
A Bayesian deep learning model with consolidation-to-tumor ratio (CTR) prior revolutionizes the prediction of spread through air spaces (STAS) in stage IA lung adenocarcinoma: a large-scale diagnostic study
Highlight box
Key findings
• This study developed the STAS-DLPrior CTR model, which integrates doctors’ knowledge via the consolidation-to-tumor ratio (CTR) as a prior to predict spread through air spaces (STAS) in stage IA lung adenocarcinoma (LUAD). This model demonstrated superior predictive performance compared to traditional deep learning (DL) approaches, achieving an area under the curve (AUC) of 0.858 and significantly enhancing decision-making for surgical interventions.
What is known and what is new?
• Prior research has identified STAS as a negative prognostic factor in LUAD, with previous attempts to predict STAS using CT features and radiomics being less standardized and subjective.
• This study is novel as it is the first to utilize a Bayesian framework that incorporates clinical priors (CTR) in DL to predict STAS in stage IA LUAD, providing a more accurate and objective method for STAS prediction.
What is the implication, and what should change now?
• The findings suggest that our proposed STAS-DLPrior CTR model offers significant advantages in preoperatively predicting STAS status in patients with stage IA LUAD, and integrating clinical knowledge into DL models can greatly improve their utility in clinical settings.
• There should be a shift towards standardized imaging protocols and validation of the model in multicenter studies to enhance its applicability, ultimately refining surgical planning for LUAD patients and potentially improving outcomes.
Introduction
Lung cancer is one of the most common malignancies worldwide, with the highest incidence and mortality among the 36 cancers surveyed, according to the Global Cancer Statistics 2022 report (1). In 2015, spread through air spaces (STAS) was recognized as a novel mechanism of invasion in lung cancer by the World Health Organization (WHO) (2). Previous studies have indicated that STAS is a significant adverse prognostic factor for early-stage lung adenocarcinoma (LUAD), associated with a high risk of recurrence and poor survival, especially in patients who underwent sublobar resection (3-7). Consequently, lobectomy should remain the standard treatment option for patients with STAS-positive early-stage LUAD. Currently, postoperative pathological examination is the main method for evaluating STAS status and intraoperative frozen section examination is less accurate for STAS detection (8,9). Therefore, non-invasive imaging methods for assessing STAS status before surgery are urgently required for selecting the appropriate surgical approach and improving patient outcomes.
Several studies have attempted to predict STAS using computed tomography (CT) features (10-17) and radiomics (18-29), demonstrating a certain predictive ability. However, the extraction of image features often depends on the subjective experience of doctors, lacking a standardized approach, which may compromise the objectivity of these models. In contrast, deep learning (DL) has recently shown great potential in accurately performing diagnostic tasks on medical imaging (30). DL methods have been applied to build models for predicting STAS and offer potential advantages in this regard (31-34). DL technology can effectively overcome the limitations of CT features and radiomics, thereby enhancing model repeatability and generalizability. However, existing DL models do not incorporate doctors’ knowledge as priors when predicting STAS, which may impair the predictive performance of the model.
Doctors’ knowledge can serve as priors to guide the development of DL models. Previous research has confirmed that there is a significant correlation between consolidation-to-tumor ratio (CTR) and STAS (15). Based on this prior knowledge, we hypothesized that the CTR prior distribution of the model can significantly enhance its predictive performance for STAS in stage IA LUAD. In this study, using a variational Bayesian inference framework, we aimed to develop a DL model (STAS-DLPrior CTR) based on the CTR prior to predict STAS in patients with stage IA LUAD before surgery to provide decision support for clinical surgical planning. This manuscript is presented in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-890/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University, Chengdu, China (No. 2024-1511). The research was registered at the China Clinical Research Registration Center, and the registration number is ChiCTR2400088227. Informed consent was waived by the ethics committee due to the retrospective nature of this study. Data were retrieved from the Western China Lung Cancer Database, which has been collecting data from lung cancer patients who underwent surgery at the Department of Thoracic Surgery, West China Hospital, Sichuan University since August 2005.
Study population
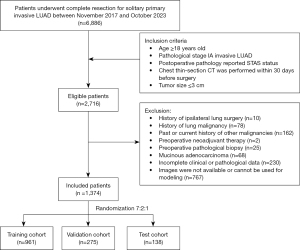
Between November 2017 and October 2023, patients with solitary primary invasive LUAD who underwent complete resection at the Department of Thoracic Surgery, West China Hospital were consecutively retrospectively enrolled. The inclusion criteria were as follows: (I) age ≥18 years old; (II) pathological stage IA invasive LUAD; (III) postoperative pathology reported STAS status; (IV) chest thin-section CT was performed within 30 days before surgery; (V) tumor size ≤3 cm. The exclusion criteria were as follows: (I) history of ipsilateral lung surgery; (II) history of lung malignancy; (III) past or current history of other malignancies; (IV) preoperative neoadjuvant therapy; (V) preoperative pathological biopsy; (VI) mucinous adenocarcinoma; (VII) incomplete clinical or pathological data; (VIII) images are not available or cannot be used for modeling. Included patients were randomly allocated to training, validation, and test cohorts in a 7:2:1 ratio using computerized random numbers. The flowchart of this process is shown in Figure 1. Demographic and clinicopathologic variables, including age, gender, smoking history, clinical T stage, surgical procedure, tumor location, tumor size, STAS status, histopathologic subtype, differentiation grade, and pathologic stage, were collected from medical records. All tumors were restaged according to the eighth edition of the tumor-node-metastasis classification by the International Association for the Study of Lung Cancer.
CT image acquisition and interpretation
Preoperative chest CT scans were performed using scanners from the following manufacturers: Revolution CT and Revolution Apex (GE Medical Systems, Milwaukee, USA), uCT 780 and uCT 960+ (UIH Medical Systems, Shanghai, China), SOMATOM Definition Flash and SOMATOM Definition AS+ (Siemens Medical Systems, Munich, Germany). CT parameters were as follows: slice thickness, 0.625–1.0 mm; tube voltage, 100–140 kVp; tube current, 131–611 mAs; matrix, 512×512, and pixel spacing, 0.53–0.96 mm.
Two senior thoracic surgeons (Z.P. and N.C., with 5 and 6 years of experience in chest CT interpretation, respectively) independently analyzed the CT images, and disagreements would be settled by a third senior thoracic surgeon (J.M.). All thoracic surgeons were blinded to the STAS status. At 1,500 Hounsfield unit (HU) window width and −600 HU window level on the lung reconstructions, thoracic surgeons assessed CT features including tumor size, lobulated sign, spicules sign, vessel concentrate sign, pleural indentation sign, vacuolar sign, and air bronchogram. CTR was calculated by the DL method we designed.
Construction of the DL model (STAS-DLPrior CTR)
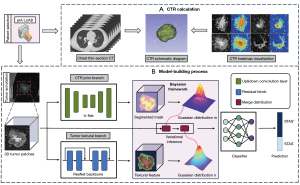
In contrast to traditional variational Bayesian approaches (35), we decomposed the three-dimensional patches into two branch variables and jointly modeled them using deep neural networks. To enhance interpretability and generalizability, one branch of the variables was associated with clinical prior knowledge and the other with textural features. Specifically, to avoid losing the location information and shape characteristics of the solid area when calculating the numerical value of the proportion, we use the Gaussian distribution of the solid area in the tumor as a hint to participate in the model’s prediction analysis of STAS based on the Bayesian prior framework, making it converge faster while improving accuracy. The model (STAS-DLPrior CTR) establishment process is illustrated in Figure 2. Details regarding the data preprocessing and model development are provided in Appendix 1. We also compared this model with another DL model without the CTR prior (STAS-DLNon-prior CTR) to validate our proposed theory. Although CTR is not directly used as input to the model, it was calculated based on a DL method developed by ourselves, and Figure 2 shows a schematic diagram of CTR calculation and heat map visualization.
STAS assessment
STAS status was evaluated based on WHO-defined criteria. STAS is defined as the presence of tumor cells in the lung parenchyma beyond the edge of the main tumor in the form of micropapillary clusters, solid nests, or single tumor cell (2). The three forms are described below: (I) micropapillary clusters, consisting of micropapillary structures lacking fibrous vascular axes, occasionally forming annular structures in the airway; (II) solid nests, composed of tumor cells in a solid mass nest; and (III) single cells, consisting of scattered, loose individual tumor cells. STAS status was obtained from final standard pathological reports, and hematoxylin and eosin stained histological sections were reviewed by two expert pathologists to verify the histological diagnosis.
Statistical analysis
All analyses were performed using R (version 4.4.1). All data were checked for normality (Shapiro-Wilk test) and homoscedasticity (Levene’s test). Continuous variables were presented as median [interquartile range (IQR)], and categorical variables were shown as percentages. Demographic, clinicopathological, and CT features were compared using the Chi-squared test or Fisher’s exact test for categorical variables and the Mann-Whitney U test or Kruskal-Wallis test for continuous variables. Model performance was evaluated using receiver operating characteristic (ROC) curves, areas under the ROC curves (AUCs), balanced accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and Matthews correlation coefficient (MCC). Differences between AUCs were compared using the DeLong test, while sensitivity and specificity were compared using the McNemar test. The bootstrap method with 1,000 bootstrap replications was used to evaluate balanced accuracy, PPV, NPV, and MCC. Model calibration was assessed using calibration curves and the Brier score. Decision curve analysis (DCA) and clinical impact curve (CIC) were employed to evaluate the model’s clinical benefit. Tables were created using the “tableone” package, ROC curves were performed using the “pROC” package, calibration curves were done using the “rms” package, and DCA and CIC were applied using the “rmda” package. A two-tailed P<0.05 was considered statistically significant.
Results
Baseline characteristics by STAS status
Between November 2017 and October 2023, 6,886 patients underwent complete resection for solitary primary invasive LUAD. After applying inclusion and exclusion criteria, 1,374 patients were included in this study. Among these, 207 patients (15.1%) were STAS-positive, and 1,167 patients (84.9%) were STAS-negative. The demographic, clinicopathological, and CT features of both groups are summarized in Table 1.
Table 1
| Variables | STAS status | P | ||
|---|---|---|---|---|
| All patients (n=1,374) | STAS-negative (n=1,167) | STAS-positive (n=207) | ||
| Demographic and clinical features | ||||
| Age (years) | 58.00 [51.00, 66.00] | 58.00 [51.00, 66.00] | 61.00 [53.00, 67.00] | 0.007 |
| Gender | 0.006 | |||
| Male | 543 (39.5) | 443 (38.0) | 100 (48.3) | |
| Female | 831 (60.5) | 724 (62.0) | 107 (51.7) | |
| Smoking history | 0.003 | |||
| No | 1,090 (79.3) | 942 (80.7) | 148 (71.5) | |
| Yes | 284 (20.7) | 225 (19.3) | 59 (28.5) | |
| Clinical T stage | <0.001 | |||
| T1a | 175 (12.7) | 168 (14.4) | 7 (3.4) | |
| T1b | 860 (62.6) | 729 (62.5) | 131 (63.3) | |
| T1c | 339 (24.7) | 270 (23.1) | 69 (33.3) | |
| Surgical procedure | <0.001 | |||
| Wedge resection | 155 (11.3) | 148 (12.7) | 7 (3.4) | |
| Segmentectomy | 452 (32.9) | 410 (35.1) | 42 (20.3) | |
| Lobectomy | 767 (55.8) | 609 (52.2) | 158 (76.3) | |
| Tumor location | 0.048 | |||
| Left upper lobe | 367 (26.7) | 315 (27.0) | 52 (25.1) | |
| Left lower lobe | 204 (14.8) | 163 (14.0) | 41 (19.8) | |
| Right upper lobe | 492 (35.8) | 430 (36.8) | 62 (30.0) | |
| Right middle lobe | 92 (6.7) | 81 (6.9) | 11 (5.3) | |
| Right lower lobe | 219 (15.9) | 178 (15.3) | 41 (19.8) | |
| CT features | ||||
| Tumor size (cm) | 1.60 [1.20, 2.00] | 1.50 [1.20, 2.00] | 1.80 [1.40, 2.20] | <0.001 |
| CTR† | 0.38 [0.20, 0.65] | 0.35 [0.19, 0.60] | 0.63 [0.36, 0.98] | <0.001 |
| Lobulated sign | <0.001 | |||
| Negative | 1,088 (79.2) | 956 (81.9) | 132 (63.8) | |
| Positive | 286 (20.8) | 211 (18.1) | 75 (36.2) | |
| Spicules sign | <0.001 | |||
| Negative | 1,026 (74.7) | 901 (77.2) | 125 (60.4) | |
| Positive | 348 (25.3) | 266 (22.8) | 82 (39.6) | |
| Vessel concentrate sign | 0.95 | |||
| Negative | 1,350 (98.3) | 1,146 (98.2) | 204 (98.6) | |
| Positive | 24 (1.7) | 21 (1.8) | 3 (1.4) | |
| Pleural indentation sign | 0.02 | |||
| Negative | 1,118 (81.4) | 962 (82.4) | 156 (75.4) | |
| Positive | 256 (18.6) | 205 (17.6) | 51 (24.6) | |
| Vacuolar sign | >0.99 | |||
| Negative | 1,169 (85.1) | 993 (85.1) | 176 (85.0) | |
| Positive | 205 (14.9) | 174 (14.9) | 31 (15.0) | |
| Air bronchogram | 0.02 | |||
| Negative | 1,298 (94.5) | 1,110 (95.1) | 188 (90.8) | |
| Positive | 76 (5.5) | 57 (4.9) | 19 (9.2) | |
| Pathological features | ||||
| Predominant subtype | <0.001 | |||
| Lepidic | 685 (49.9) | 648 (55.5) | 37 (17.9) | |
| Acinar | 569 (41.4) | 446 (38.2) | 123 (59.4) | |
| Papillary | 86 (6.3) | 59 (5.1) | 27 (13.0) | |
| Micropapillary | 4 (0.3) | 0 (0.0) | 4 (1.9) | |
| Solid | 25 (1.8) | 14 (1.2) | 11 (5.3) | |
| Complex glandular pattern | 5 (0.4) | 0 (0.0) | 5 (2.4) | |
| High-grade histological type‡ | <0.001 | |||
| Negative | 1,169 (85.1) | 1,060 (90.8) | 109 (52.7) | |
| Positive | 205 (14.9) | 107 (9.2) | 98 (47.3) | |
| Differentiation grade | <0.001 | |||
| Poor | 124 (9.0) | 42 (3.6) | 82 (39.6) | |
| Middle | 916 (66.7) | 793 (68.0) | 123 (59.4) | |
| High | 334 (24.3) | 332 (28.4) | 2 (1.0) | |
| Pathologic stage | <0.001 | |||
| IA1 | 183 (13.3) | 175 (15.0) | 8 (3.9) | |
| IA2 | 851 (61.9) | 722 (61.9) | 129 (62.3) | |
| IA3 | 340 (24.7) | 270 (23.1) | 70 (33.8) | |
Data are presented as median [IQR] or n (%). †, CTR is calculated by the deep learning method we designed; ‡, high-grade histological type contains at least one of the following histological types: micropapillary, solid, complex glandular pattern, and cribriform pattern. CT, computed tomography; CTR, consolidation-to-tumor ratio; IQR, interquartile range; STAS, spread through air spaces.
When comparing demographic and clinical features, the STAS-positive group was older than the STAS-negative group [61.00 (IQR, 53.00, 67.00) vs. 58.00 (IQR, 51.00, 66.00), P=0.007]. Male patients (48.3% vs. 38.0%, P=0.006) and those with a history of smoking (28.5% vs. 19.3%, P=0.003) were more common in the STAS-positive group. Additionally, the STAS-positive group had a higher proportion of patients with clinical T1b/T1c stage (96.6% vs. 85.6%, P<0.001). Lobectomy was more frequently performed in the STAS-positive group (76.3% vs. 52.2%, P<0.001), there were more tumors located in the lower lobes of both lungs in the STAS-positive group (39.6% vs. 29.3%, P=0.048).
Regarding CT features, tumors in the STAS-positive group were larger [1.80 (IQR, 1.40, 2.20) vs. 1.50 (IQR, 1.20, 2.00), P<0.001] and had a higher CTR [0.63 (IQR, 0.36, 0.98) vs. 0.35 (IQR, 0.19, 0.60), P<0.001]. STAS was associated with the lobulated sign (P<0.001), spicules sign (P<0.001), pleural indentation sign (P=0.02), and air bronchogram (P=0.02). However, no significant differences were found between the two groups in terms of vessel concentrate sign (P=0.95) and vacuolar sign (P>0.99).
In terms of pathological features, lepidic subtypes were most common in the STAS-negative group (55.5%), while acinar subtypes were predominant in the STAS-positive group (59.4%, P<0.001). STAS was associated with high-grade histological type (P<0.001). Poor differentiation grade was more prevalent in the STAS-positive group (39.6% vs. 3.6%, P<0.001). Additionally, a higher proportion of patients in the STAS-positive group had pathologic IA2/IA3 stage (96.1% vs. 85.0%, P<0.001).
Among the 1,374 patients, 961 were randomly allocated to the training cohort, 275 to the validation cohort, and 138 to the test cohort. As shown in Table S1, the three cohorts had similar baseline characteristics.
Performance evaluation of STAS-DLPrior CTR
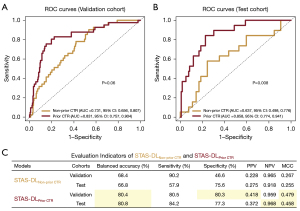
Figure 3 shows the ROC curve, AUC value, and other evaluation indicators resulting from evaluating the two models on the validation and test sets. STAS-DLPrior CTR achieved an AUC of 0.831 [95% confidence interval (CI): 0.757, 0.904; Figure 3A] and the balanced accuracy was 80.4% (95% CI: 75.7%, 85.1%), sensitivity 80.5% (33 of 41, 95% CI: 68.3%, 92.6%), specificity 80.3% (188 of 234, 95% CI: 75.2%, 85.4%), PPV 0.418 (33 of 79, 95% CI: 0.309, 0.526), NPV 0.959 (188 of 196, 95% CI: 0.931, 0.987), MCC 0.479 (95% CI: 0.362, 0.593) (Figure 3C) in the validation cohort. In the test cohort, STAS-DLPrior CTR yielded an AUC of 0.858 (95% CI: 0.774, 0.941; Figure 3B) with a balanced accuracy of 80.8% (95% CI: 74.2%, 87.3%), sensitivity of 84.2% (16 of 19, 95% CI: 67.8%, 100.6%), specificity of 77.3% (92 of 119, 95% CI: 69.8%, 84.8%), PPV of 0.372 (16 of 43, 95% CI: 0.228, 0.517), NPV of 0.968 (92 of 95, 95% CI: 0.933, 1.004), and MCC of 0.458 (95% CI: 0.284, 0.600) (Figure 3C). These results suggest that the model has excellent discrimination capabilities.
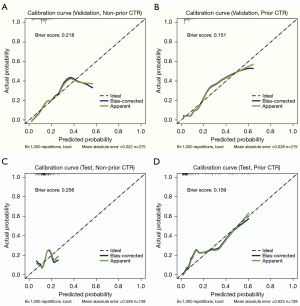
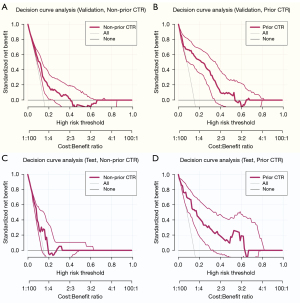
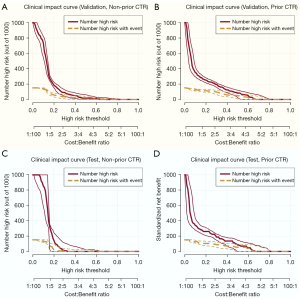
The calibration curve and adjusted calibration curve are presented in Figure 4, showing that STAS-DLPrior CTR exhibited good calibration in both the validation cohort (Figure 4B) and the test cohort (Figure 4D). The Brier score was 0.151 in the validation cohort and 0.159 in the test cohort, both close to 0, indicating an excellent prediction performance. We used DCA (Figure 5) and CIC (Figure 6) to evaluate whether STAS-DLPrior CTR offers clinical benefits. In the validation cohort, the DCA curve showed that if the threshold probabilities ranged between 0.03 and 0.53, using STAS-DLPrior CTR to decide whether to undergo lobectomy added more net benefit than treating either all patients or none (Figure 5B) and the beneficial cutoff range was between 0.02 and 0.63 in the test cohort (Figure 5D). The results of DCA indicated that STAS-positive patients clearly benefited from decisions based on STAS-DLPrior CTR. The CIC of STAS-DLPrior CTR depicted the predicted number of SATS patients and true positive patients at different threshold probabilities in both the validation (Figure 6B) and test cohorts (Figure 6D), demonstrating that the predictive robustness of STAS-DLPrior CTR was reliable. Consequently, our model demonstrated a positive net benefit without increasing the number of false positives.
Comparing STAS-DLNon-prior CTR with STAS-DLPrior CTR
We compared the performance of STAS-DLNon-prior CTR and STAS-DLPrior CTR in predicting STAS status and clinical application. As shown in Figure 3A, STAS-DLNon-prior CTR achieved an AUC of 0.731 (95% CI: 0.656, 0.807), and the balanced accuracy was 68.4% (95% CI: 62.9%, 73.9%), sensitivity 90.2% (37 of 41, 95% CI: 81.2%, 99.3%), specificity 46.6% (109 of 234, 95% CI: 40.2%, 53.0%), PPV 0.228 (37 of 162, 95% CI: 0.164, 0.293), NPV 0.965 (109 of 113, 95% CI: 0.931, 0.999), and MCC 0.267 (95% CI: 0.180, 0.350) in the validation cohort (Figure 3C). Compared to STAS-DLNon-prior CTR, the AUC tends to be higher for STAS-DLPrior CTR (0.831 vs. 0.731, P=0.06) in the validation cohort. STAS-DLPrior CTR had a higher specificity (80.3% vs. 46.6%, P<0.001), but there was no significant difference in sensitivity between the two models (P=0.34). The bootstrap method with 1,000 bootstrap replications showed that STAS-DLPrior CTR had a significantly higher balanced accuracy (80.4% vs. 68.4%, 95% CI: 0.032, 0.195), PPV (0.418 vs. 0.228, 95% CI: 0.113, 0.261), and MCC (0.479 vs. 0.267, 95% CI: 0.078, 0.327), but no difference in NPV (95% CI: −0.044, 0.031). In the test cohort, STAS-DLNon-prior CTR yielded an AUC of 0.637 (95% CI: 0.498, 0.776; Figure 3B) with a balanced accuracy of 66.8% (95% CI: 58.9%, 74.6%), sensitivity of 57.9% (11 of 19, 95% CI: 35.7%, 80.1%), specificity of 75.6% (90 of 119, 95% CI: 67.9%, 83.3%), PPV of 0.275 (11 of 40, 95% CI: 0.137, 0.413), NPV of 0.918 (90 of 98, 95% CI: 0.864, 0.973), and MCC of 0.255 (95% CI: 0.048, 0.436) (Figure 3C). The AUC of STAS-DLPrior CTR was significantly higher than that of STAS-DLNon-prior CTR (0.858 vs. 0.637, P=0.008). However, there was no significant difference in sensitivity (P=0.13) or specificity (P=0.84) between the two models. STAS-DLPrior CTR had a significantly higher balanced accuracy (80.8% vs. 66.8%, 95% CI: 0.004, 0.274), NPV (0.968 vs. 0.918, 95% CI: 0.001, 0.100), and MCC (0.458 vs. 0.255, 95% CI: 0.005, 0.402), but no difference in PPV (95% CI: −0.012, 0.215).
Compared to STAS-DLNon-prior CTR, STAS-DLPrior CTR’s calibration curves were closer to ideal curves, and its Brier scores were lower in both the validation and test cohorts, indicating better fitness and predictive ability (Figure 4). The clinical benefit of the two models was evaluated and compared using DCA and CIC. On DCA, STAS-DLPrior CTR showed superior net benefit across a wider range of threshold probabilities in both the validation and test cohorts (Figure 5). Similarly, CIC visually indicated that STAS-DLPrior CTR conferred high clinical net benefit (Figure 6). These findings suggested that STAS-DLPrior CTR provided better clinical utility than STAS-DLNon-prior CTR.
Discussion
To the best of our knowledge, this is the largest cohort study on this topic and the first to use DL to predict STAS in stage IA LUAD. Additionally, it is the first study to apply a Bayesian framework incorporating doctors’ knowledge as priors in model construction, achieving the best predictive performance to date.
In this large-scale diagnostic study, we developed the STAS-DLPrior CTR model, which incorporates the CTR as a prior for predicting STAS in stage IA LUAD. We then compared it with another DL model that did not take the CTR as a prior (STAS-DLNon-prior CTR). The results demonstrated that STAS-DLPrior CTR achieved a higher balanced accuracy, specificity, PPV, and MCC in the validation cohort. In the test cohort, STAS-DLPrior CTR yielded a higher AUC, balanced accuracy, NPV, and MCC. In addition, calibration curves, Brier scores, DCA, and CIC confirmed that STAS-DLPrior CTR performed better clinical utility.
With the popularization of low-dose spiral CT and people’s increasing concern about their health, more patients with early-stage lung cancer have been diagnosed (36). Previous studies have demonstrated that sublobar resection offers significant benefits in treating early-stage lung cancer. However, the presence of STAS is associated with a higher recurrence rate and shorter survival in patients who undergo sublobar resection (3). This highlights the urgent need for non-invasive and accurate methods for detecting STAS. However, no studies have focused on using DL to predict STAS in stage IA LUAD. It has been reported that the incidence of STAS in LUAD varies widely, ranging from 15% to 73% (37), and the incidence of STAS in early-stage LUAD is very low. In our center, the incidence of STAS in stage IA LUAD was 15.1%, which aligns with the lower end of the reported range. Therefore, it is very difficult to predict STAS in stage IA LUAD, but it is the most meaningful and crucial for selecting appropriate surgical methods and improving patient outcomes. In this study, the DL model incorporating the CTR as a prior improved the AUC by 34.7% compared to the traditional DL model in the test group. Additionally, the balanced accuracy increased by 14%, meaning that our proposed model would enable 140 out of every 1,000 patients to be accurately reclassified, allowing for the selection of the most appropriate surgical method and thereby avoiding both excessive surgery and insufficient surgical resection.
Previous studies have identified the CTR as a strong predictor of STAS (10-12,14-16,24,38-40). CTR is calculated as the ratio of the maximum diameter of the largest solid component to the maximum diameter of the lesion, a measurement typically performed by doctors on preoperative CT images. However, this measurement can be inconsistent among doctors with varying experience levels, making the measurement more subjective. Additionally, due to the irregular shapes of solid components and tumors, the CTR calculated by traditional methods often lacks representativeness. To address these issues, we designed a DL algorithm to measure CTR as the ratio of the volume of the solid component to the volume of the entire tumor. This method allows for automatic calculation of CTR, which is not only fast and stable but also free from human subjectivity and unaffected by tumor morphology. Our results showed that the CTR of the STAS-positive group was significantly higher than that of the STAS-negative group, consistent with previous research findings.
Incorporating doctors’ knowledge as priors to enhance the effectiveness of DL models for STAS prediction, as well as determining how to integrate this knowledge, are current research hotspots. However, no satisfactory solution has been found. To solve this problem, we propose a novel method based on a Bayesian framework that integrates prior knowledge with DL features. Our results demonstrate that incorporating the CTR prior significantly improves the model’s predictive ability for STAS. This study serves as an important example of how integrating doctors’ knowledge can greatly enhance the effectiveness of medical DL techniques in addressing real-world clinical challenges. Our approach also introduces a novel direction for future research in DL model construction.
Despite the aforementioned strengths, this study also has some important limitations and suggests several directions for future studies. First, this study was a single-center, retrospective study, which may lead to an unavoidable risk of selection bias, and the predictive model may require further validation in a multicenter, prospective study. Second, it should be noted that as the chest thin-CT was performed on different scanners, these differences in scanner types and image acquisition protocols inherently might lead to some bias. Imaging protocols should be standardized for the most efficient use possible. We recommend that image acquisition should be standardized to obtain the most reliable conclusions although it is not always possible.
Conclusions
In conclusion, our proposed STAS-DLPrior CTR model offers significant advantages in preoperatively predicting STAS status in patients with stage IA LUAD. Moreover, our study demonstrates that incorporating doctors’ knowledge as priors can effectively guide the development of DL models, significantly improving their performance.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-890/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-890/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-890/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-890/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University, Chengdu, China (No. 2024-1511) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Kadota K, Nitadori JI, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread Through Air Spaces Is a Prognostic Factor in Sublobar Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;106:354-60. [Crossref] [PubMed]
- Kadota K, Kushida Y, Kagawa S, et al. Limited Resection Is Associated With a Higher Risk of Locoregional Recurrence than Lobectomy in Stage I Lung Adenocarcinoma With Tumor Spread Through Air Spaces. Am J Surg Pathol 2019;43:1033-41. [Crossref] [PubMed]
- Ren Y, Xie H, Dai C, et al. Prognostic Impact of Tumor Spread Through Air Spaces in Sublobar Resection for 1A Lung Adenocarcinoma Patients. Ann Surg Oncol 2019;26:1901-8. [Crossref] [PubMed]
- Walts AE, Marchevsky AM. Current Evidence Does Not Warrant Frozen Section Evaluation for the Presence of Tumor Spread Through Alveolar Spaces. Arch Pathol Lab Med 2018;142:59-63. [Crossref] [PubMed]
- Villalba JA, Shih AR, Sayo TMS, et al. Accuracy and Reproducibility of Intraoperative Assessment on Tumor Spread Through Air Spaces in Stage 1 Lung Adenocarcinomas. J Thorac Oncol 2021;16:619-29. [Crossref] [PubMed]
- Chen Y, Jiang C, Kang W, et al. Development and validation of a CT-based nomogram to predict spread through air space (STAS) in peripheral stage IA lung adenocarcinoma. Jpn J Radiol 2022;40:586-94. [Crossref] [PubMed]
- Ding Y, Chen Y, Wen H, et al. Pretreatment prediction of tumour spread through air spaces in clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2022;62:ezac248. [Crossref] [PubMed]
- Qi L, Xue K, Cai Y, et al. Predictors of CT Morphologic Features to Identify Spread Through Air Spaces Preoperatively in Small-Sized Lung Adenocarcinoma. Front Oncol 2020;10:548430. [Crossref] [PubMed]
- Qin L, Sun Y, Zhu R, et al. Clinicopathological and CT features of tumor spread through air space in invasive lung adenocarcinoma. Front Oncol 2022;12:959113. [Crossref] [PubMed]
- Yamamoto M, Tamura M, Miyazaki R, et al. Mean computed tomography value to predict spread through air spaces in clinical N0 lung adenocarcinoma. J Cardiothorac Surg 2024;19:260. [Crossref] [PubMed]
- Kim SK, Kim TJ, Chung MJ, et al. Lung Adenocarcinoma: CT Features Associated with Spread through Air Spaces. Radiology 2018;289:831-40. [Crossref] [PubMed]
- Wang Y, Lyu D, Zhang D, et al. Nomogram based on clinical characteristics and radiological features for the preoperative prediction of spread through air spaces in patients with clinical stage IA non-small cell lung cancer: a multicenter study. Diagn Interv Radiol 2023;29:771-85. [Crossref] [PubMed]
- Zhang Z, Liu Z, Feng H, et al. Predictive value of radiological features on spread through air space in stage cIA lung adenocarcinoma. J Thorac Dis 2020;12:6494-504. [Crossref] [PubMed]
- Bassi M, Russomando A, Vannucci J, et al. Role of radiomics in predicting lung cancer spread through air spaces in a heterogeneous dataset. Transl Lung Cancer Res 2022;11:560-71. [Crossref] [PubMed]
- Chen D, She Y, Wang T, et al. Radiomics-based prediction for tumour spread through air spaces in stage I lung adenocarcinoma using machine learning. Eur J Cardiothorac Surg 2020;58:51-8. [Crossref] [PubMed]
- Chen LW, Lin MW, Hsieh MS, et al. Radiomic Values from High-Grade Subtypes to Predict Spread Through Air Spaces in Lung Adenocarcinoma. Ann Thorac Surg 2022;114:999-1006. [Crossref] [PubMed]
- Gong J, Yin R, Sun L, et al. CT-based radiomics model to predict spread through air space in resectable lung cancer. Cancer Med 2023;12:18755-66. [Crossref] [PubMed]
- Han X, Fan J, Zheng Y, et al. The Value of CT-Based Radiomics for Predicting Spread Through Air Spaces in Stage IA Lung Adenocarcinoma. Front Oncol 2022;12:757389. [Crossref] [PubMed]
- Jiang C, Luo Y, Yuan J, et al. CT-based radiomics and machine learning to predict spread through air space in lung adenocarcinoma. Eur Radiol 2020;30:4050-7. [Crossref] [PubMed]
- Liao G, Huang L, Wu S, et al. Preoperative CT-based peritumoral and tumoral radiomic features prediction for tumor spread through air spaces in clinical stage I lung adenocarcinoma. Lung Cancer 2022;163:87-95. [Crossref] [PubMed]
- Onozato Y, Nakajima T, Yokota H, et al. Radiomics is feasible for prediction of spread through air spaces in patients with nonsmall cell lung cancer. Sci Rep 2021;11:13526. [Crossref] [PubMed]
- Suh YJ, Han K, Kwon Y, et al. Computed Tomography Radiomics for Preoperative Prediction of Spread Through Air Spaces in the Early Stage of Surgically Resected Lung Adenocarcinomas. Yonsei Med J 2024;65:163-73. [Crossref] [PubMed]
- Takehana K, Sakamoto R, Fujimoto K, et al. Peritumoral radiomics features on preoperative thin-slice CT images can predict the spread through air spaces of lung adenocarcinoma. Sci Rep 2022;12:10323. [Crossref] [PubMed]
- Wang Y, Lyu D, Hu L, et al. CT-Based Intratumoral and Peritumoral Radiomics Nomograms for the Preoperative Prediction of Spread Through Air Spaces in Clinical Stage IA Non-small Cell Lung Cancer. J Imaging Inform Med 2024;37:520-35. [Crossref] [PubMed]
- Qi L, Li X, He L, et al. Comparison of Diagnostic Performance of Spread Through Airspaces of Lung Adenocarcinoma Based on Morphological Analysis and Perinodular and Intranodular Radiomic Features on Chest CT Images. Front Oncol 2021;11:654413. [Crossref] [PubMed]
- Suzuki K. Overview of deep learning in medical imaging. Radiol Phys Technol 2017;10:257-73. [Crossref] [PubMed]
- Jin W, Shen L, Tian Y, et al. Improving the prediction of Spreading Through Air Spaces (STAS) in primary lung cancer with a dynamic dual-delta hybrid machine learning model: a multicenter cohort study. Biomark Res 2023;11:102. [Crossref] [PubMed]
- Lin MW, Chen LW, Yang SM, et al. CT-Based Deep-Learning Model for Spread-Through-Air-Spaces Prediction in Ground Glass-Predominant Lung Adenocarcinoma. Ann Surg Oncol 2024;31:1536-45. [Crossref] [PubMed]
- Tao J, Liang C, Yin K, et al. 3D convolutional neural network model from contrast-enhanced CT to predict spread through air spaces in non-small cell lung cancer. Diagn Interv Imaging 2022;103:535-44. [Crossref] [PubMed]
- Wang S, Liu X, Jiang C, et al. CT-Based Super-Resolution Deep Learning Models with Attention Mechanisms for Predicting Spread Through Air Spaces of Solid or Part-Solid Lung Adenocarcinoma. Acad Radiol 2024;31:2601-9. [Crossref] [PubMed]
- Frazier DT, Loaiza-Maya R, Martin GM, et al. Loss-Based Variational Bayes Prediction. J Comput Graph Stat 2025;34:84-95.
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Gutierrez-Sainz L, López-Muñoz S, Cruz-Castellanos P, et al. Retrospective analysis of the prognostic implications of tumor spread through air spaces in lung adenocarcinoma patients treated with surgery. ESMO Open 2022;7:100568. [Crossref] [PubMed]
- Liu BC, Ma HY, Huang J, et al. Does dual-layer spectral detector CT provide added value in predicting spread through air spaces in lung adenocarcinoma? A preliminary study. Eur Radiol 2024;34:4176-86. [Crossref] [PubMed]
- Wang J, Yao Y, Tang D, et al. An individualized nomogram for predicting and validating spread through air space (STAS) in surgically resected lung adenocarcinoma: a single center retrospective analysis. J Cardiothorac Surg 2023;18:337. [Crossref] [PubMed]
- Wang Y, Lyu D, Cheng C, et al. Preoperative nomogram for predicting spread through air spaces in clinical-stage IA non-small cell lung cancer using (18)F-fluorodeoxyglucose positron emission tomography/computed tomography. J Cancer Res Clin Oncol 2024;150:185. [Crossref] [PubMed]


