
Ivonescimab plus chemotherapy in advanced or metastatic non‑squamous non‑small cell lung cancer with EGFR variant in China: a cost-effectiveness analysis
Highlight box
Key findings
• In the context of the healthcare system in China, ivonescimab and chemotherapy for treating non-squamous non-small cell lung cancer (nsq-NSCLC) patients with epidermal growth factor receptor (EGFR) variants showing disease progression during treatment with EGFR-tyrosine kinase inhibitor (EGFR-TKI) therapy is unlikely to be a cost-effective option in the absence of price adjustment or any current charitable aid program.
What is known and what is new?
• Ivonescimab plus chemotherapy greatly enhanced progression-free survival in patients with nsq-NSCLC previously treated with EGFR-TKI with an acceptable safety profile. However, the high cost of ivonescimab has raised concerns among researchers and healthcare policymakers about its economic affordability, especially for ordinary patients who have already borne the high costs of EGFR-TKI therapy in China.
• The study firstly systemically reported the economic outcomes of ivonescimab treatment in patients with advanced or metastatic nsq-NSCLC with EGFR variant whose disease progressed while receiving EGFR-TKI therapy using a state-transition Markov model.
What is the implication, and what should change now?
• The bispecific antibody ivonescimab with chemotherapy is unlikely to be a cost-effective option for EGFR variant patients whose disease progressed while receiving EGFR-TKI therapy. Incorporating value-based pricing models, conditional reimbursement agreements, or volume-based discount strategies would be valuable for negotiations with the pharmaceutical industry. These approaches could significantly enhance the economic feasibility of ivonescimab within the Chinese healthcare system.
Introduction
Lung cancer is responsible for the majority of cancer-associated deaths in China (1). In 2022 alone, there were 1.06 million new diagnoses, representing 21.98% of all cancer diagnoses, together with 733,300 deaths (2). The most common subtype is non-small cell lung cancer (NSCLC), representing 85% of lung cancer cases, with non-squamous histology accounting for 40% of all NSCLC histological subtypes (3,4). Due to the high invasiveness of NSCLC and the absence of noticeable symptoms in its early stages, close to 70% of diagnoses occur when the disease is already advanced, despite extensive efforts by clinicians to develop screening protocols (4,5). Mutations in epidermal growth factor receptor (EGFR) are frequent in non-squamous non-small cell lung cancer (nsq-NSCLC), with a positive mutation rate of approximately 50% in East Asian populations (6). These variants can alter signaling in metabolic processes, increasing tumor proliferation and disrupting the cell cycle, leading to uncontrolled growth (7). The first-line therapy for cases with advanced/metastatic NSCLC with EGFR variants is the use of EGFR tyrosine kinase inhibitors (EGFR-TKIs) represented by osimertinib (8,9). However, despite significant enhancement in both survival and quality of life with these therapies, acquired resistance to EGFR-TKIs is common, and the 5-year survival for these patients is only 15% (10,11).
Immunotherapy targeting the programmed death 1/programmed death ligand-1 (PD-1/PD-L1) axis has transformed the treatment standards for various tumors, including driver gene-negative NSCLC. However, it has limited efficacy in EGFR-variant nsq-NSCLC cases that have responded poorly to EGFR-TKIs, presumably indicating the presence of additional inhibitory mechanisms that reduce the effectiveness of immunotherapy (12,13). The development of new treatment strategies targeting other immunosuppressive mechanisms may maximize the effectiveness of immunotherapy and benefit patients resistant to immune checkpoint inhibitors (ICIs) (14). Vascular endothelial growth factor (VEGF) is known to be key for angiogenesis in tumors, with VEGF-driven angiogenesis in the tumor microenvironment (TME) being a critical driver of immunosuppression (11,15). Several preclinical studies have reported synergistic effects of anti-VEGF agents and ICIs in enhancing the effectiveness of chemoimmunotherapy by promoting the recruitment of lymphocytes in the TME and counteracting immunosuppression induced by VEGF, suggesting a potential application in the treatment of nsq-NSCLC (16-18). The ORIENT-31 trial was the first prospective, double-blind, placebo-controlled, Phase 3 trial to assess the combined effects of PD-1 inhibitors, anti-VEGF, and chemotherapy, showing that for patients experiencing progression when treated with EGFR-TKIs, the combination of sintilimab, bevacizumab biosimilar, and chemotherapy markedly extended progression-free survival (PFS) relative to chemotherapy alone, with good tolerability and no new safety signals. However, disappointingly, further follow-up analysis showed no corresponding benefit in overall survival (OS) (19). In China, patients with advanced/metastatic nsq-NSCLC showing disease progression during treatment with EGFR-TKIs, especially third-generation EGFR-TKIs, there are limited treatment options.
Ivonescimab is a bispecific humanized antibody that targets both PD-1 and VEGF-A to counteract immune suppression and angiogenesis in the TME, thereby suppressing tumor growth (20). A recent large Phase 3 clinical trial, the HARMONi-A study, revealed that the combination of ivonescimab with chemotherapy showed significantly improved efficacy in cases with advanced/metastatic EGFR-variant nsq-NSCLC with progression following EGFR-TKI treatment, particularly in terms of PFS, prolonging PFS by a median of 2.3 months [hazard ratio (HR) 0.46, 95% confidence interval (CI): 0.34–0.62] (21). The safety profile was manageable and no unexpected treatment-related adverse events (TRAEs). Treatment with ivonescimab combined with carboplatin and pemetrexed has recently been approved in China, and is classified in the latest guidelines as a Category I recommendation for treating advanced or metastatic nsq-NSCLC after EGFR-TKI therapy, establishing it as a new standard treatment option (22).
However, despite the clinical benefits of ivonescimab plus chemotherapy in treating these patients, the high cost of ivonescimab has raised concerns among researchers and healthcare policymakers about its economic affordability, especially for ordinary patients who have already borne the high costs of EGFR-TKI therapy in China. Chinese healthcare system relies on public hospitals and a diversified medical model, with costs shared by social medical insurance, individuals, and the government. Social and commercial medical insurance complement each other, creating a multi-tiered system aimed at providing equitable, efficient, and high-quality services to meet the needs of the majority. To date, there has been no analysis of the cost-effectiveness of this treatment combination in this patient group. Therefore, our study aimed to assess the cost-effectiveness of ivonescimab combined with chemotherapy relative to chemotherapy alone in the treatment of advanced/metastatic EGFR-variant nsq-NSCLC with disease progression during EGFR-TKI therapy. We present this article in accordance with the CHEERS reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1053/rc).
Methods
This study used data from previously published investigations analysis and did not conduct research on humans or human or animal tissues or samples. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Patients and interventions
Using data from the HARMONi-A study, eligible patients with EGFR variants who had experienced disease progression during EGFR-TKI treatment were randomly allocated (1:1 ratio) via a web/voice interaction system to the ivonescimab or placebo groups (20 mg/kg) combined with chemotherapy [pemetrexed 500 mg/m2, carboplatin at an area under the curve (AUC) 5 mg/mL/min]. The drugs were administered intravenously on day 1 of a three-week cycle for a total of four cycles, after which maintenance therapy of ivonescimab or placebo plus pemetrexed was given until disease progression, death, intolerable toxicity, or discontinuation resulting from specific reasons. All patients in both groups underwent systematic tumor imaging assessments every 6 weeks and those with progressive disease (PD) received subsequent-line systemic antitumor therapy based on the recommendations of the Chinese Society of Clinical Oncology [152 cases (94.4%) and 157 cases (97.5%) in the two groups, respectively] (8). Patients who did not undergo subsequent treatment were considered to have received best supportive care (BSC) before death, and there were no deaths from treatment-related causes. Details of the drug dosages and unit prices are provided in Table S1.
Model construction
The simulated total costs (efficacy) of patients in the ivonescimab and chemotherapy groups were integrated in the construction of a Markov model simulating the natural course of patients in the two groups from the perspective of Chinese society by using the TreeAge Pro version 2022. Three health states, namely, PFS, PD, and death, were used. Initially, all patients were classified as PFS, while at the end of each treatment cycle, they were either reclassified to the PD or death states or remained in the PFS state (Figure S1).
Based on the HARMONi-A study dosing regimens, frequency, and follow-up time, a three-week cycle length was used in the Markov model, with 10 years as the horizon during which over 99% of patients would be expected to have died. The predicted life years (LYs), quality-adjusted life years (QALYs), incremental cost-effectiveness ratios (ICERs), and incremental net health benefits (INHBs) were regarded as the key outcomes. Based on previous reports and guidelines, a willingness-to-pay (WTP) threshold $36,997 was used [three times the 2023 per capita gross domestic product (GDP) in China] (23,24). It remained lower than these developed countries such as the United States, South Korea, and Germany, reflecting relatively low and uneven economic development (25,26). Additionally, a 5% discount was applied annually to medical benefits and costs (27).
The OS and PFS Kaplan-Meier (KM) curves of the study population were used to calculate the likelihood of transitions between the different health-related states to estimate transition probabilities, using GetData Graph Digitizer version 2.26. In terms of the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) for estimating prediction errors, the exponential, log-logistic, log-normal, Gompertz, and Weibull distributions were used for fitting the survival curves of the two groups. Visual analysis revealed that the Weibull distribution was the most effective for the reconstruction of individual patient survival data (Figure S2 and Table S2). The model parameters γ (scale) and λ (shape) were determined using R Studio version 1.2.5042.
Costs and utility input
The study considered only direct medical costs. Given that the median annual salary was $13,044 in China for 2023, medical insurance covers only part of the treatment cost, leaving patients to bear a significant portion. These included drugs, administration, required laboratory and radiological tests, as well as severe TRAEs, subsequent-line treatments, BSC, and hospice care. Drug prices were sourced from local hospital. The costs associated with TRAEs included management expenses for TRAEs of grade 3 and above that occurred in more than 5% of the participants in the two groups, including anemia and reduced white blood cell, neutrophil, and platelet counts (21). The unit costs for TRAEs as well as other related costs were derived from previous reports (28-30). Drug doses were calculated according to the average characteristics of the trial population, and assumed a typical Chinese patient to be 60 years old, with heights and weights of 1.64 m and 65 kg, respectively, body surface area (BSA) of 1.72 m2, and serum creatinine of 1 mg/dL. Due to government regulation, healthcare costs in China are protected from inflation, and all costs in this analysis were calculated using an exchange rate of $1=¥7.2459 (June 2024).
Health utility values represent the health-related quality of life (HRQOL) of a patient during the natural progression of a disease, ranging from 1 (perfect health) to 0 (death). Regrettably, the HARMONi-A study did not report data on quality of life, and thus a model of the cost-effectiveness of sintilimab versus docetaxel as a second-line treatment for advanced/metastatic squamous NSCLC in China was used as a reference, using PFS and PD health utility values of 0.789 and 0.674, respectively (31). Negative utility values associated with TRAEs were also considered for adjustment (28,30). The key parameters were informed by previously published sources, as summarized in Table 1. It was assumed that the cost data followed a gamma distribution, while the health utility values, and TRAE incidence rates followed a beta distribution (32).
Table 1
| Variable | Mean value (range) | Reference | Distribution |
|---|---|---|---|
| Clinical parameters | |||
| Weibull survival model for ivonescimab plus chemotherapy | |||
| OS | Scale =0.008652, shape =1.503374 | – | – |
| PFS | Scale =0.013913, shape =1.90058 | – | – |
| Weibull survival model for chemotherapy | |||
| OS | Scale =0.009565, shape =1.55284 | – | – |
| PFS | Scale =0.038851, shape =1.750756 | – | – |
| Rate of post-discontinuation therapy | |||
| Ivonescimab plus chemotherapy group | 0.944 | (21) | Beta |
| Chemotherapy group | 0.975 | (21) | Beta |
| Risk for severe TRAEs in ivonescimab plus chemotherapy | |||
| Anemia | 0.137 (0.1096–0.1644) | ||
| White blood cell count decreased | 0.199 (0.1592–0.2388) | (21) | Beta |
| Neutrophil count decreased | 0.298 (0.2384–0.3576) | (21) | Beta |
| Platelet count decreased | 0.161 (0.1288–0.1932) | (21) | Beta |
| Risk for severe TRAEs in chemotherapy group | |||
| Anemia | 0.124 (0.0992–0.1488) | ||
| White blood cell count decreased | 0.168 (0.1344–0.2016) | (21) | Beta |
| Neutrophil count decreased | 0.193 (0.1544–0.2316) | (21) | Beta |
| Platelet count decreased | 0.118 (0.0944–0.1416) | (21) | Beta |
| Disutility and utility | |||
| Disutility of WBC count decreased | 0.090 (0.072–0.108) | (28) | Beta |
| Disutility of neutrophil count decreased | 0.090 (0.072–0.108) | (28) | Beta |
| Disutility of anemia | 0.070 (0.056–0.084) | (30) | Beta |
| Disutility of platelet count decreased | 0.110 (0.088–0.132) | (30) | Beta |
| Utility of PFS | 0.789 (0.6312–0.9468) | (31) | Beta |
| Utility of PD | 0.674 (0.5392–0.8088) | (31) | Beta |
| Cost, $/per cycle | |||
| Treatment cost | |||
| Ivonescimab | 4,125 (3,300–4,950) | Real world | Gamma |
| Pemetrexed | 479 (383.2–574.8) | Real world | Gamma |
| Carboplatin | 35 (28–42) | Real world | Gamma |
| Cost of TRAEs | |||
| Ivonescimab plus chemotherapy group | 280 (224–336) | (30) | Gamma |
| Chemotherapy group | 207 (165.6–248.4) | (30) | Gamma |
| Laboratory | 75 (60–90) | (28) | Gamma |
| Radiological test | 236 (188.8–283.2) | (28) | Gamma |
| Administration | 17 (13.6–20.4) | (28) | Gamma |
| Best supportive care | 339 (271.2–406.8) | (29) | Gamma |
| Hospice care per patient | 2,475 (1,980–2,970) | (29) | Gamma |
| Discount rate | 0.05 (0–0.08) | (27) | Uniform |
Adapted from our previous publication in Sci Rep (24). OS, overall survival; PD, progressive disease; PFS, progression-free survival; TRAEs, treatment-related adverse events; WBC, white blood cell.
Statistical analysis
In the baseline analysis, we evaluated the cost-effectiveness of ivonescimab plus chemotherapy versus chemotherapy alone in advanced/metastatic EGFR-variant nsq-NSCLC with disease progression during EGFR-TKI therapy. In accordance with the Chinese Pharmacoeconomic Evaluation Guidelines, three times the GDP per capita ($36,997/QALY) was used as the WTP threshold, and the ICER values were compared with the WTP values to identify the most cost-effective intervention (23).
Various sensitivity analyses were performed to examine the model’s performance and stability and thus reduce the likelihood of decision bias. The one-way analysis involved the individual adjustment of parameters within their upper and lower limits (baseline value ±20%) to identify those that significantly influenced the ICER value; these results were shown in a tornado diagram (33). Furthermore, to simulate the effect of simultaneously varying multiple uncertain parameters, probabilistic sensitivity analysis (PSA) with 10,000 Monte Carlo simulations was conducted, with random variation of the parameters within given ranges (33). The PSA results were presented using scatter plots and cost-effectiveness acceptability curves. Furthermore, following the recommendations of Hoyle et al., subgroup analyses based on stratified patient characteristics were also undertaken to examine the influence of clinical features on the outcome (34). Finally, a scenario analysis was performed, gradually adjusting the prices of key therapeutic agents to examine the potential cost-effectiveness of the ivonescimab-chemotherapy combination in treating this specific patient population.
Results
Base-case analyses
These results are illustrated in Table 2. Relative to the use of chemotherapy alone, the combination of chemotherapy with ivonescimab resulted in an additional 0.13 QALY (0.17 LYs) with a marginal cost of $35,166 and an ICER of $277,594 per additional QALY, which far exceeded the pre-set WTP threshold of $36,997/QALY. Furthermore, the INHB of ivonescimab plus chemotherapy relative to chemotherapy alone was −0.04. These suggested that from the viewpoint of the healthcare system in China, the ivonescimab-chemotherapy combination is not cost-effective in comparison with chemotherapy only for treating advanced or metastatic EGFR-variant nsq-NSCLC cases where disease progression has occurred during EGFR-TKI therapy.
Table 2
| Treatment | Total cost ($) |
Overall LYs |
Overall QALYs |
ICER, $ | INHB, QALY |
|
|---|---|---|---|---|---|---|
| Per LY | Per QALY | |||||
| Chemotherapy | 6,187 | 1.09 | 0.77 | Reference | Reference | Reference |
| Ivonescimab plus chemotherapy | 41,354 | 1.26 | 0.90 | 203,744 | 277,594 | −0.82 |
Adapted from our previous publication in Sci Rep (24). LYs, life-years; ICER, incremental cost-effectiveness ratio; INHB, incremental net health benefits; QALYs, quality-adjusted life-years.
Sensitivity analyses
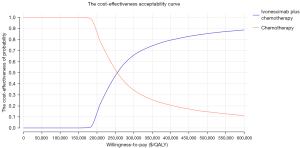
The tornado plot in Figure 1 shows the one-way sensitivity analysis results. This shows that the most critical parameters affecting the model output were the cost of ivonescimab and the PFS utility value. Parameters such as the incidence and costs associated with TRAEs had a relatively limited impact on the model’s results. Any parameter variations within the specified range led to an ICER below the WTP threshold of $36,997 per QALY, indicating that the model was robust. In the PSA, all ICER scatter points in the cost-effectiveness scatter plot fell above the WTP threshold, indicating that at a WTP cutoff of $36,997 per QALY, the probability that ivonescimab-chemotherapy treatment is cost-effective compared to chemotherapy alone was 0% (Figure 2 and Figure S3).
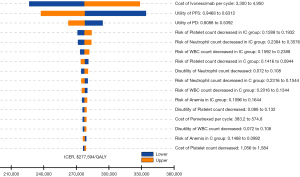
It was found that the ivonescimab-chemotherapy combination was superior in reducing the likelihood of disease progression in most subgroups, with a reduction of over 50%. The ICER for ivonescimab plus chemotherapy versus chemotherapy alone ranged between $238,558 and $326,760 per QALY, with INHB values between −1.24 and −0.68. The ICERs for all patient subgroups were higher than the WTP threshold, accompanied by negative INHB values (Table 3).
Table 3
| Subgroup | PFS, HR (95% CI) | ICER, $/QALY | INHB, QALYs | Cost-effectiveness probability of ivonescimab plus chemotherapy at WTP ($36,997/QALY) (%) |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 0.45 (0.31–0.64) | 289,341 | −0.87 | 0 |
| ≥65 | 0.54 (0.31–0.95) | 267,923 | −0.78 | 0 |
| Sex | ||||
| Male | 0.41 (0.27–0.64) | 300,471 | −0.91 | 0 |
| Female | 0.52 (0.34–0.80) | 272,310 | −0.80 | 0 |
| Clinical stage at study entry | ||||
| IV | 0.47 (0.34–0.63) | 284,184 | −0.85 | 0 |
| No. of distant metastasis sites at baseline | ||||
| <3 | 0.33 (0.21–0.51) | 326,760 | −1.02 | 0 |
| ≥3 | 0.70 (0.46–1.08) | 238,558 | −0.68 | 0 |
| Liver metastasis | ||||
| Presence | 0.64 (0.29–1.41) | 248,530 | −0.72 | 0 |
| Absence | 0.44 (0.32–0.61) | 292,018 | −0.88 | 0 |
| Smoking history | ||||
| Yes | 0.50 (0.29–0.87) | 276,896 | −0.82 | 0 |
| No | 0.45 (0.32–0.65) | 289,341 | −0.87 | 0 |
| Baseline ECOG score | ||||
| 0 | 0.46 (0.22–0.97) | 286,731 | −0.86 | 0 |
| 1 | 0.47 (0.33–0.65) | 284,184 | −0.85 | 0 |
| Baseline EGFR mutation | ||||
| Exon 19 deletion | 0.48 (0.32–0.73) | 281,697 | −0.84 | 0 |
| L858R | 0.43 (0.27–0.67) | 294,763 | −0.89 | 0 |
| Other | 0.40 (0.20–0.81) | 303,441 | −0.93 | 0 |
| T790M variation status | ||||
| Negative | 0.46 (0.21–1.01) | 286,731 | −0.86 | 0 |
| Positive | 0.22 (0.09–0.54) | 375,951 | −1.24 | 0 |
| Baseline brain metastasis | ||||
| Presence | 0.40 (0.22–0.73) | 303,441 | −0.93 | 0 |
| Absence | 0.48 (0.34–0.69) | 281,697 | −0.84 | 0 |
| Previously received EGFR-TKI treatment | ||||
| One line | 0.47 (0.30–0.73) | 284,184 | −0.85 | 0 |
| Two or more lines | 0.46 (0.31–0.69) | 286,731 | −0.86 | 0 |
| Previous treatment with third-generation EGFR-TKI | ||||
| Received | 0.48 (0.35–0.66) | 281,697 | −0.84 | 0 |
| Not received | 0.40 (0.17–0.95) | 303,441 | −0.93 | 0 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; INHB, incremental net health benefits; PFS, progression-free survival; QALY, quality-adjusted life-year; WTP, willingness-to-pay.
Since ivonescimab has only recently been introduced in China and given its significant impact on the ICER due to its price, it is possible that price adjustments or the development of charitable aid programs might be implemented in the future based on the realities of the Chinese medical market. Thus, a scenario analysis was performed, with gradual adjustment of the price of ivonescimab to examine the potential cost-effectiveness of the ivonescimab-chemotherapy combination in treating this particular subset of patients (Table S3). This indicated that, under the current conditions, to achieve economic feasibility in terms of cost-effectiveness, the unit price of ivonescimab would need to be reduced by 95% (approximately $0.1586 per mg) or a suitable charitable aid program would need to be established.
Discussion
The past decade has seen significant changes in the treatment of malignant tumors, especially in terms of NSCLC, where an increasing number of genetic mutations have been identified and are being successfully treated (35). Mutations in EGFR are found in approximately 50% of east Asian populations and are frequently observed in nsq-NSCLC (6). The standard first-line management of these cases is EGFR-TKIs such as osimertinib, which has significantly improved both survival and quality of life. Unfortunately, the majority of these patients eventually develop resistance (10). Increased knowledge of the functioning of the immune microenvironment of tumors has led to the development of various forms of immunotherapy, showing promise for NSCLC with oncogenic driver mutations. Immunotherapy has already transformed treatment strategies for solid tumors, including NSCLC, without driver gene mutations (12). Notably, some patients continue to experience long-lasting clinical benefits even after discontinuing treatment, offering significant hope for potential cures (36). However, many patients do not experience lasting responses with current immunotherapies, and monotherapies are often not successful. Thus, oncologists are turning to the use of combination immunotherapy approaches, in which combinations of ICIs or the application of bispecific antibodies represent promising strategies for improving outcomes (37). However, ICI combinations are more expensive and are frequently linked with a higher incidence of TRAEs relative to monotherapy. Bispecific antibodies that target different epitopes on separate antigens offer a potential treatment approach to enhance immune responses while potentially reducing toxicity (38,39).
Ivonescimab, a humanized tetravalent bispecific antibody, can specifically and synergistically interact with both human PD-1 and VEGF-A. These interactions can block PD-1-mediated immunosuppression in the TME as well as angiogenesis, suggesting its potential for tumor treatment (20). A phase 2 trial on NSCLC patients reported that ivonescimab with chemotherapy was efficacious in cases with disease progression during EGFR-TKI treatment, with an objective response rate (ORR) of 49.8% and a median PFS of 12 months, as well as a manageable safety profile (40). The HARMONi-A investigation is the first large Phase 3 trial analyzing the efficacy of treatment with ivonescimab together with chemotherapy against chemotherapy only in cases with advanced/metastatic EGFR-variant nsq-NSCLC with disease progression following EGFR-TKI treatment. The median PFS in the ivonescimab group was 7.1 months, extending by 2.3 months compared to the chemotherapy group (HR 0.46, 95% CI: 0.34−0.62). Subgroup analyses indicated that in nearly all subgroups, the PFS of patients receiving ivonescimab plus chemotherapy were longer than those treated with chemotherapy alone, with an over 50% lower risk of disease progression and death. This was especially notable in subgroups with disease progression during treatment with third-generation EGFR-TKIs and those with brain metastasis. Although the current OS data is as yet immature, a favorable trend toward improved OS was noticeable in the ivonescimab group (HR 0.80, 95% CI: 0.59−1.08). The safety profiles in both groups were generally manageable, with low discontinuation rates, while the most common TRAE was reduced neutrophil counts and no unexpected TRAEs were observed (21). With its recent approval in China, the ivonescimab-chemotherapy combination is thus a new standard treatment option for nsq-NSCLC cases where disease has progressed following EGFR-TKI treatment, as recommended in the guideline (22).
There have few cost-effectiveness analyses for treating EGFR-variant advanced/metastatic nsq-NSCLC unresponsive to EGFR-TKIs. In 2017, Wu et al. were the first to examine osimertinib cost-effectiveness for NSCLC with the EGFR T790M mutation after administration of first- and second-generation EGFR-TKIs, based on the AURA3 trial, concluding that it was doubtful that osimertinib treatment for T790M-mutant NSCLC would be cost-effective in either the USA or China (41). The present analysis is the first to systemically report the economic outcomes of ivonescimab treatment of advanced/metastatic nsq-NSCLC with EGFR variants where disease progression has occurred during EGFR-TKI administration from the viewpoint of healthcare system in China. Over a 10-year life cycle, ivonescimab could provide additional health benefits with marginal costs, leading to an average survival improvement of life expectancy of 0.17 LYs (2.04 months) per patient. In terms of improving the HRQOL of patients, the incremental cost of ivonescimab amounted to $35,166 (0.13 QALYs) due to improved clinical efficacy, leading to an ICER of $277,594 per QALY, significantly exceeding the WTP threshold in China Sensitivity analysis indicated that the cost of ivonescimab was the major driver of the model outcomes. At a WTP threshold of $36,997/QALY, it is doubtful that the ivonescimab-chemotherapy combination would be cost-effective relative to chemotherapy only for this patient population. The scenario analysis indicated that if the unit price of ivonescimab were to be reduced to 5%, the overall cost of the combination treatment would be reduced by 75%, yielding an ICER of $35,860 per QALY, making ivonescimab plus chemotherapy a more cost-effective treatment option (Table S3), which explicitly demonstrated that a significant price reduction in ivonescimab improved its cost-effectiveness from a practical perspective. This finding suggests that although bispecific antibodies have significant potential in clinical application, the economic aspect remains a critical hindrance when considering personalized treatment. Price adjustments or the establishment of charitable aid programs could enhance its cost-effectiveness, making the treatment more affordable for patients and balancing the fiscal expenditure on the national healthcare system. To explore the differential benefits in specific subpopulations and identify those who may be prioritized for treatment, subgroup analyses based on stratified patient characteristics were also undertaken. It was found that the ICER for all patient subgroups were higher than the WTP threshold, accompanied by negative INHB values, which indicated that the ivonescimab was not cost-effective in all subgroups. Additional trials and potential settings for the application of ivonescimab in the future could be associated with a more favorable cost-effectiveness.
This analysis has several strengths worth highlighting. First, it is the first evaluation of the potential cost-effectiveness of using the bispecific antibody ivonescimab combined with chemotherapy for treating advanced/metastatic EGFR-variant nsq-NSCLC where disease progression has occurred during treatment with EGFR-TKIs, both in China and globally. Second, the participants in the HARMONi-A study were exclusively Chinese, enabling a direct analysis of the cost-effectiveness of this treatment within the specific context of the Chinese healthcare system, which is particularly beneficial for decision-making by Chinese oncologists. Additionally, pharmacoeconomic analysis is critically involved in the “National Health Insurance Negotiations,” providing an essential reference for the inclusion of innovative drugs in the national health insurance structure. The results of this analysis offer robust evidence for negotiations involving the national government, healthcare financial institutions, and pharmaceutical companies to improve the economic feasibility of ivonescimab within the Chinese healthcare system, especially in exploring value-based pricing models, conditional reimbursement agreements, or volume-based discounts.
Several limitations should be discussed. Due to the relatively short follow-up period in the phase 3 HARMONi-A study, data on the median OS are not yet fully mature. Though statistical fitting can be used for the prediction of long-term survival, this may result in discrepancies between the model and the real world. Despite conducting sensitivity analyses to assess this uncertainty, such limitations are inevitable in model-based economic analyses of anti-tumor therapies. Second, according to the guideline, it was assumed that all patients experiencing disease progression would receive docetaxel as the subsequent-line treatment; however, this may not be an accurate reflection of clinical practice, as oncologists might choose alternative treatments based on the specificities of the case (8). Nonetheless, further sensitivity analysis found that the cost of subsequent-line treatments had a minimal impact on the model. Third, the analysis considered only the management costs of severe TRAEs (grade 3 or higher with ≥5% incidence rates), which might have introduced some bias in direct medical costs. However, the sensitivity analysis indicated that the model outputs remained stable. Finally, since the HARMONi-A study did not report health utility data, the utility values for PFS and PD were drawn from an economic evaluation of Chinese patients with advanced/metastatic squamous NSCLC, which may have introduced some bias into the model results.
Conclusions
In the context of the healthcare system in China, the combination of the bispecific antibody ivonescimab and chemotherapy for treating NSCLC patients with EGFR variants showing disease progression during treatment with EGFR-TKIs therapy is unlikely to be a cost-effective option in the absence of price adjustment or any current charitable aid program. Incorporating value-based pricing models, conditional reimbursement agreements, or volume-based discount strategies would be valuable for negotiations with the pharmaceutical industry. These approaches could significantly enhance the economic feasibility of ivonescimab within the Chinese healthcare system.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1053/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1053/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1053/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4:47-53. [Crossref] [PubMed]
- Zhang Y, Vaccarella S, Morgan E, et al. Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol 2023;24:1206-18. [Crossref] [PubMed]
- Losanno T, Gridelli C. Safety profiles of first-line therapies for metastatic non-squamous non-small-cell lung cancer. Expert Opin Drug Saf 2016;15:837-51. [Crossref] [PubMed]
- Ortega MA, Pekarek L, Navarro F, et al. Updated Views in Targeted Therapy in the Patient with Non-Small Cell Lung Cancer. J Pers Med 2023;13:167. [Crossref] [PubMed]
- Society of Cancer Precision Medicine of Chinese Anti-Cancer Association. Chinese expert consensus on immunotherapy for advanced non-small lung cancer with oncogenic driver mutations (2023 edition). Zhonghua Zhong Liu Za Zhi 2023;45:717-40. [Crossref] [PubMed]
- da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol 2011;6:49-69. [Crossref] [PubMed]
- Guidelines of Chinese Society of Clinical Oncology (CSCO Guidelines). Non-small cell lung cancer. Available online: http://www.csco.org.cn/cn/index.aspx (accessed July 25, 2024).
- NCCN (2024). NCCN guidelines, non-small cell lung cancer. Available online: https://www.nccn.org/guidelines/category_1 (accessed July 25, 2024).
- Fu K, Xie F, Wang F, et al. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J Hematol Oncol 2022;15:173. [Crossref] [PubMed]
- Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients With EGFR-Mutant NSCLC. J Thorac Oncol 2021;16:205-15. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Hegde PS, Chen DS. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020;52:17-35. [Crossref] [PubMed]
- Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front Immunol 2020;11:598877. [Crossref] [PubMed]
- Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. [Crossref] [PubMed]
- Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017;9:eaak9679. [Crossref] [PubMed]
- Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 2018;52:117-24. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Lu S, Wu L, Jian H, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med 2023;11:624-36. [Crossref] [PubMed]
- Frentzas S, Austria Mislang AR, Lemech C, et al. Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors. J Immunother Cancer 2024;12:e008037. [Crossref] [PubMed]
- Zhang L, Fang W, Zhao Y, et al. Ivonescimab combined with chemotherapy in patients with EGFR-mutant non-squamous non-small cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor treatment (HARMONi-A): A randomized, double-blind, multi-center, phase 3 trial. J Clin Oncol 2024;42:abstr 8508.
- Medical Oncology Branch of China International Exchange and Promotive Association for Medical and Health Care. China clinical practice guideline for stage IV primary lung cancer (2024 edition). Zhonghua Zhong Liu Za Zhi 2024;46:595-636. [Crossref] [PubMed]
- China guidelines for pharmacoeconomic evaluations 2020 (Chinese-English Version). Beijing: China Market Publishing Corp.; 2020.
- Long R, Guo H, Chen K. Cost-effectiveness analysis of nimotuzumab combined with gemcitabine for K-Ras wild type locally advanced or metastatic pancreatic cancer in China. Sci Rep 2025;15:6429. [Crossref] [PubMed]
- Kim J, Lim J, Lee SW, et al. Immune checkpoint inhibitors with chemotherapy for primary advanced mismatch repair-deficient endometrial cancer: A cost-effectiveness analysis. Gynecol Oncol 2023;179:106-14. [Crossref] [PubMed]
- Rieger C, Schlüchtermann J, Lehmann M, et al. Cost-effectiveness Analysis in the New Era of Treatment Strategies in Metastatic Urothelial Carcinoma Based on Checkmate-901 and EV302/Keynote-A39. Eur Urol Oncol 2024;S2588-9311(24)00231-1.
- Zhu Y, Liu K, Zhu H. Immune checkpoint inhibitor for patients with advanced biliary tract cancer: A cost-effectiveness analysis. Liver Int 2023;43:2292-301. [Crossref] [PubMed]
- Zhu Y, Liu K, Qin Q, et al. Serplulimab plus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer: A cost-effectiveness analysis. Front Immunol 2022;13:1044678. [Crossref] [PubMed]
- Lu T, Huang Y, Cai Z, et al. The cost-effectiveness of cemiplimab plus chemotherapy as the first-line treatment for advanced non-small cell lung cancer. Front Pharmacol 2023;14:1171302. [Crossref] [PubMed]
- Shi Y, Qian D, Li Y, et al. Comparing the cost-effectiveness of sintilimab + pemetrexed plus platinum and pemetrexed plus platinum alone as a first-line therapy for Chinese patients with nonsquamous non-small cell lung cancer. Transl Cancer Res 2023;12:928-38. [Crossref] [PubMed]
- Liao M, Kang S. Economic evaluation of sintilimab versus docetaxel as second-line treatment for patients with advanced or metastatic squamous non-small-cell lung cancer in China: a model-based cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res 2024;24:161-6. [Crossref] [PubMed]
- Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making 2012;32:722-32. [Crossref] [PubMed]
- Wan X, Zhang Y, Tan C, et al. First-line Nivolumab Plus Ipilimumab vs Sunitinib for Metastatic Renal Cell Carcinoma: A Cost-effectiveness Analysis. JAMA Oncol 2019;5:491-6. [Crossref] [PubMed]
- Hoyle M, Green C, Thompson-Coon J, et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health 2010;13:61-8. [Crossref] [PubMed]
- Dammeijer F, Dumoulin DW, Aerts JGJV. Anti-vascular endothelial growth factor/programmed cell death protein 1 bispecific antibodies: using nunchucks to fight an old adversary. J Thorac Oncol 2024;19:366-9. [Crossref] [PubMed]
- Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801. [Crossref] [PubMed]
- Upadhaya S, Neftelinov ST, Hodge J, et al. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov 2022;21:482-3. [Crossref] [PubMed]
- Burton EM, Tawbi HA. Bispecific Antibodies to PD-1 and CTLA4: Doubling Down on T Cells to Decouple Efficacy from Toxicity. Cancer Discov 2021;11:1008-10. [Crossref] [PubMed]
- Ma Y, Xue J, Zhao Y, et al. Phase I trial of KN046, a novel bispecific antibody targeting PD-L1 and CTLA-4 in patients with advanced solid tumors. J Immunother Cancer 2023;11:e006654. [Crossref] [PubMed]
- Zhao Y, Chen G, Chen J, et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. EClinicalMedicine 2023;62:102106. [Crossref] [PubMed]
- Wu B, Gu X, Zhang Q. Cost-Effectiveness of Osimertinib for EGFR Mutation-Positive Non-Small Cell Lung Cancer after Progression following First-Line EGFR TKI Therapy. J Thorac Oncol 2018;13:184-93. [Crossref] [PubMed]


