
Impaired natural killer cell maturation in lung adenocarcinoma driven by FABP4 and SPON2 downregulation through disrupted lipid metabolism
Highlight box
Key findings
• Through bioinformatics analysis, natural killer (NK) cells are significantly reduced in the tumor microenvironment of lung adenocarcinoma, with markedly decreased expression of FABP4 and SPON2, and NK cell maturation in tumor tissues was hindered. Further experiments demonstrated that FABP4 downregulation in NK cells affected cellular maturation and function through disrupted lipid metabolism.
What is known and what is new?
• NK cells are essential components of innate immunity and play a pivotal role in antitumor responses. Previous studies have mainly focused on their infiltration and functional inhibition, while the metabolic changes driving their dysfunction in lung adenocarcinoma remain poorly understood.
• This study presents new evidence showing that NK cells might display a phenotype of developmental stagnation in lung adenocarcinoma, and low FABP4 and SPON2 expression may impair NK cell maturity by affecting lipid metabolism.
What is the implication, and what should change now?
• This work highlights the crucial role of FABP4 and SPON2 in maintaining NK cell function, offering new insights into immune dysregulation in lung cancer. Targeting FABP4 and SPON2 may serve as a promising strategy for restoring immune function and enhancing NK cell-based immunotherapies in patients with lung cancer.
Introduction
Lung cancer is a leading cause of cancer-associated mortality worldwide (1). Immunological analysis of the tumor microenvironment (TME) has shown great promise for improving prognosis and predicting patient response to immunotherapy. The role of the host immune system is critical in predicting the benefits of immune checkpoint inhibitors. Understanding the mechanisms influencing the diversity and function of immune cells in the TME will pave the way for the development of more effective immunomodulatory strategies. Most prior studies have focused on stromal cells, cancer cells, T cells, or macrophages within the TME.
As key players in the innate immune system, natural killer (NK) cells play a critical role in combating tumor development and progression. However, the reasons for impaired NK cell function in the hostile TME remain unclear, particularly regarding their infiltration into tumors, phenotypic heterogeneity, and dysregulation within the TME (2,3). Better understanding of the regional features of NK cells in the TME is required to fully exploit the potential of NK-based checkpoint immunotherapy. It has been demonstrated that TIGIT and TIM3 exert inhibitory functions on NK cells within the TME (4,5). Some studies have identified a deficiency and dysfunction of NK cells interacting with LAMP3+ dendritic cells and TREM2 macrophages in lung cancer (6,7).
The metabolic reprogramming of NK cells in anti-tumor immunity has also garnered widespread attention (8,9). The TME drives metabolic dysfunction in NK cells to impair antitumor immunity (10) and modulating lipid metabolism could enhance tumor immunotherapy (11). The metabolic pathway of glycolysis and oxidative phosphorylation (OXPHOS) plays a key role in maintaining proliferation and cytotoxicity of NK cells (9). A study has reported NK cell dysfunction in the TME is due to suppression of glucose metabolism via lipid peroxidation-associated oxidative stress, activation of the Nrf2 antioxidant pathway restored NK cell metabolism and function (12). MEF2C is also a crucial orchestrator of NK cell antiviral immunity by regulating lipid metabolism (13). Modulating the metabolites and enzymes involved in sphingolipid metabolism helps maintain immune system balance, for instance, sphingomyelin sustains the cytotoxicity of NK cells (14). A study has highlighted that fatty acid oxidation promotes metabolic resilience in NK cells against cancer (15). Interleukin-15 (IL-15) can serve as a key regulatory factor for metabolic reprogramming, restoring mitochondrial function in NK cells and enhancing their anti-tumor capabilities (10). Thus,exploring the mechanism of NK cell metabolic reprogramming is expected to provide new insights for optimizing NK cell immunotherapy.
The present study aim to investigate the metabolic modification of NK cells in lung adenocarcinoma. To explore the mechanisms underlying diminished or immature NK cells in lung adenocarcinoma, a comprehensive single-cell transcriptomic profiling analysis were conducted on NK cell populations from 13 clinical specimens with lung adenocarcinoma. The findings were validated in clinical samples and an NK cell model. The present study has important theoretical significance for understanding NK cell dysregulation in tumor formation. We present this article in accordance with the MDAR reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-1027/rc).
Methods
Cell clustering, visualization, and cell type annotation
Single-cell transcriptome data from the E-MTAB-6149, EMTAB-653, and GSE131907 datasets were integrated to explore the TME characteristics in lung adenocarcinoma. The data included 13 pairs of cancer and adjacent samples (Table S1). The R package Seurat (v4.1.0) was used to cluster the cells in a merged matrix of single-cell data from 26 lung tissues. Low-quality cells were initially excluded based on specific thresholds: those with fewer than 200 or more than 5,000 detected transcripts, as well as cells where mitochondrial genome transcripts exceeded 15% of the total. Following this filtering step, the gene expression matrices from the remaining cells were normalized according to the total Unique Molecular Identifier (UMI) counts per cell. The normalized data was then transformed using the natural logarithm for further analysis. The FindVariableFeatures function was used to obtain the top 2,000 highly variable genes from the corrected expression matrix, which were then centered and scaled after regression cell cycle (S and G2M) score calculation using the CellCycleScoring function in Seurat. Principal component analysis (PCA) was then performed on the highly variable genes using the RunPCA function. The RunHarmony function with default parameters in Seurat was utilized to correct for batch effects in the two datasets. The primary cell cluster was determined by applying the Louvain-Jaccard graph-based approach. This followed PCA, which was used to reduce dimensionality and construct k-nearest neighbor graphs (k=15) for the cells. The FindNeighbors function, utilizing Euclidean distance in the 50-dimensional principal component (PC) space, facilitated this process. To classify all filtered cells, the clustering resolution was set to 0.4 using the FindClusters function in Seurat. Subsequently, the RunUMAP function in Seurat, with dimension parameters set to 1:15, was employed to project the high-dimensional data into two dimensions for visualization purposes. Finally, the Seurat FindAllMarkers function, using default settings, was applied to detect genes uniquely expressed in each cluster. The statistical significance of gene expression differences was assessed using the Wilcoxon rank sum test with Bonferroni correction, and cell types were manually annotated based on the identified cluster markers.
Cell-type subclustering
Subclustering was performed on T cell and NK cell cluster. The same functions described above were used to obtain the subclusters. The normalization, dimensionality reduction, and clustering steps were repeated. Subclusters were identified and annotated as different specific cell subtypes. Different cell types overexpress distinct marker genes. Macrophages overexpress CD14, CD68, and FCGR3A. Epithelial cells overexpress EPCAM, KRT8, and SFTPA1. Endothelial cells show high expression of PECAM1, VWF, and IGFBP7, while B cells express high levels of CD79A, CD19, and MS4A1. As for NK cells, the three traditional NK cell subpopulations exhibit high expression levels of KLRD1 and TYROBP. The NKT cell subpopulation overexpress three genes, including CD3E, CD8A (a conventional CD8+ T cell marker), and KLRD1 (a traditional NK cell marker). Both immature CD4+ and CD8+ T cells express high levels of IL7R and GPR183.
Cell ratio calculation
The sample composition based on cell type was determined by quantifying the number of cells belonging to each cell type within every sample. These counts were subsequently normalized by dividing them by the total cell count for the corresponding sample and then converted into percentages, scaling the values to 100% for each cell type.
Differentially expressed gene (DEG) analysis
To identify DEGs between tumor and adjacent tissue for each cell type, the FindMarkers function in Seurat was utilized. The statistical significance of these gene expression differences was assessed using the Wilcoxon rank sum test, with adjustments made using the Bonferroni correction. DEGs between the two groups within each subcluster were defined based on the following thresholds: (I) the gene must be expressed in more than 10% of cells in at least one or both groups; (II) the absolute value of the log2 fold change (|log2 fold change (FC)|) must exceed 0.25; and (III) the adjusted P value from the Wilcoxon rank sum test must be less than 0.05.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
The computation of enrichment scores, represented as P values, for selected GO and Kyoto KEGG terms was performed utilizing the clusterProfiler R package (version 3.14.3). This process involved the application of a hypergeometric statistical test, adopting a significance threshold of 0.05. To mitigate the risk of false discoveries, the Benjamini-Hochberg procedure was employed for adjustment. The analysis focused on DEGs identified within subclusters, with the entire set of genes cataloged in the org.Hs.eg.db database serving as the comparative background. For the purpose of result illustration, a barplot function was utilized to graphically represent the findings.
Single-cell metabolism quantification
The single-cell metabolic activity of NK subtype cells was calculated using the scMetabolism package (v0.2.1). The analysis was performed with the “VISION” method, and KEGG metabolic gene sets were employed as the reference. To visualize the results, a heatmap depicting the metabolic activity scores was created using the pheatmap package in R.
Pseudotime trajectory construction
To investigate the transitions between cell states in NK1 clusters, trajectory analysis was independently conducted using the Monocle2 R package (v 2.14.0). The process began by constructing a CellDataSet object from raw count matrices of the relevant cell types, applying the negbinomial.size parameter with default configurations. Normalization was performed using the estimateSizeFactors and estimateDispersions functions, maintaining default settings. DEGs identified in each group via the Seurat FindAllMarkers function (with default parameters) were utilized to arrange cells along a pseudotime axis. The DDRTree method was employed for dimensionality reduction, and the orderCells function was used to organize the cells. The resulting trajectory was visualized using the plot_cell_trajectory function. Furthermore, changes in gene expression over pseudotime were assessed using the differentialGeneTest function with default parameters. Significant DEGs (with an adjusted P value, q value <0.0001) were displayed using the plot_cell_trajectory and plot_pseudotime_heatmap functions within Monocle2.
Cell lines and human tissues
NK92 cells were obtained from the Cellverse Co., Ltd (cat. No. iCell-h0388, Shanghai, China). NK92 cells were grown in alpha minimum essential medium supplemented with 12.5% fetal bovine serum, 12.5% horse serum, 100–200 U/mL rhIL-2, 0.2 mM inositol, 0.1 mM β-mercaptoethanol, and 0.02 mM folic acid in an incubator containing 5% CO2 at 37 ℃. Five patients with lung adenocarcinoma at the Guangdong Lung Cancer Institute were enrolled in 2022. Clinical features of patients are provided in Table S2. The study protocol was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. KY2024-078-01). All patients provided written informed consent to participate. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Immunohistochemistry (IHC)
To assess the expression of target genes, anti-FABP4 rabbit monoclonal antibodies (Abcam, MA, UK) and anti-SPON2 rabbit polyclonal antibodies (Proteintech, IL, USA) were employed. Tumor tissue specimens were preserved in formalin and subsequently embedded in paraffin. Sections of non-small cell lung cancer (NSCLC) tissues, cut to a thickness of 3–5 µm, were dried for 60 minutes in a dehydration oven set at 60 ℃. Antigen retrieval was conducted using citrate buffer (pH 6.0) in a microwave oven. To block non-specific binding, the tissue sections were treated with 10% normal goat serum (Tian Gen Biotech, Beijing, China) for 30 minutes. The slides were then incubated overnight at 4 ℃ with anti-FABP4 monoclonal antibodies (diluted 1:100) and anti-SPON2 polyclonal antibodies (diluted 1:100). This was followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (diluted 1:2,000; Abcam) for 45 minutes at 37 ℃. Nuclei were counterstained with hematoxylin for 1 minute. Finally, the slides were scanned under white light using an Olympus CX31 microscope (Japan).
Multicolor immunofluorescence (IF) and laser confocal imaging
Multicolor IF confocal imaging was used to verify FABP4 and SPON2 expression in NK cells within lung cancer tissue samples. To detect FABP4 and SPON2 gene expression of NK cell in lung adenocarcinoma, multicolor fluorescence staining was performed with anti-CD3 human mAb (cat. No. 60181-1-IG; Proteintech), anti-CD56 human mAb (cat. No. AF2408; Novus, Colorado, USA), anti-FABP4 mAb and anti-SPON2 pAb. Tyramine signal amplification (TSA) technology was utilized. The slides were examined under a laser confocal microscope (ZEISS, BW, Germany). Cancerous and adjacent tissues were compared. Repeat three slides for each sample.
Lentivirus construction
FABP4 knockdown lentiviruses were obtained from OBiO Technology Co., Ltd. (Shanghai, China). The following three shRNA target sequences for FABP4 (NM_001442) were designed: hFABP4 (shRNA1): GCCAGGAATTTGACGAAGTCA; hFABP4 (shRNA2): GTGATCACCATTAAATCTGAA; and hFABP4 (shRNA3): GCATGGCCAAACCTAACAT. The hFABP4 shRNAs were inserted into the pSLenti-U6-shRNA-CMV-EGFP-F2A-Puro-WPRE vector. Lentivirus plasmids LV-hFABP4 (shRNA1,2,3) were constructed and transfected into E. coli DH5α competent cells. Then, positive transformants were identified using colony polymerase chain reaction (PCR), the plasmids were extracted, and sequencing was performed for validation. Recombinant lentiviruses were generated using 293T cell transfection. To prepare viral stocks, the supernatant was harvested 48 h after infection. Viral stocks were frozen at –80 ℃ and thawed before use.
Lentiviral treatment and transfection
Before infection, NK92 cells were seeded in six-well plates overnight. Then, 1 mL of fresh medium containing lentivirus [multiplicity of infection (MOI): 100] and 7 µL of polybrene-plus reagent (GLPBIO Biotech, Beijing, China) were added to each well. The culture medium was replenished after 16–18 h. FABP4 expression in infected NK92 cells was evaluated using a fluorescence microscope and quantitative PCR (qPCR) until the fluorescence reached 100%. Successfully transfected cells were screened using 2 µg/mL puromycin. Repeat three wells for each sample.
Nucleic acid extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from NK92 cells using TRIzol reagent (Invitrogen, CA, USA). Subsequently, 1 µg of RNA was reverse transcribed using a reverse transcription-PCR kit (Takara, Dalian, China) and primer sequences were designed. Then, qPCR was performed with gene-specific primers and a PowerUpTM SYBR Green assay (Applied Biosystems, CA, USA) in an ABI PRISM® 7500 Sequence Detection System (Applied Biosystems). The primers were purchased (Invitrogen, Shanghai, China). Primer details are provided in Table S3. The reaction conditions were as follows: 50 ℃ for 2 min; 95 ℃ for 2 min; and 40 cycles of 95 ℃ for 15 s and 60 ℃ for 30 s. Repeat three wells for each sample. The dissolution curve was subsequently determined. Relative expression was normalized to the geometric mean of the housekeeping gene expression and was calculated with the 2-ΔΔCt method.
Western blotting (WB)
Briefly, cells were lysed in radio immunoprecipitation assay (RIPA) lysis buffer (Biyuntian Biotech, Shanghai, China). The extracts were run on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Then, proteins were transferred to PVDF membranes (Millipore,MA, USA) and blocked with 5% milk. FABP4 (1:1,000; Abcam) and GAPDH (1:10,000; Kangcheng Biotech, Guangzhou, China) antibodies were used. Repeat three times for each sample. Blots were visualized with chemiluminescence (ECL) Prime (Millipore) and imaged using a ChemiDoc Touch apparatus (Bio-Rad, CA, USA).
NK cell cytotoxicity assays
NK92 cells exhibit cytotoxic effects and were utilized in the antibody-independent cytotoxicity assay. NK92 cells were treated with an FABP4 inhibitor BMS-309403 (20 µmol/L; APExBIO, Houston, TX, USA) for 48 h at 37 ℃. For NK cell cytotoxicity assays, NK92-shFABP4 cells, NK92-FABP4-inhibited cells (Product Name: BMS-309403; cat. No. APEB7794), and H1650 lung cancer cells were individually cultured for 24 h at 37 ℃ under 5% CO2. Subsequently, NK92 cells were co-cultured with H1650 cells in 12-well plates at a final effect cell:target cell ratio of 5:1 for 4 h. Then, NK92 cell degranulation was measured using flow cytometry (FCM) detection of CD107a in viable cells. Repeat three wells for each sample.
FCM
FCM was performed on co-cultured NK cells from wild-type NK92, NK92-shRNA-control, NK92-shFABP4, and NK92-FABP4-inhibited cells. For extracellular staining, ~1×106 cells were washed with phosphate-buffered saline (PBS), incubated with antibodies in 100 µL of PBS with 5 µL 100 µg/mL of mouse anti-human CD107A mAb (cat. No. 562623; BD, NJ, USA), 20 µL 25 µg/mL APC mouse Anti-Human CD56 mAb (cat. No. 555518; BD) and 5 µL 0.2 mg/mL PE-CY5 mouse Anti-Human CD326 mAb (cat. No. 624350; BD) for 20 min at room temperature protected from light, and then washed with PBS again. CD56 mAb labeled NK cells, and CD326 mAb labeled tumor cells. Repeat three times for each sample. FCM was performed using a BD FACSAriaTM II instrument. Data were analyzed in FlowJo (BD).
Non-targeted lipid metabolism analysis
Furthermore, lipid metabolism was compared among wild-type NK92, NK92-shRNA-control, NK92-shFABP4, and NK92-FABP4-inhibited cells. Cell samples (200 µL) were collected and 400 µL of glacial methyl tert-butyl ether solution and 80 µL of glacial methanol were added to a 2.0-mL tube, shaken, and mixed thoroughly. Samples were then centrifuged at 1,000 g for 15 min and 200 µL of supernatant was transferred to another EP tube. After the supernatant was freeze-dried, 200 µL of dichloromethane and methanol (1:1) was added for reconstitution. After centrifugation at 3,000 rpm for 15 min, the supernatant was transferred to a sample vial for ultra-performance liquid chromatography (UPLC)-high resolution mass spectrometry (HRMS) detection. A 10–20-µL extract volume was mixed into quality control samples for UPLC-HRMS detection. The entire operation was performed on ice. A Waters Ultra-High Pressure Liquid Phase System ACQUITY UPLC System Class was used for data acquisition. The column type was ACQUITY UPLC CSH C18 (1.7 µm 100 mm × 2.1 mm, Waters, UK). The column temperature was set to 40 ℃ at the time of acquisition, while the flow rate was 0.3 mL/min. Acquisition was performed on a Triple TOF 6600 (SCIEX, Framingham, MA, USA) high-resolution time of flight mass spectrometer. Each sample was acquired in positive mode and in negative ion mode. Data collection was performed in information-dependent collection mode. Repeat three times for each sample.
Statistical analysis
Statistical analysis and visualization were performed using R language (v4.2.0). Differential gene expression was analyzed with the Wilcoxon rank-sum test. Continuous variables were evaluated using GraphPad Prism Software (v9.0). The significance level was set at P<0.05.
Results
Immune cell subset composition assessment
Bioinformatics analyses were conducted, and IF, IHC, and cell function experiments were performed for verification. The study process is illustrated in Figure 1A. A total of 90,234 cells were filtered from 13 pairs of single-cell data samples for subsequent integration analysis, which allowed to categorize all cells into the following ten subsets: T-NK cells [37,318], macrophages [24,784], epithelial cells [11,153], fibroblasts (3,995), endothelial cells [3,651], B cells [3,080], mast cells [2,656], plasma cells [1,776], proliferating cells [1,541], and plasma cell-like dendritic cells [280] (Figure 1B,1C). The major immune cells expressed the canonical cell markers (Figure 1D). There were significant differences in the proportions of some cell types between cancer samples and adjacent tissues. Specifically, the proportion of macrophages and endothelial cells was significantly lower in cancer samples, whereas the proportion of B cells and plasma cells was significantly higher in cancer samples (Figure 1E).
Diminished NK cell numbers in lung adenocarcinoma microenvironment
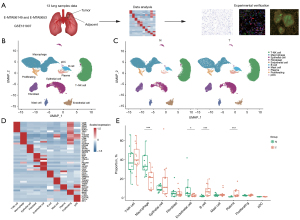
To further investigate the molecular characteristics of NK cells in lung adenocarcinoma and their changes during the disease occurrence, T and NK cell subpopulations were subdivided. CD4+ T cells were categorized into immature CD4+ T cells, two groups of effector memory CD4+ T cells, Treg CD4+ T cells, and follicular CD4+ T helper cells. CD8+ T cells were divided into immature CD8+ T cells, effector memory CD8+ T cells, effector CD8+ T cells, and resident CD8+ T cells. NK cells were categorized into three subgroups of NK cells and one group of NKT cells (Figure 2A,2B). The NK cell subsets expressed distinct canonical cell markers (Figure 2C). The proportions of NK and T cells showed an inverse trend in both adjacent and cancer samples. Cancer samples exhibited a notably higher proportion of most T cell subsets compared to adjacent samples, while the proportion of NK cells was notably reduced in cancer samples (Figure 2D). Gene expression difference analysis in NK cell subpopulations in cancer and adjacent samples showed that NK cells in cancer samples expressed higher levels of genes, such as GZMK, CXCR4, BIRC3, and RGCC, compared to the levels observed in the adjacent samples. They also expressed lower levels of FABP4, SPON2, and KLRF1 (Figure 2E). According to the GO term analysis of DEGs, the highly expressed genes were concentrated in gene modules associated with apoptosis and hypoxia response, whereas the weakly expressed genes were associated primarily with fatty acid biosynthesis, immune response regulation, and NK cell-mediated cytotoxic response (Figure 2F). Single-cell metabolism quantification indicated that the score for fatty acid metabolism activity was lower in the tumor group of the NK subgroup, particularly the NK1 subgroup (Figure 2G).
Pseudotime ordering and pathway analysis of NK1 clusters
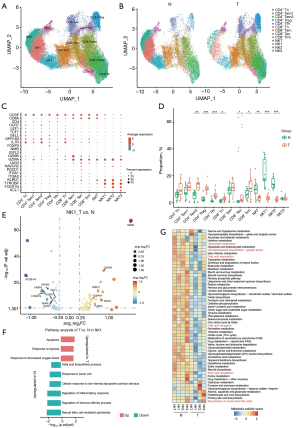
Time-simulating analysis was used to examine the changes in NK cells within the lung adenocarcinoma TME. The development and maturation process of NK cells is depicted from right to left in Figure 3A, with colors transitioning from dark to light. NK cells from samples adjacent to the tumors spanned the entire developmental trajectory, whereas most NK cells from cancer samples clustered near the starting point of this trajectory and showed minimal presence near the endpoint (Figure 3B). These results indicated hindered development and maturation of NK cells in cancer samples. To determine the factors contributing to this inhibition, trends in gene expression levels during NK cell maturation were investigated. Genes showing dynamic changes throughout the maturation process were categorized into two modules. Genes in module 1 were highly expressed at the developmental starting point, with expression gradually decreasing as development progressed. In contrast, genes in module 2 were expressed at low levels during the early stages of development. Their expression gradually increased and peaked at the developmental endpoint (Figure 3C). Module 2 comprised 103 genes. GO term analysis revealed that these genes are involved primarily in the biological synthesis of unsaturated fatty acids and fatty acid derivatives, as well as lipoprotein particle responses, among other functional modules (Figure 3D,3E). Taking FABP4 and SPON2 as examples, both genes were expressed at low levels near the developmental initiation point but showed high expression at the developmental endpoint (Figure 3F). It was speculated that the inhibition of module 2 gene expression in NK cells from cancer samples and low expression state maintenance impeded the development and maturation process, thus leading to NK cells remaining in an immature state.
FABP4 and SPON2 expression verification in tissues and NK cells
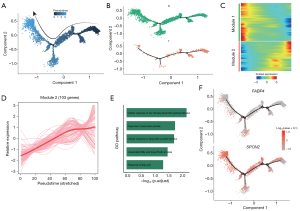
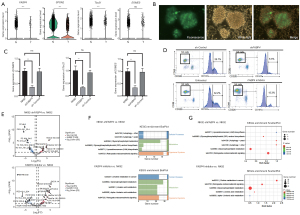
The results of multicolor IF confirmed the previous bioinformatics analysis, indicating that the expression of both FABP4 and SPON2 in the immune microenvironment of lung cancer tissue samples was significantly lower than that in the adjacent tissues (Figure 4A-4C). In addition, limited infiltration of NK cells was observed in lung cancer tissues. FABP4 and SPON2 expression was further analyzed using TCGA data. FABP4 expression was significantly lower in lung cancer tissues than in adjacent tissues, whereas no difference was observed in SPON2 expression (Figure 4D). This result was further validated using IHC in five pairs of lung cancer tissue samples, which confirmed that FABP4 expression was significantly lower in lung cancer tissues than in adjacent tissues, whereas no significant difference was found in SPON2 expression (Figure 4E,4F).
Inhibited function of FABP4-deficient NK cells
Bioinformatics analysis of NK cells in tumors and adjacent tissues indicated that FABP4, SPON2, and Tbx21 expression in tumor NK cells was significantly decreased (Figure 5A). Lentiviral shFABP4 was transduced into NK92 cells (Figure 5B). Decreased FABP4, Tbx21, and EOMES expression was verified using qPCR or WB in NK92-shFABP4 cells (Figure 5C; Figure S1). The percentage of degranulated NK cells (CD56+ CD107a+) of the shFABP4 and FABP4 inhibitor groups (BMS-309403) were significantly lower (6.3% and 15.3%, respectively) than the wild-type group (32.2%) (Figure 5D). Non targeted lipid metabolism analysis showed a total of 36 lipids, including hexCer 42:3;O2, hexCer 34:1;O2, phosphatidyl ethanolamine (18:0/18:1), and phosphatidylcholine (20:5/0:0), were upregulated in NK92-shFABP4 cells compared to wild-type NK cells. In contrast, 59 lipids, such as phosphatidylcholine 46:6, phosphatidyl ethanolamine O-18:1_22:4, phosphatidylcholine 18:4_24:6, phosphatidylcholine 21:0_21:0, were downregulated (Figure 5E). In addition, inclusion of the BMS-309403 inhibitor also induced lipid metabolic disorders in NK92 cells, thus leading to the upregulation of 65 lipids, including diacylglycerol 51:14, HexCer 42:3;O2, lysophosphatidylcholine 20:4 and sphingomyelin 29:5;O2; and the downregulation of 23 lipids, including sphingomyelin 48:9;O2, phosphatidyl ethanolamine (ether bond) 18:1_22:4, phosphatidylinositol (18:1/0:0) and triglyceride 18:2_18:2_22:3;O (Figure 5E). Enrichment analysis of these differentially expressed lipids was subsequently conducted. Differentially expressed lipids in NK92-shFABP4 cells were primarily enriched in metabolic pathways, such as glycerophospholipid metabolism. Similarly, NK cells in the BMS-309403 inhibition group also exhibited enrichment in comparable pathways (Figure 5F,5G).
Discussion
NK cells belong to a key class of innate immune lymphocytes and play a central role in mediating the body’s immune surveillance and defense mechanisms against tumor cells by secreting specific cytokines and chemokines (16,17). There are notable differences in NK cell infiltration across various tumor types (6). The presence of NK cells within the TME may be associated with favorable prognosis. Anti-programmed death ligand 1 (PD-L1) antibodies have been reported to inhibit the growth of PD-L1-negative tumors in vivo by activating PD-L1+ NK cells (18). In addition, a recent clinical trial has shown that pembrolizumab combined with NK cells significantly prolongs survival in patients with advanced PD-L1+ NSCLC (19). These results suggest that changes in NK cell percentage may be associated with immune treatment effectiveness.
The proportion of NK cells has been found to be significantly lower in NSCLC lesions than in uninvolved normal lung tissue (20,21). Consistent with previous research, the current study revealed that the percentage of NK cells was markedly reduced in cancer samples compared to adjacent samples. Another study comparing the immune microenvironments of smoking and non-smoking lung cancer has confirmed that tumor tissues have a lower proportion of NK cells and a higher proportion of B lymphocytes than normal lung tissues. Notably, NK cells in non-smoking tumor tissue show a more dramatic decline than those in smoking tumors (22). Our analysis identified three distinct NK cell subgroups: human Cytomegalovirus (HCMV)-driven adaptive NK cells (NK1), CD56dim NK cells (NK2), and CD56bright NK cells (NK3), which is consistent with previous studies (23) (Figure S2). The percentage of CD56dim NK cells was notably lower in tumor tissues than in normal tissues (P<0.001) (Figure 2D), as aligned with prior report (6). Pseudotime analysis revealed that NK cells in the TME were significantly enriched in the early developmental stages, while terminally differentiated subset was nearly absent (Figure 3B). CD56dim NK cells are characterized by terminal differentiation markers and are known for their greater cytotoxicity and higher secretion of granzymes, perforin, and cytolytic granules (6,24-26). Since CD56dim NK cells are generally defined as fully differentiated cells (27-29), their reduction in tumors may indicate that the maturation of NK cells is potentially being hindered. To identify a specific cause, gene expression in NK cells from cancer samples was compared to that from adjacent tissue cells. FABP4 and SPON2 gene expression in NK cells from cancer tissues was decreased.
Recent research focuses on the dysregulation of lipid metabolism within the immune microenvironment in lung adenocarcinoma (30-32). As a key regulator of intracellular lipid transport, the FABP family has attracted increasing attention due to its role in tumor occurrence and progression (33,34). In NSCLC, SIRT5 promotes cell development by decreasing FABP4 acetylation levels (33). Some studies have also associated elevated FABP4 level with poor prognosis in NSCLC (35). FABP4 has been widely observed to be differentially expressed in various cell types, including immune cells, adipocytes, endothelial cells, and bronchial epithelial cells. This diversity of expression patterns does not only reveal the broad distribution of FABP family but also predicts its specific functions in different tissue environments (35-39). For example, FABP4 supports CD8+ tissue-resident memory T cells in effectively using free fatty acids (FFAs) from the environment, while also ensuring cell persistence in specific tissues by promoting the oxidative FFA metabolism, thus mediating a strong protective immune response (40). In macrophages, FABP4 promotes obesity-associated pancreatic cancer progression via the NLRP3/IL-1β axis (41). In summary, FABP4 is a key regulator of metabolic signaling pathways and gene expression. Its pleiotropic effects and extensive network provide important insights for deeper understanding of cell metabolism and tumor biology (42). FABP4 expression in the present study was significantly decreased in both tumor tissues and NK cells within tumor tissues.
SPON2 is an extracellular matrix protein composed primarily of two structures: the N-terminal F-spondin domain and the C-terminal thrombospondin type 1 repeat, which plays a role in SPON2’s function as a pathogen pattern recognition molecule, thereby enabling it to recognize and bind specific pathogen components and trigger an immune response (43-45). Recent studies increasingly indicate that SPON2 expression is significantly elevated in lung adenocarcinoma (46), breast cancer (47), gastric cancer (48), and ovarian cancer (49) and is notably associated with poor prognosis. Additionally, SPON2 has been found to promote bone metastasis in lung adenocarcinoma by activating the NF-κB signaling pathway (50). However, detailed investigations on the specific role and expression of SPON2 in immune cells remain limited. No significant difference in SPON2 expression was present between tumor and normal tissues. However, SPON2 levels were significantly decreased in NK cells within tumor tissues, which was in agreement with previous study results (6).
Initial bioinformatics analysis showed that FABP4 and SPON2 were associated with the lipid metabolism pathway in NK cells in lung cancer. Furthermore, NK92-shFABP4 cells were constructed to explore the mechanisms and biological consequences related to lipid metabolic reprogramming. Among the metabolic alterations observed in lung cancer, those associated with lipid metabolism are garnering increasing attention and recognition (51,52). Although lipid metabolism in tumor cells has been extensively discussed, the effects of these changes on tumor immunity remain poorly defined. One study demonstrated that FABP5-mediated lipid homeostasis positively regulates NK cell maturation, thereby preventing tumor metastasis in the lungs (53). The present study indicated that the expression of FABP4, a member of the FABP family, was significantly decreased in NK cells from cancer samples. Based on these findings, it can be speculated that FABP4 might also mediate immunosuppressive effects by regulating NK cell maturation. On one hand, the lack of FABP4 decreases the uptake and oxidative metabolism of FFA in NK cells, thus decreasing adenosine triphosphate (ATP), Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), and other important intermediate levels and affecting NK cell maturation and function (54). On the other hand, FABP4 inhibition enhances PPARγ levels and activity. Activation of the PPAR pathway has been extensively studied and confirmed to inhibit the mammalian target of rapamycin (mTOR) signaling pathway, thus interfering with activation-induced metabolic reprogramming and decreasing the expression of effector molecules in NK cells. This cascade of molecular events ultimately leads to NK cell deficiencies in patients, as evidenced by decreased cytotoxicity and significant inhibition of p-S6, which is crucial for NK cell maturation (55-58). To explore the function of the FABP4 gene in NK cells, lentiviral knockdown and small molecule inhibitor BMS309403 were used to suppress endogenous FABP4 expression. In the present study, FABP4-deficient NK cells exhibited low expression of Tbx21 and EOMES in NK92 cells transfected with shFABP4. Sustained expression of EOMES and Tbx21 is critical for orchestrating mature NK cell function and identity (53,59). FCM results showed that cytotoxic function was impaired in NK92-shFABP4 and NK92-FABP4-inhibited cells (BMS-309403). Non-targeted lipid metabolism analysis indicated that severe lipid metabolism disorders occurred in both NK92-shFABP4 and NK92-FABP4-inhibited cells (BMS-309403). Differentially expressed lipids were concentrated in pathways associated with autophagy, glycerophospholipid metabolism, linoleic acid metabolism, and other signaling pathways. These findings suggested that low FABP4 gene expression might hinder NK cell maturation by affecting lipid metabolism in lung adenocarcinoma.
The present study findings elucidate the mechanisms underlying the decreased NK cell number and impaired function in the TME, thereby offering a new perspective for restoring immune function in patients with lung cancer. However, there are several limitations in this study. First, FABP4 expression upregulation in NK cells was not explored in primary lung cancer cells or animal models, both of which could provide a better understanding of the effects of FABP4 on NK cell function. Second, further exploration of the involved signaling pathway network is necessary to fully understand how FABP4 regulates the function and maturation of NK cells.
Conclusions
The number of NK cells was reduced in the TME of lung adenocarcinoma and FABP4 and SPON2 expression was diminished. The low FABP4 expression in NK cells in lung cancer tissues affected cellular maturation and function. The present study revealed novel findings indicating that low FABP4 and SPON2 expression may hinder NK cell maturity by affecting lipid metabolism in lung adenocarcinoma, thus providing a new perspective for restoring immune function in patients with lung cancer.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-1027/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-1027/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-1027/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-1027/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. KY2024-078-01). All patients provided written informed consent to participate. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Habif G, Crinier A, André P, et al. Targeting natural killer cells in solid tumors. Cell Mol Immunol 2019;16:415-22. [Crossref] [PubMed]
- Melaiu O, Lucarini V, Cifaldi L, et al. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front Immunol 2019;10:3038. [Crossref] [PubMed]
- Bi J, Tian Z. NK Cell Dysfunction and Checkpoint Immunotherapy. Front Immunol 2019;10:1999. [Crossref] [PubMed]
- Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018;19:723-32. [Crossref] [PubMed]
- Tang F, Li J, Qi L, et al. A pan-cancer single-cell panorama of human natural killer cells. Cell 2023;186:4235-4251.e20. [Crossref] [PubMed]
- Park MD, Reyes-Torres I, LeBerichel J, et al. TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer. Nat Immunol 2023;24:792-801. [Crossref] [PubMed]
- Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer 2020;20:516-31. [Crossref] [PubMed]
- Jiang M, Fang H, Tian H. Metabolism of cancer cells and immune cells in the initiation, progression, and metastasis of cancer. Theranostics 2025;15:155-88. [Crossref] [PubMed]
- Tumino N, Nava Lauson CB, Tiberti S, et al. The tumor microenvironment drives NK cell metabolic dysfunction leading to impaired antitumor activity. Int J Cancer 2023;152:1698-706. [Crossref] [PubMed]
- Ping Y, Fan Q, Zhang Y. Modulating lipid metabolism improves tumor immunotherapy. J Immunother Cancer 2025;13:e010824. [Crossref] [PubMed]
- Poznanski SM, Singh K, Ritchie TM, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab 2021;33:1205-1220.e5. [Crossref] [PubMed]
- Li JH, Zhou A, Lee CD, et al. MEF2C regulates NK cell effector functions through control of lipid metabolism. Nat Immunol 2024;25:778-89. [Crossref] [PubMed]
- Zheng X, Hou Z, Qian Y, et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat Immunol 2023;24:802-13. [Crossref] [PubMed]
- Sheppard S, Srpan K, Lin W, et al. Fatty acid oxidation fuels natural killer cell responses against infection and cancer. Proc Natl Acad Sci U S A 2024;121:e2319254121. [Crossref] [PubMed]
- Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discov 2022;21:559-77. [Crossref] [PubMed]
- Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011;331:44-9. [Crossref] [PubMed]
- Cózar B, Greppi M, Carpentier S, et al. Tumor-Infiltrating Natural Killer Cells. Cancer Discov 2021;11:34-44. [Crossref] [PubMed]
- Lin M, Luo H, Liang S, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest 2020;130:2560-9. [Crossref] [PubMed]
- Leader AM, Grout JA, Maier BB, et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell 2021;39:1594-1609.e12. [Crossref] [PubMed]
- Zhao J, Lu Y, Wang Z, et al. Tumor immune microenvironment analysis of non-small cell lung cancer development through multiplex immunofluorescence. Transl Lung Cancer Res 2024;13:2395-410. [Crossref] [PubMed]
- Luo W, Zeng Z, Jin Y, et al. Distinct immune microenvironment of lung adenocarcinoma in never-smokers from smokers. Cell Rep Med 2023;4:101078. [Crossref] [PubMed]
- Rebuffet L, Melsen JE, Escalière B, et al. High-dimensional single-cell analysis of human natural killer cell heterogeneity. Nat Immunol 2024;25:1474-88. [Crossref] [PubMed]
- Pickering H, Sen S, Arakawa-Hoyt J, et al. NK and CD8+ T cell phenotypes predict onset and control of CMV viremia after kidney transplant. JCI Insight 2021;6:e153175. [Crossref] [PubMed]
- Chen S, Zhu H, Jounaidi Y. Comprehensive snapshots of natural killer cells functions, signaling, molecular mechanisms and clinical utilization. Signal Transduct Target Ther 2024;9:302. [Crossref] [PubMed]
- Blanquart E, Ekren R, Rigaud B, et al. NK cells with adhesion defects and reduced cytotoxic functions are associated with a poor prognosis in multiple myeloma. Blood 2024;144:1271-83. [Crossref] [PubMed]
- Seymour F, Cavenagh JD, Mathews J, et al. NK cells CD56bright and CD56dim subset cytokine loss and exhaustion is associated with impaired survival in myeloma. Blood Adv 2022;6:5152-9. [Crossref] [PubMed]
- Netskar H, Pfefferle A, Goodridge JP, et al. Pan-cancer profiling of tumor-infiltrating natural killer cells through transcriptional reference mapping. Nat Immunol 2024;25:1445-59. [Crossref] [PubMed]
- Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015;42:443-56. [Crossref] [PubMed]
- Kuhlmann-Hogan A, Cordes T, Xu Z, et al. EGFR-Driven Lung Adenocarcinomas Co-opt Alveolar Macrophage Metabolism and Function to Support EGFR Signaling and Growth. Cancer Discov 2024; Epub ahead of print. [Crossref]
- Jin HR, Wang J, Wang ZJ, et al. Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics. J Hematol Oncol 2023;16:103. [Crossref] [PubMed]
- Chen Y, Zhou Y, Ren R, et al. Harnessing lipid metabolism modulation for improved immunotherapy outcomes in lung adenocarcinoma. J Immunother Cancer 2024;12:e008811. [Crossref] [PubMed]
- McKillop IH, Girardi CA, Thompson KJ. Role of fatty acid binding proteins (FABPs) in cancer development and progression. Cell Signal 2019;62:109336. [Crossref] [PubMed]
- Li Z, Yu DP, Wang N, et al. SIRT5 promotes non-small cell lung cancer progression by reducing FABP4 acetylation level. Neoplasma 2022;69:909-17. [Crossref] [PubMed]
- Erbay E, Babaev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 2009;15:1383-91. [Crossref] [PubMed]
- Li B, Hao J, Zeng J, et al. SnapShot FABP Functions. Cell 2020;182:1066-1066.e1. [Crossref] [PubMed]
- Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 2001;7:699-705. [Crossref] [PubMed]
- Yang X, Deng B, Zhao W, et al. FABP5(+) lipid-loaded macrophages process tumour-derived unsaturated fatty acid signal to suppress T-cell antitumour immunity. J Hepatol 2025;82:676-89. [Crossref] [PubMed]
- Liang X, Gupta K, Quintero JR, et al. Macrophage FABP4 is required for neutrophil recruitment and bacterial clearance in Pseudomonas aeruginosa pneumonia. FASEB J 2019;33:3562-74. [Crossref] [PubMed]
- Pan Y, Tian T, Park CO, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017;543:252-6. [Crossref] [PubMed]
- Yang J, Liu S, Li Y, et al. FABP4 in macrophages facilitates obesity-associated pancreatic cancer progression via the NLRP3/IL-1β axis. Cancer Lett 2023;575:216403. [Crossref] [PubMed]
- Furuhashi M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J Atheroscler Thromb 2019;26:216-32. [Crossref] [PubMed]
- Zhang YL, Li Q, Yang XM, et al. SPON2 Promotes M1-like Macrophage Recruitment and Inhibits Hepatocellular Carcinoma Metastasis by Distinct Integrin-Rho GTPase-Hippo Pathways. Cancer Res 2018;78:2305-17. [Crossref] [PubMed]
- Huang C, Ou R, Chen X, et al. Tumor cell-derived SPON2 promotes M2-polarized tumor-associated macrophage infiltration and cancer progression by activating PYK2 in CRC. J Exp Clin Cancer Res 2021;40:304. [Crossref] [PubMed]
- Zhang J, Liu G, Liu Y, et al. The biological functions and related signaling pathways of SPON2. Front Oncol 2023;13:1323744. [Crossref] [PubMed]
- Yuan X, Bian T, Liu J, et al. Spondin2 is a new prognostic biomarker for lung adenocarcinoma. Oncotarget 2017;8:59324-32. [Crossref] [PubMed]
- Hu X, Su C, Wei J. Knockdown of SPON2 inhibits the growth of triple-negative breast cancer. Front Oncol 2023;13:1141417. [Crossref] [PubMed]
- Lu H, Feng Y, Hu Y, et al. Spondin 2 promotes the proliferation, migration and invasion of gastric cancer cells. J Cell Mol Med 2020;24:98-113. [Crossref] [PubMed]
- Anderson GL, McIntosh M, Wu L, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst 2010;102:26-38. [Crossref] [PubMed]
- Wu M, Kong D, Zhang Y. SPON2 promotes the bone metastasis of lung adenocarcinoma via activation of the NF-κB signaling pathway. Bone 2023;167:116630. [Crossref] [PubMed]
- Wu Y, Ma J, Yang X, et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell 2024;187:1422-1439.e24. [Crossref] [PubMed]
- Mendes C, Serpa J. Metabolic Remodelling: An Accomplice for New Therapeutic Strategies to Fight Lung Cancer. Antioxidants (Basel) 2019;8:603. [Crossref] [PubMed]
- Yang S, Kobayashi S, Sekino K, et al. Fatty acid-binding protein 5 controls lung tumor metastasis by regulating the maturation of natural killer cells in the lung. FEBS Lett 2021;595:1797-805. [Crossref] [PubMed]
- Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer 2020;122:4-22. [Crossref] [PubMed]
- Garin-Shkolnik T, Rudich A, Hotamisligil GS, et al. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes 2014;63:900-11. [Crossref] [PubMed]
- Michelet X, Dyck L, Hogan A, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 2018;19:1330-40. [Crossref] [PubMed]
- Ringel AE, Drijvers JM, Baker GJ, et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020;183:1848-1866.e26. [Crossref] [PubMed]
- Marçais A, Cherfils-Vicini J, Viant C, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol 2014;15:749-57. [Crossref] [PubMed]
- Wong P, Foltz JA, Chang L, et al. T-BET and EOMES sustain mature human NK cell identity and antitumor function. J Clin Invest 2023;133:e162530. [Crossref] [PubMed]


