
Clinicopathological factors vs. molecular model for predicting adjuvant EGFR-TKI benefit in stage I EGFR-mutant non-small cell lung cancer
Highlight box
Key findings
• The 14-gene molecular assay outperformed clinicopathological factors in predicting postoperative recurrence and guiding adjuvant epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in stage I non-small cell lung cancer (NSCLC) patients.
• Molecular high-risk patients benefited significantly from adjuvant EGFR-TKIs, achieving a marked improvement in 5-year disease-free survival, whereas molecular low-risk patients showed no benefit.
What is known and what is new?
• Clinicopathological high-risk factors are commonly used for risk stratification and guiding adjuvant therapy in early-stage NSCLC, but the prognostic accuracy is limited, and a significant proportion of patients are misclassified.
• This study demonstrates that the 14-gene assay provides superior prognostic accuracy by identifying high-risk patients who benefit from adjuvant EGFR-TKIs and low-risk patients who can avoid unnecessary treatment.
What is the implication, and what should change now?
• The integration of molecular risk assessment into the standard management of stage I NSCLC is recommended to refine treatment decisions. Adjuvant EGFR-TKIs should be selectively administered to molecular high-risk patients, while avoiding unnecessary treatment in molecular low-risk patients.
Introduction
The pathological staging system serves as the gold standard for treatment planning and prognosis prediction for patients with lung cancer. Currently, stage I non-small cell lung cancer (NSCLC) shows significant heterogeneity in clinical outcomes, with approximately 20–40% of patients experiencing local recurrence or distant metastasis within 5 years after surgery (1). This may stem from undetected residual viable tumor cells either locally or systemically (2), making adjuvant therapy for stage I NSCLC a significant clinical challenge.
Currently, the National Comprehensive Cancer Network (NCCN) guidelines for adjuvant therapy in early-stage NSCLC are based on the identification of patients at high risk of recurrence (3). However, the NCCN guidelines lack explicit criteria defining for “high-risk” patients, instead offering clinicopathologic features that may suggest a higher risk of recurrence. There are limitations with these population-level indicators, as a notable proportion of patients classified as low-risk based on these parameters still experience relapse, while some categorized as high-risk remain disease-free for extended periods.
Considerable efforts have been devoted to exploring the potential role of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) as adjuvant treatment for early-stage EGFR-mutant NSCLC. For instance, exploratory analyses from the ADAURA and CORIN studies have revealed a decreased risk of recurrence in stage IB patients (4,5). Additionally, our previous retrospective study has provided supporting evidence for the use of adjuvant EGFR-TKIs in stage I patients, including those in stage IA (6). However, according to the current NCCN guidelines, routine adjuvant treatment is not recommended for stage IA patients. For patients with stage IB, the selective recommendation of adjuvant treatment is contingent upon clinicians accurately identifying high-risk factors.
The aforementioned findings indicate that the existing pathological staging system exhibits inadequate prognostic accuracy and fails to effectively identify which patients would experience advantages from adjuvant EGFR-TKIs. Consequently, there is an urgent need for novel stratification approaches that can improve prognosis prediction and identify individuals who would derive the greatest benefits from adjuvant EGFR-TKIs.
A 14-gene molecular assay has been developed to stratify the risk of recurrence in NSCLC patients after surgical resection (7,8). Previous studies have demonstrated that patients classified as molecular high-risk who received adjuvant chemotherapy experienced notably prolonged disease-free survival (DFS) compared to those who did not (9). This assay holds the potential benefits in accurately identifying patients at high risk who could benefit the most from adjuvant therapy. Additionally, it may help identify patients with a low risk of recurrence who are more likely to be cured through surgical resection alone, thus potentially eliminating the need for additional interventions.
In this study, we compared the performance of the 14-gene assay and NCCN clinicopathological factors to assess recurrence events and distinguish patients who may benefit from adjuvant EGFR-TKIs in stage I NSCLC, aiming to provide a more precise approach for guiding treatment decisions and ultimately improving patient outcomes. We present this article in accordance with the STROCSS reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-20/rc) (10).
Methods
Study design and patients
The study design is presented in Figure 1. In this retrospective cohort study, we included patients with resected stage I NSCLC from March 2013 to February 2019. The inclusion criteria were as follows: (I) aged ≥18 years; (II) confirmed pathology of primary non-squamous NSCLC; (III) pathological stage I according to the 8th edition AJCC/UICC classification; (IV) harbored a sensitizing EGFR mutation (19Del or L858R); (V) underwent complete surgical resections via lobectomy or pneumonectomy with systemic intrathoracic lymph node dissection; (VI) available clinicopathological data; and (VII) complete postoperative follow-up of at least 5 years for patients without disease progression. For those experiencing disease progressions, their follow-up duration was determined by the time until progression within the 5-year period.
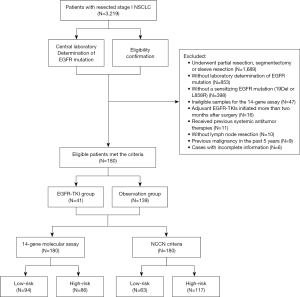
The exclusion criteria were as follows: (I) incomplete surgical resection, as outlined in the Chinese guidelines on the diagnosis and treatment of primary lung cancer [2019]; (II) initiation of adjuvant EGFR-TKIs more than two months after surgery; (III) combination with other malignant tumors; (IV) received previous preoperative therapy (chemotherapy, radiotherapy, or targeted therapy).
The administration of adjuvant EGFR-TKIs was determined through a collaborative decision-making process involving patients and attending physicians. This process entailed a comprehensive evaluation of the patient’s financial circumstances and treatment preferences, while also considering guidance from the NCCN guidelines, which identify specific high-risk factors. By incorporating these factors, personalized recommendations were formulated regarding the use of adjuvant EGFR-TKIs. The prescribed daily dosages for icotinib, erlotinib, and gefitinib were 125 mg three times, 150 mg once, and 250 mg once, respectively.
This retrospective cohort study was registered at the Research Registry (UIN: researchregistry10105). This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2022181) and individual consent for this retrospective analysis was waived due to the retrospective nature.
Data collection
Data for the study were obtained by extracting information from the electronic medical records system, which included baseline clinical data and postoperative follow-up details. Demographic information including age, sex, and smoking status, along with cancer-related details including tumor location, size, tumor-node-metastasis (TNM) classification stage, pathology, histology, tumor differentiation, number of examined lymph nodes, and EGFR mutation status, were collected. EGFR mutations were detected via amplification-refractory mutation system polymerase chain reaction (ARMS-PCR) or next-generation sequencing (NGS) based on institutional availability during the study period. Both are validated and concordant for exon 19/21 mutations. Postoperative information included survival status, disease progression, and any subsequent treatments received.
Patients underwent regular postoperative surveillance with low-dose computed tomography (LDCT) scans every 3 months during treatment and every 6 months thereafter for up to five years. Additional contrast-enhanced CT or positron emission tomography/computed tomography (PET/CT) scans were performed as needed based on clinical symptoms or imaging findings, as determined by the treating oncologist or surgeon. Disease recurrence was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
To minimize selection and recall bias, we included all consecutive patients who satisfied the eligibility criteria during the predefined study period. Data were retrieved from the institution’s standardized electronic medical record system. Two independent researchers abstracted the data following a standardized protocol, and any discrepancies were resolved by consensus. Follow-up data were collected via scheduled outpatient visits and telephone interviews using a uniform questionnaire. While telephone interviews may not provide an equivalent level of comprehensive information as the electronic medical records system, they do offer significant insights into patient-reported outcomes, overall well-being, and disease status. No major clinical or pathological variable had missing data.
Definition of clinicopathological high-risk factors
According to the NCCN guidelines, clinicopathological high-risk factors include: (I) tumor size >4 cm; (II) poorly differentiated histology; (III) vascular invasion; (IV) wedge resection; (V) visceral pleural invasion; and (VI) unknown lymph node status (Nx) (3).
The biological characteristics of lung adenocarcinoma (LUAD) patients at the same stage exhibit significant heterogeneity. In 2011, the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) proposed a new histological classification based on major growth patterns and prognostic relevance, identifying five main subtypes: lepidic, acinar, papillary, micropapillary, and solid (11). This classification was adopted and further refined by the World Health Organization (WHO) in 2015 (12). Numerous studies have confirmed that LUADs dominated by micropapillary or solid patterns is associated with poorer prognosis, and these are considered high-grade patterns (13). Additionally, complex glandular patterns, such as cribriform and fused glands, are also considered high-grade acinar components, exhibiting similar poor survival rates when compared to micropapillary and solid patterns (14).
Therefore, the six clinical high-risk factors defined by the NCCN guidelines and the solid, micropapillary subtypes, as well as complex glandular patterns, were recognized as clinicopathological high-risk factors in this study. High-risk histological subtypes (solid, micropapillary, or complex glandular patterns) were determined by two experienced pulmonary pathologists according to the according to the IASLC/ATS/ERS classification, with any discrepancies resolved by consensus of a third senior pathologist.
14-gene molecular assay
The 14-gene quantitative PCR expression assay (DetermaRx™, Burning Rock Biotech) is a CLIA-certified molecular test developed and validated globally for assessing the recurrence risk of NSCLC after surgical resection. The assay quantifies the expression levels of 14 genes using quantitative reverse transcription PCR (qPCR) on total RNA extracted from formalin-fixed, paraffin-embedded tumor tissues. The RNA extraction, quality control, qPCR procedures, and analytical validation have been comprehensively documented in previous studies. A proprietary algorithm then calculates a risk score based on the gene expression data, classifying patients into low-, intermediate-, or high-risk groups according to validated cutoff values (7,15).
To evaluate the predictive significance of this assay in the context of adjuvant EGFR-TKI treatment, we retrospectively collected formalin-fixed, paraffin-embedded tumor samples submitted for molecular testing. Patients were stratified into low-, intermediate-, or high-risk categories based on the molecular prognostic assay. Since both intermediate- and high-risk patients were considered to have a higher risk of recurrence, they were grouped together and designated as molecular high-risk.
Outcome
The primary endpoint assessed in this study was the 5-year DFS, calculated from the date of surgery to the occurrence of the first recurrence at any site or all-cause death. Patients who did not experience recurrence, were no longer being monitored, or survived without documented recurrence were censored at their last available assessment. Follow-up information was last updated on July 31, 2022 or on the date of death.
Statistical analysis
To summarize patient characteristics, continuous variables were summarized using means and standard deviations for normally distributed data, and median with interquartile range (IQR) for non-normally distributed data. Differences between groups were assessed using either Student’s t-test or Mann-Whitney U-test. Categorical variables were presented as frequencies and percentages, and comparisons between groups were conducted using Pearson’s Chi-squared test or Fisher’s exact test. The estimation of DFS was performed utilizing the Kaplan-Meier method and subsequently compared employing the log-rank test. Additionally, Cox proportional hazards regression was employed to calculate hazard ratios (HRs) accompanied by 95% confidence intervals (CIs). Statistical significance was established at a P value below 0.05, and all statistical analyses were executed using IBM SPSS Statistics (version 26.0), R software (version 4.2.2), and GraphPad Prism (version 9.5.1).
Results
Study population
A total of 180 eligible patients were included in the study, with 41 (22.8%) receiving adjuvant EGFR-TKIs therapy and 139 (77.2%) in the observation group. The median follow-up duration was 76.9 (IQR, 67.0–88.6) months. The median age of the study cohort was 61 years. All patients underwent R0 surgical resection by lobectomy with negative margins. Among them, 90 (50.0%) patients were classified as stage IA, while the remaining 90 (50.0%) were classified as stage IB. Leu858Arg and 19Del mutation were identified in 117 (65.0%) and 63 (35.0%) patients, respectively. Cancer cell differentiation was categorized as poor in 67 patients (37.2%), moderate in 82 patients (45.6%), and well in 31 patients (17.2%). A comprehensive summary of the demographic and clinical characteristics of all patients are presented in Table 1.
Table 1
| Characteristic | All patients (n=180) | Clinicopathological factors | 14-gene assay | |||||
|---|---|---|---|---|---|---|---|---|
| High-risk (n=118) | Low-risk (n=62) | P value | High-risk (n=86) | Low-risk (n=94) | P value | |||
| Age (years) | 0.36 | 0.57 | ||||||
| <60 | 73 (40.6) | 45 (38.1) | 28 (45.2) | 33 (38.4) | 40 (42.6) | |||
| ≥60 | 107 (59.4) | 73 (61.9) | 34 (54.8) | 53 (61.6) | 54 (57.4) | |||
| Sex | 0.71 | 0.03* | ||||||
| Female | 105 (58.3) | 70 (59.3) | 35 (56.5) | 43 (50.0) | 62 (66.0) | |||
| Male | 75 (41.7) | 48 (40.7) | 27 (43.5) | 43 (50.0) | 32 (34.0) | |||
| Smoking | 0.91 | 0.77 | ||||||
| Never | 146 (81.1) | 96 (81.4) | 50 (80.6) | 69 (80.2) | 77 (81.9) | |||
| Ever | 34 (18.9) | 22 (18.6) | 12 (19.4) | 17 (19.8) | 17 (18.1) | |||
| Location | 0.95 | 0.16 | ||||||
| Upper | 106 (58.9) | 70 (59.3) | 36 (58.1) | 57 (66.3) | 49 (52.1) | |||
| Middle | 13 (7.2) | 8 (6.8) | 5 (8.1) | 5 (5.8) | 8 (8.5) | |||
| Lower | 61 (33.9) | 40 (33.9) | 21 (33.9) | 24 (27.9) | 37 (39.4) | |||
| ELN | 0.75 | 0.64 | ||||||
| <16 | 87 (48.3) | 56 (47.5) | 31 (50.0) | 40 (46.5) | 47 (50.0) | |||
| ≥16 | 93 (51.7) | 62 (52.5) | 31 (50.0) | 46 (53.5) | 47 (50.0) | |||
| Pathology stage | <0.001* | 0.77 | ||||||
| IA | 90 (50.0) | 29 (24.6) | 61 (98.4) | 42 (48.8) | 48 (51.1) | |||
| IB | 90 (50.0) | 89 (75.4) | 1 (1.6) | 44 (51.2) | 46 (48.9) | |||
| Differentiation grade | <0.001* | <0.001* | ||||||
| Well | 31 (17.2) | 14 (11.9) | 17 (27.4) | 8 (9.3) | 23 (24.5) | |||
| Moderate | 82 (45.6) | 37 (31.4) | 45 (72.6) | 31 (36.0) | 51 (54.3) | |||
| Poor | 67 (37.2) | 67 (56.8) | 0 (0.0) | 47 (54.7) | 20 (21.3) | |||
| Histology | >0.99 | 0.48 | ||||||
| Adenocarcinoma | 179 (99.4) | 117 (99.2) | 62 (100.0) | 85 (98.8) | 94 (100.0) | |||
| Adenosquamous carcinoma | 1 (0.6) | 1 (0.8) | 0 (0.0) | 1 (1.2) | 0 (0.0) | |||
| EGFR mutation | 0.92 | 0.98 | ||||||
| Exon 19 deletions | 63 (35.0) | 41 (34.7) | 22 (35.5) | 30 (34.9) | 33 (35.1) | |||
| Exon 21 Leu858Arg | 117 (65.0) | 77 (65.3) | 40 (64.5) | 56 (65.1) | 61 (64.9) | |||
| VPI | <0.001* | 0.68 | ||||||
| Yes | 85 (47.2) | 85 (72.0) | 0 (0.0) | 42 (48.8) | 43 (45.7) | |||
| No | 95 (52.8) | 33 (28.0) | 62 (100.0) | 44 (51.2) | 51 (54.3) | |||
| GGO | 0.04* | 0.13 | ||||||
| Yes | 47 (26.1) | 25 (21.2) | 22 (35.5) | 18 (20.9) | 29 (30.9) | |||
| No | 133 (73.9) | 93 (78.8) | 40 (64.5) | 68 (79.1) | 65 (69.1) | |||
| MPLC | 0.99 | 0.38 | ||||||
| Yes | 55 (30.6) | 36 (30.5) | 19 (30.6) | 29 (33.7) | 26 (27.7) | |||
| No | 125 (69.4) | 82 (69.5) | 43 (69.4) | 57 (66.3) | 68 (72.3) | |||
| LVI | 0.06 | 0.41 | ||||||
| Yes | 9 (5.0) | 9 (7.6) | 0 (0.0) | 6 (7.0) | 3 (3.2) | |||
| No | 171 (95.0) | 109 (92.4) | 62 (100.0) | 80 (93.0) | 91 (96.8) | |||
| Adjuvant therapy | 0.06 | 0.054 | ||||||
| EGFR-TKIs | 41 (22.8) | 32 (27.1) | 9 (14.5) | 25 (29.1) | 16 (17.0) | |||
| Observation | 139 (77.2) | 86 (72.9) | 53 (85.5) | 61 (70.9) | 78 (83.0) | |||
Data are presented as n (%). Statistical significance is indicated by *P<0.05. EGFR, epidermal growth factor receptor; ELN, examined lymph node; GGO, ground glass opacity; LVI, lympho-vascular invasion; MPLC, multiple primary lung cancer; TKI, tyrosine kinase inhibitor; VPI, visceral pleural invasion.
The clinicopathological high-risk group showed a higher incidence of stage IB disease (75.4%) compared to the low-risk group, where stage IA disease predominated (98.4%). Moreover, high-risk patients were more frequently associated with poorly differentiated tumors (56.8%), while the low-risk group all presented with well/moderately differentiated tumors (100.0%). In terms of adjuvant therapy, a higher proportion of high-risk patients received EGFR-TKIs (27.1%) compared to the low-risk group (14.5%).
Conversely, there were no significant differences observed in TNM stage, vascular invasion, ground glass opacity, or the administration of adjuvant EGFR-TKIs between molecular high-risk and low-risk patients. However, the 14-gene assay revealed significant associations with tumor differentiation grade. The molecular high-risk group exhibited a notably higher prevalence of poorly differentiated tumors (54.7%) compared to the low-risk group (21.3%), which underscores its ability to capture molecular variations influencing tumor aggressiveness.
Predicting postoperative prognosis
In terms of predicting postoperative prognosis, the 14-gene molecular prognostic testing stratified 94 (52.2%) patients as low-risk for 5-year mortality and 86 (47.8%) patients as high-risk. NCCN criteria identified 118 (65.6%) patients as high-risk and 62 (34.4%) patients as low-risk. A total of 82 patients in the cohort had discordant risk results: 57 (31.7%) of 180 patients had high clinical risk and low molecular risk, and 25 (13.9%) of 180 patients had low clinical risk and high molecular risk.
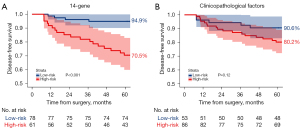
After 5 years of follow-up, the differentiation in 5-year DFS among patients who did not receive adjuvant EGFR-TKIs, as delineated by the 14-gene assay and NCCN-defined clinicopathological high-risk factors, exhibited different performances. The 14-gene assay revealed that patients in molecular high-risk group showed a notably reduced DFS compared to those in the low-risk group [HR =6.49 (95% CI: 2.78–15.2); P<0.001]. The 5-year DFS rates for the molecular low-risk and high-risk group were 94.9% (95% CI: 90.1–99.9%) and 70.5% (95% CI: 59.9–82.9%), indicating a discrimination capacity of 24.4% (P<0.001) (Figure 2A). In comparison, according to NCCN-defined clinicopathological risk factors, clinical high-risk patients experienced a lower DFS compared to low-risk patients (HR =2.18; 95% CI: 0.93–5.14; P=0.12). The 5-year DFS rates for the clinicopathological low- and high-risk group were 90.6% (95% CI: 83.0–98.8%) and 80.2% (95% CI: 72.2–89.1%), reflecting a discrimination capacity of 10.4% (P=0.12) (Figure 2B).
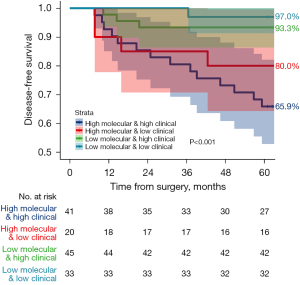
Among 139 patients who did not receive adjuvant EGFR-TKIs, they were divided into four main groups based on their clinicopathological and molecular risk profiles: low clinicopathological risk and low molecular risk, which included 33 patients (23.7%); low clinicopathological risk and high molecular risk, which included 20 patients (14.4%); high clinicopathological risk and low molecular risk, which included 45 patients (32.4%); and high clinicopathological risk and high molecular risk, which included 41 patients (29.5%). For discordant-risk groups, patients with high clinicopathological risk but low molecular risk exhibited a 5-year DFS of 93.3% (95% CI: 86.3–100.0%), while those with low clinicopathological risk but high molecular risk had a 5-year DFS of 80.0% (95% CI: 64.3–99.6%) (P<0.001) (Figure 3). These findings suggested that high molecular risk, regardless of clinicopathological risk, was associated with suboptimal prognosis. Conversely, patients with low molecular risk maintained relatively favorable outcomes even with high clinicopathological risk.
Predicting adjuvant EGFR-TKIs benefit
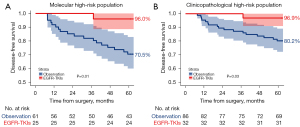
Among 86 patients categorized as molecular high-risk, 25 patients underwent adjuvant EGFR-TKIs, resulting in a 5-year DFS rate of 96.0% (95% CI: 88.6–100.0%). Conversely, patients not receiving adjuvant EGFR-TKIs had a 5-year DFS rate of 70.5% (95% CI: 59.9–82.9%), indicating a 25.5% decline compared to the EGFR-TKIs group (P=0.01) (Figure 4A). Similarly, among 118 patients identified as high clinicopathological risk, 32 patients received adjuvant EGFR-TKIs, achieving a 5-year DFS rate of 96.9% (95% CI: 91.0–100.0%). In contrast, those who did not receive adjuvant EGFR-TKIs had a 5-year DFS rate of 80.2% (95% CI: 72.2–89.1%), representing a 16.7% decrease compared to the EGFR-TKIs group (P=0.03) (Figure 4B). These findings underscore the utility of both NCCN high-risk criteria and the 14-gene assay in guiding decisions regarding adjuvant EGFR-TKIs therapy.
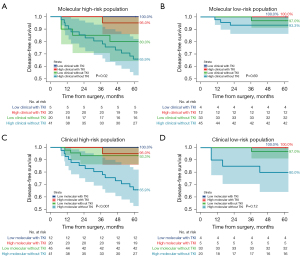
Among patients in the discordant risk groups, molecular high-risk, rather than clinicopathological high-risk factors, proved more predictive of the benefits of adjuvant EGFR-TKIs. Specifically, for molecular high-risk patients, adjuvant EGFR-TKIs significantly improved 5-year DFS rates from 65.9% (95% CI: 52.8–82.1%) to 95.0% (95% CI: 85.9–100.0%) (P=0.02) in clinically high-risk subgroups and from 80.0% (95% CI: 64.3–99.6%) to 100.0% (95% CI: 100.0–100.0%) (P=0.04) in clinically low-risk subgroups (Figure 5A). However, molecular low-risk patients showed no significant benefit from adjuvant EGFR-TKIs, regardless of clinicopathological high-risk [DFS rate increased from 93.3% (95% CI: 86.3–100.0%) to 100% (95% CI: 100.0–100.0%); P=0.37] or low-risk subgroups [97.0% (95% CI: 91.3–100.0%) to 100.0% (95% CI: 100.0–100.0%); P=0.73] (Figure 5B). In other words, patients categorized as clinically high-risk but molecular low-risk could potentially avoid additional postoperative treatment [93.3% (95% CI: 86.3–100.0%) to 100.0% (95% CI: 100.0–100.0%); P=0.37] (Figure 5C). Conversely, clinically low-risk individuals with molecular high-risk profiles still benefited from adjuvant EGFR-TKIs [80.0% (95% CI: 64.3–99.6%) to 100.0% (95% CI: 100.0–100.0%); P=0.04] (Figure 5D).
Discussion
In our study, we compared NCCN clinicopathological factors and the 14-gene assay in predicting postoperative prognosis and guiding adjuvant EGFR-TKIs decisions for stage I NSCLC. We found that the molecular risk stratification was independent of clinical and pathological factors and outperformed in both prognostic and predictive accuracy. Specifically, the 14-gene assay identified a subset of molecular high-risk patients within the clinically low-risk group who significantly benefited from adjuvant therapy, resulting in a 20% increase in 5-year DFS rates. Also, it identified a molecular low-risk population within the clinically high-risk group that showed limited benefit from adjuvant therapy. This finding holds considerable significance as it figured out the predominant population that could benefit from adjuvant EGFR-TKIs that has previously been overlooked and low-risk recurrence patients, sparing them from unnecessary interventions.
The 14-gene assay effectively stratified both clinical high-risk and low-risk patients with greater accuracy than the NCCN criteria alone. The improved predictive accuracy may stem from the inherent limitations of clinical factors, which mainly reflect external manifestations rather than intrinsic molecular characteristics. A large proportion of postoperative deaths in stage I NSCLC were attributed to distant recurrence, suggesting that many patients classified as stage I by traditional standards actually have occult metastases or circulating tumor cells at the time of resection (16). The genes within the 14-gene assay are related to classic lung cancer oncogenic molecular pathways and prognostic gene characteristics (17-21), enabling reliable identification of patient subgroups with different survival outcomes.
The clinical significance of the 14-gene assay lies in its ability to provide more accurate prognostic information and guide personalized treatment strategies. Molecular risk status, independent of clinical risk, effectively stratifies patients into high- and low-risk groups among both clinically high-risk and low-risk patients. Compared to untreated patients, molecularly high-risk patients who received adjuvant EGFR-TKI therapy showed significantly improved 5-year DFS. As reported in previous literature, the 14-gene assay has the ability to identify high-risk patients who benefit from adjuvant chemotherapy (7,9,22,23). In our study, we demonstrated the clinical utility of the 14-gene prognostic assay in guiding adjuvant EGFR-TKIs. These findings highlight the importance of molecular risk assessment in optimizing treatment decisions, ensuring that adjuvant EGFR-TKI is selectively given to patients most likely to derive clinical benefit, thereby minimizing the burden of unnecessary treatment for low molecular risk patients.
While genomic testing, including the 14-gene assay, enhances prognostic accuracy and aids in identifying patients who may benefit from adjuvant EGFR-TKI therapy, molecular residual disease (MRD) monitoring also offers valuable insights into tumor recurrence, metastasis, and treatment efficacy (24-27). However, the challenge with MRD lies in its insufficient sensitivity, potentially yielding false-negative results, particularly in stage I patients on whom the concentration of circulating tumor DNA (ctDNA) may fall below the detection limit (28). In such cases, the 14-gene molecular assay serves as a valuable supplement. Also, the MRD detection methods and assessment standards still need to be standardized. Looking ahead, the combination of molecular markers, clinical risk factors, and MRD assessment may enhance the precision of adjuvant therapy recommendations, potentially leading to improved survival outcomes and reduced cancer-related mortality. Further research and standardization efforts are essential to optimize the integration of these modalities into clinical practice, ultimately improving patient outcomes in early-stage NSCLC management.
Despite the notable findings, this study has several limitations. First, the focus on stage I NSCLC patients receiving off-label adjuvant EGFR-TKI therapy resulted in a small eligible population and limited recurrence events. Consequently, our study could not assess whether the coexistence of multiple high-risk features increases recurrence risk or enhances treatment benefits. Additionally, the retrospective design may have introduced selection bias. Nevertheless, this study demonstrated the prognostic and predictive value of the 14-gene assay with sufficient statistical power. Further validation in larger prospective cohorts is ongoing to address these limitations and confirm the assay’s utility in guiding treatment decisions.
Conclusions
Our study demonstrates that the 14-gene assay offers superior prognostic value and predictive power compared to clinicopathological factors in stage I NSCLC patients. These findings highlight the potential for a more refined and personalized approach to treatment decision-making in this population, ultimately contributing to improved patient outcomes. Further research is needed to validate these results and establish the clinical utility of the 14-gene assay in routine practice.
Acknowledgments
The primary results of the abstract in this study were presented as a meeting poster (P2.07A.01) in the World Conference on Lung Cancer 2024.
Footnote
Reporting Checklist: The authors have completed the STROCSS reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-20/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-20/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-20/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-20/coif). W.L. serves as an associate Editor-in-Chief of Translational Lung Cancer Research from May 2024 to April 2025. S.C. is from Guangzhou Burning Rock Biotech Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study received approval from the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (No. 2022181), and individual consent for this retrospective analysis was waived due to the retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Park JH, Lee CT, Lee HW, et al. Postoperative adjuvant chemotherapy for stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2005;27:1086-91. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw 2023;21:340-50. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Ou W, Li N, Wang BX, et al. Adjuvant icotinib versus observation in patients with completely resected EGFR-mutated stage IB NSCLC (GASTO1003, CORIN): a randomised, open-label, phase 2 trial. EClinicalMedicine 2023;57:101839. [Crossref] [PubMed]
- Jiang Y, Lin Y, Fu W, et al. The impact of adjuvant EGFR-TKIs and 14-gene molecular assay on stage I non-small cell lung cancer with sensitive EGFR mutations. EClinicalMedicine 2023;64:102205. [Crossref] [PubMed]
- Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [Crossref] [PubMed]
- Kratz JR, Van den Eeden SK, He J, et al. A prognostic assay to identify patients at high risk of mortality despite small, node-negative lung tumors. JAMA 2012;308:1629-31. [Crossref] [PubMed]
- Woodard GA, Wang SX, Kratz JR, et al. Adjuvant Chemotherapy Guided by Molecular Profiling and Improved Outcomes in Early Stage, Non-Small-Cell Lung Cancer. Clin Lung Cancer 2018;19:58-64. [Crossref] [PubMed]
- Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Wang Y, Yang X, Liu B, et al. Percentage of Newly Proposed High-Grade Patterns Is Associated with Prognosis of Pathological T1-2N0M0 Lung Adenocarcinoma. Ann Surg Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski C, et al. Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol 2015;10:638-44. [Crossref] [PubMed]
- Kratz JR, Tham PT, Mulvihill MS, et al. Analytical validation of a practical molecular assay prognostic of survival in nonsquamous non-small cell lung cancer. Diagn Mol Pathol 2013;22:65-9. [Crossref] [PubMed]
- Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer 2009;115:5218-27. [Crossref] [PubMed]
- Sun Z, Wigle DA, Yang P. Non-overlapping and non-cell-type-specific gene expression signatures predict lung cancer survival. J Clin Oncol 2008;26:877-83. [Crossref] [PubMed]
- Director's Challenge Consortium for the Molecular Classification of Lung Adenocarcinoma. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med 2008;14:822-7. [Crossref] [PubMed]
- Raponi M, Zhang Y, Yu J, et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res 2006;66:7466-72. [Crossref] [PubMed]
- Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med 2007;356:11-20. [Crossref] [PubMed]
- Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med 2002;8:816-24. [Crossref] [PubMed]
- Woodard GA, Gubens MA, Jahan TM, et al. Prognostic molecular assay might improve identification of patients at risk for recurrence in early-stage non-small-cell lung cancer. Clin Lung Cancer 2014;15:426-32. [Crossref] [PubMed]
- Woodard GA, Kratz JR, Haro G, et al. Molecular Risk Stratification is Independent of EGFR Mutation Status in Identifying Early-Stage Non-Squamous Non-Small Cell Lung Cancer Patients at Risk for Recurrence and Likely to Benefit From Adjuvant Chemotherapy. Clin Lung Cancer 2021;22:587-95. [Crossref] [PubMed]
- Qiu B, Guo W, Zhang F, et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat Commun 2021;12:6770. [Crossref] [PubMed]
- Moding EJ, Liu Y, Nabet BY, et al. Circulating Tumor DNA Dynamics Predict Benefit from Consolidation Immunotherapy in Locally Advanced Non-Small Cell Lung Cancer. Nat Cancer 2020;1:176-83. [Crossref] [PubMed]
- Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov 2017;7:1394-403. [Crossref] [PubMed]
- Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017;545:446-51. [Crossref] [PubMed]
- Chen K, Yang F, Shen H, et al. Individualized tumor-informed circulating tumor DNA analysis for postoperative monitoring of non-small cell lung cancer. Cancer Cell 2023;41:1749-1762.e6. [Crossref] [PubMed]


