with advanced imaging are evolving and a high diagnostic yield can be obtained even in relatively small and peripherally localized pulmonary nodules (24,25). Notably, in the remaining 194 patients (24.0% of the total study population) without upfront NSCLC confirmation stratified as clinical stage I, no relevant implications were noticed: when considered feasible, these patients will yet undergo primary surgery without any neoadjuvant or perioperative treatment according to the current standard of care in the Netherlands (10).
Stage migration was noticed in almost half of patients. These results are similar compared with earlier findings. For example, in an American study with 315 cases of resected NSCLC [2016], clinicopathological correlation was investigated across stage I, II and III disease resulting in 50% correct clinical to pathological staging (26). However, neither subcategories in the staging procedures were provided nor etiological factors underlying present staging discrepancies.
Relevant stage migration occurred in 19.5% of our total study population with resectable NSCLC. To our knowledge, relevant stage migration potentially altering neoadjuvant ICI administration in resectable NSCLC has not been investigated in previous relevant literature. Our study provides clinicopathological migration rates in the era before the introduction of neoadjuvant chemoimmunotherapy. In essence, we intended to create awareness for clinical staging optimization in order to improve future neoadjuvant treatment decision-making. Regarding the patient cohort with an upfront pathological diagnosis of NSCLC, relevant stage migration occurred in 105 patients (19.7%). We considered this finding being substantial, as it implicates over- or undertreatment of neoadjuvant treatment in one-fifth of patients if these findings still being actual.
Time-to-treatment was hypothesized to be a potential confounder of relevant clinicopathological upstaging. Nevertheless, we demonstrated time-to-treatment being equally balanced across the up- and downstaging cohorts with application of both our definitions regarding date of diagnosis. These findings encountered time being a confounder in the upstaging subgroup. In a Dutch nation-wide observation cohort study [2023] Klarenbeek and colleagues demonstrated an increased risk of death due to extended time-to-treatment in patients with resectable NSCLC (27). Notably, this increased mortality risk was noticed in clinical stage II that turned out to be pathological stage I after surgery. At this point, no appropriate explanation was found.
Regarding our subgroup of patients with at least clinical stage IB disease, stage migration was significantly more present in patients with absent invasive staging versus present invasive staging. Moreover, in only two-third of patients with lymph node discrepancies as etiological factor of stage migration, invasive staging was performed. Efforts to indicate and perform EBUS-TBNA adequately could reduce these numbers. However, part of lymph node discrepancies is difficult to overcome. For instance, discovery of micrometastases in the lymph node resection specimen could easily be undetected on initial CT or PET imaging and during invasive preoperative lymph node procedures. Tsai and colleagues [2022] investigated the incidence rate of lymph nodal upstaging after surgical resection. In patients with clinical T1a-bN0M0 adenocarcinoma, in 8% at least N1 lymph nodes were observed after resection (28). Furthermore, we noticed relevant clinicopathological stage migration only explained by at least one satellite lesion, parietal pleural invasion or chest wall invasion in 20% of the total relevant migration population. Relevant stage migration between the initial clinical and final pathological stage is therefore partly inevitable.
As we expected, mOS was significantly higher in the correctly staged stage I subgroup in comparison with the relevant upstaging subgroup eventually classified as stage IIA–IIIB disease. Bott and colleagues [2015] found comparable results with a median survival of 39 months in the upstaged cohort versus 73 months (P<0.001) (29). Our study provided additional data, as we did not only investigate histopathological upstaging but also downstaging. From a different perspective, Park and colleagues [2019] investigated prognosis in patients with clinical N0 disease and nodal upstaging to N1-2 disease after resection compared with preoperative pathologically proven N1–2 disease. Postoperative nodal upstaging was not a significant prognostic factor compared to the subgroup with positive nodal staging upfront. Again, only clinicopathological upstaging was investigated here, whereas our study provided up- and downstaging analyses (30). In conclusion, these findings again emphasize the importance of adequate clinical staging: clinicopathological upstaging leads to suboptimal patient care regarding treatment preference and life expectation management.
Implications and actions needed
The implications of our study results are bifold. First, we suggest optimizing efforts in order to obtain pathological confirmation of NSCLC before resection. These efforts will ultimately tend to increase due to the urgency of diagnosing NSCLC preoperatively in order to administer neoadjuvant treatment if indicated. Whether the rate of pathologically confirmed NSCLC will actually increase in the landscape of neoadjuvant treatment should be investigated in future cohorts and could be compared with our study results. In order to optimize the diagnostic yield, new techniques can be used. For example, cone beam CT navigation bronchoscopy is a relatively new strategy in lung cancer diagnostics. The Dutch National Healthcare Institute acknowledged this technique to be feasible when a pulmonary lesion suspect to be malignant cannot be reached by transthoracic puncture or conventional bronchoscopy (31). Second, indicating and performing invasive staging procedures systematically, for example EBUS-TBNA should be optimized in order to improve staging accuracy. Evison and colleagues investigated standards in EBUS-guided mediastinal lung cancer staging. The introduction of standards was associated with a significant increase (P<0.001) in sampling of key mediastinal lymph node stations and a reduction in the variability of staging sensitivity between centres (17). In addition, Bousema and colleagues showed that confirmatory mediastinoscopy after negative systematic endosonography can be omitted in patients with resectable NSCLC and an indication for mediastinal staging, based on a non-inferiority margin in the rate of unforeseen N2 disease (32). Third, new developments in imaging techniques will lead to improved detection of lung cancer. For example, photon-counting CT provides ultrahigh resolution (0.2–0.4 millimeters) images and is considered an innovation in CT technology (33). In addition, alternative promising PET tracers have been studied beyond F18 fluorodeoxyglucose (FDG) in PET imaging with the increasing knowledge of tumor heterogeneity and biological characteristics of lung cancer (34). However, clinical applications are currently limited due to a specificity of most of these PET tracers.
Conclusions
In the era before the introduction of neoadjuvant chemoimmunotherapy as new standard of care, absent preoperative NSCLC confirmation or inaccurate staging occurred in nearly a quarter of potential candidates for neoadjuvant treatment. These two limiting factors need to be optimized in order to correctly administer neoadjuvant therapy in patients with resectable NSCLC.
Acknowledgments
We would like to thank Drs. H.M. van Groningen (Department of Pulmonology, Elkerliek Hospital, Helmond, the Netherlands), Drs. M.C.M. Bunnik (Department of Pulmonology, Pantein, Boxmeer, the Netherlands), Drs. J.P.H. van den Bogart (Department of Pulmonology, Bernhoven, Uden, the Netherlands), Dr. B. Biesma (Department of Pulmonology, Jeroen Bosch Hospital, ‘s-Hertogenbosch, the Netherlands), Dr. A. Mulders (Department of Pulmonology, Gelderse Vallei, Ede, the Netherlands), Dr. F.H.W. Hermens (Department of Pulmonology, Slingeland Hospital, Doetinchem, the Netherlands) and Dr. Y. Berk (Department of Pulmonology, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands) for facilitating data collection and data management.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1120/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1120/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1120/prf
Funding: This work was financially supported by Merck Sharp & Dohme (MSD).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1120/coif). F.C. reports that he has been Chair of the Scientific and Medical Advisory Board of TRIBVN Healthcare and received advisory board fees from TRIBVN Healthcare. He is shareholder in Aiosyn B.V. M.M.v.d.H. received research funding Roche Genentech, AstraZeneca, BMS, Boehringer Ingelheim, Merck, Novartis, Pamgene, Pfizer, Roche diagnostics, Stichting Treatmeds and Takeda. He has been speaker on educationals and webinars for Amgen, AstraZeneca, BMS, Janssen Pharmaceutica, Merck, MSD, Novartis, Pfizer and Roche. He is an advisory board member of AbbVie, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Janssen, Lilly, Merck, MSD, Novartis, Pfizer, Roche, Sanofi and Takeda. Financial fees were paid to the institution. He has been the chair of the NVALT study foundation and chair of the section oncology NVALT. K.A. is an employee of Merck Sharp & Dohme, a for-profit company. All authors reported that the study received financial support from a for-profit organization, MSD. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the review board of the Dutch Clinical Research Foundation (DCRF) (MK-3475/NIS101592). The investigators refrained from asking for consent because an exception rule had been applied: asking for permission could not reasonably be required because very large numbers of patients were involved. All participating hospitals were informed and agreed the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
*According to the American Joint Committee on cancer (AJCC)/Union for International Cancer Control (IUCC)/International Association for the Study of Lung Cancer (IASLC) tumor-node-metastasis (TNM) classification 8th edition.
†Lung Cancer Network with participating centers Radboudumc Nijmegen, Canisius Wilhelmina Hospital Nijmegen, Pantein Boxmeer, Elkerliek Helmond, Jeroen Bosch Hospital’s-Hertogenbosch, Bernhoven Uden and the Alliance of Regional Optimized Care with participating centers Rijnstate Arnhem, Gelderse Vallei Ede and Slingeland Hospital Doetinchem.
References
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344-57. Erratum in: Lancet 2021;398:1686. [Crossref] [PubMed]
- Tsuboi M, Herbst RS, John T, et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 2023;389:137-47. [Crossref] [PubMed]
- Wu YL, Dziadziuszko R, Ahn JS, et al. Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2024;390:1265-76. [Crossref] [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:1672-84. [Crossref] [PubMed]
- Cascone T, Awad MM, Spicer JD, et al. Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med 2024;390:1756-69. [Crossref] [PubMed]
- Tjan-Heijnen V, Groen H, Stoger G et al. NVALT guideline ‘Non-small Cell Lung Cancer’ Federation of Medical Specialists. Publication date: 12-09-2024.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Ettinger D, Wood D, Riely G, et al. NCCN Clinical Practice Guidelines in Oncology – Non-Small Cell Lung Cancer. J Natl Compr Canc Netw 2024;22:249-74.
- Vansteenkiste J, Dooms C, De Leyn P. Early stage non-small-cell lung cancer: challenges in staging and adjuvant treatment: evidence-based staging. Ann Oncol 2010;21:vii189-95. [Crossref] [PubMed]
- Ghamati MR, Li WWL, van der Heijden EHFM, et al. Surgery without preoperative histological confirmation of lung cancer: what is the current clinical practice? J Thorac Dis 2021;13:5765-75. [Crossref] [PubMed]
- Hendriks LE, Kerr KM, Menis J, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:358-76. [Crossref] [PubMed]
- Cheng TL, Huang ZS, Zhang J, et al. Comparison of cryobiopsy and forceps biopsy for the diagnosis of mediastinal lesions: A randomised clinical trial. Pulmonology 2024;30:466-74. [Crossref] [PubMed]
- Evison M, Crosbie P, Martin J, et al. EBUS-guided mediastinal lung cancer staging: monitoring of quality standards improves performance. Thorax 2016;71:762-3. [Crossref] [PubMed]
- Cane P, Linklater K, Santis G, et al. The LungPath study: variation in the diagnostic and staging pathway for patients with lung cancer in England. Thorax 2016;71:291-3. [Crossref] [PubMed]
- Stokes SM, Massarweh NN, Stringham JR, et al. Clinical-Pathologic Correlation and Guideline Concordance in Resectable Non-Small Cell Lung Cancer. Ann Thorac Surg. 2019;108:837-44. [Crossref] [PubMed]
- Hwang JK, Page BJ, Flynn D, et al. Validation of the Eighth Edition TNM Lung Cancer Staging System. J Thorac Oncol 2020;15:649-54.
- Perioperatieve behandeling met pembrolizumab en chemotherapie bij het resectabel niet-kleincellig longcarcinoom - NVMO. Available online: www.nvmo.org/bom/perioperatieve-behandeling-met-pembrolizumab-en-chemotherapie-bij-het-resectabel-niet-kleincellig-longcarcinoom/?meta
- Charlson ME, Carrozzino D, Guidi J, et al. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother Psychosom 2022;91:8-35. [Crossref] [PubMed]
- Schwenck J, Sonanini D, Cotton JM, et al. Advances in PET imaging of cancer. Nat Rev Cancer 2023;23:474-90. [Crossref] [PubMed]
- Kops SEP, Heus P, Korevaar DA, et al. Diagnostic yield and safety of navigation bronchoscopy: A systematic review and meta-analysis. Lung Cancer 2023;180:107196. [Crossref] [PubMed]
- Bondue B, Taton O, Tannouri F, et al. High diagnostic yield of electromagnetic navigation bronchoscopy performed under cone beam CT guidance: results of a randomized Belgian monocentric study. BMC Pulm Med 2023;23:185. [Crossref] [PubMed]
- Vernon J, Andruszkiewicz N, Schneider L, et al. Comprehensive Clinical Staging for Resectable Lung Cancer: Clinicopathological Correlations and the Role of Brain MRI. J Thorac Oncol 2016;11:1970-5. [Crossref] [PubMed]
- Klarenbeek SE, Aarts MJ, van den Heuvel MM, et al. Impact of time-to-treatment on survival for early-stage non-small cell lung cancer in The Netherlands-a nationwide observational cohort study. Transl Lung Cancer Res 2023;12:2015-29. [Crossref] [PubMed]
- Tsai TM, Liu CY, Lin MW, et al. Factors Associated with Nodal Upstaging in Clinical T1a-bN0M0 Non-Small Cell Lung Cancers. Cancers (Basel) 2022;14:1277. [Crossref] [PubMed]
- Bott MJ, Patel AP, Crabtree TD, et al. Pathologic Upstaging in Patients Undergoing Resection for Stage I Non-Small Cell Lung Cancer: Are There Modifiable Predictors?. Ann Thorac Surg 2015;100:2048-53. [Crossref] [PubMed]
- Park JK, Moon Y. Prognosis of upstaged N1 and N2 disease after curative resection in patients with clinical N0 non-small cell lung cancer. J Thorac Dis 2019;11:1202-12. [Crossref] [PubMed]
- www.zorginstituutnederland.nl/publicaties/standpunten/2022/07/06/standpunt-navigatie-bronchoscopietechnieken-bij-verdenking-op-longkanker.
- Bousema JE, Dijkgraaf MGW, van der Heijden EHFM, et al. Endosonography With or Without Confirmatory Mediastinoscopy for Resectable Lung Cancer: A Randomized Clinical Trial. J Clin Oncol 2023;41:3805-15. [Crossref] [PubMed]
- Tárnoki ÁD, Tárnoki DL, Dąbrowska M, et al. New developments in the imaging of lung cancer. Breathe (Sheff) 2024;20:230176. [Crossref] [PubMed]
- Zhu J, Pan F, Cai H, et al. Positron emission tomography imaging of lung cancer: An overview of alternative positron emission tomography tracers beyond F18 fluorodeoxyglucose. Front Med (Lausanne) 2022;9:945602. [Crossref] [PubMed]


