
Re-irradiation for local recurrence after definitive stereotactic body radiotherapy for early-stage non-small cell lung cancer
Highlight box
Key findings
• Re-irradiation with stereotactic body radiotherapy (SBRT) for in-field local recurrence of non-small cell lung cancer (NSCLC) demonstrated good feasibility, achieving long-term survival and disease control, particularly in patients with adenocarcinoma.
• Toxicity was minimal, with no grade 3 or higher adverse events observed, confirming the safety of this approach.
What is known and what is new?
• Salvage treatment options for local recurrence of NSCLC after definitive SBRT remain limited and underexplored.
• Previous studies have not adequately addressed the efficacy and long-term outcomes of re-irradiation with SBRT.
• This study highlights that re-irradiation with SBRT offers promising outcomes, including prolonged survival, particularly for patients with adenocarcinoma, and demonstrates a low incidence of severe toxicity.
What is the implication, and what should change now?
• Re-irradiation with SBRT should be considered a viable salvage treatment option for in-field local recurrence of NSCLC, especially in patients with adenocarcinoma. This study data is expected to serve as fundamental evidence for designing future prospective trials.
Introduction
Stereotactic body radiotherapy (SBRT) has been a standard treatment option for early-stage non-small cell lung cancer (NSCLC), exhibiting good survival and high local control (LC) rates, with our previous studies reporting 5-year LC rates of 83–85% (1-3). However, in-field local recurrences remain a significant problem despite these favorable outcomes. Salvage treatment for local recurrences after initial SBRT remains unestablished (4). Salvage surgery after recurrence demonstrated encouraging results (5-7), but many patients who were considered inoperable before initial SBRT are generally deemed unsuitable for salvage surgery. Systemic chemotherapy alone is frequently adapted as a salvage treatment option when local therapies are contraindicated, but this is usually not considered a curative treatment (8).
Re-irradiation has been considered a high-risk treatment due to exceeding the tolerable dose for normal tissues. Re-irradiation is defined as a second course of radiotherapy that geometrically overlaps with the irradiated volume of the initial course (9). Recent studies indicated that salvage SBRT demonstrated excellent LC rates with acceptable adverse events (10-12), revealing that re-irradiation with SBRT may be a feasible and safe option for local recurrence in early-stage NSCLC. Retrospective studies have reported the feasibility of SBRT re-irradiation, but data are limited (13,14). This study aims to investigate outcomes for 35 patients receiving re-irradiation with SBRT for early-stage NSCLC, making it the largest series with the longest follow-up. Unlike our previous study (15), which included a different population (primary lung and metastatic disease lesions), this study focuses specifically on the long-term results of the re-irradiation with SBRT for NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-89/rc).
Methods
This retrospective study focused on the salvage of second SBRT for patients with in-field local recurrence after initial SBRT for NSCLC. The institutional review board of Nagoya City University Hospital approved this study (No. 60-23-0059). Written informed consent was waived due to the retrospective study design, and its content was disclosed in the form of an opt-out available on the website. This study was conducted under the guidelines of the Helsinki Declaration and its subsequent amendments. The decision to administer the second SBRT was based on clinical judgment because the SBRT protocols did not specifically define second-line treatment. In general, the main eligibility criterion was the absence of grade ≥3 radiation pneumonitis after the initial SBRT. Other criteria were consistent with those used in our previously published SBRT studies (15). The indication for surgery was determined by a tumor board consisting of respiratory physicians, respiratory surgeons, and radiation oncologists. Patients with impaired pulmonary function or comorbidities were deemed ineligible for surgery.
Patient characteristics
Thirty-five patients diagnosed with in-field local recurrence of NSCLC received a second SBRT from July 2004 to August 2021. Local recurrence was diagnosed by biopsy whenever possible. When biopsy was not feasible, the diagnosis was based on computed tomography (CT) findings combined with fluorodeoxyglucose-positron emission tomography (FDG-PET) results [maximum standardized uptake value (SUVmax) ≥5]. Recurrence was histologically confirmed in 18 of the 19 patients who underwent biopsy, whereas biopsy results were negative in one patient, but recurrence was radiologically diagnosed based on the subsequent further tumor enlargement. Sixteen patients who did not undergo a pathological examination were diagnosed with recurrence based on FDG-PET findings. Concurrent with the second SBRT, eight of 35 patients met the criteria for operability (16), but none selected surgery; whereas, the remaining 27 patients were considered inoperable. The median patient age at the second SBRT was 77 years (range, 52–95 years), with 30 men and 5 women. Patient characteristics are summarized in Table 1. Tumor location was categorized as central and peripheral based on published criteria (17), with no patients having an ultra-central tumor. None of the patients received concurrent systemic chemotherapy.
Table 1
| Characteristics | Value |
|---|---|
| Age at second SBRT (years) | 77 (52–95) |
| Gender (male/female) | 30/5 |
| Histology at initial SBRT (AD/SCC) | 19/16 |
| T stage at initial SBRT (T1a/T1b/T1c/T2a/T2b) | 2/13/13/5/2 |
| Tumor location at second SBRT (peripheral/central) | 29/6 |
| Lesion size at initial SBRT (maximum diameter) (mm) | 19 (8–41) |
| Lesion size at second SBRT (maximum diameter) (mm) | 30 (13–54) |
| Radiation pneumonitis after initial SBRT (grade 0/1/2) | 9/23/3 |
Data are presented as median (range) or number. AD, adenocarcinoma; SBRT, stereotactic body radiotherapy; SCC, squamous cell carcinoma.
SBRT methodology
Our SBRT approach for NSCLC has been extensively detailed in previous publications (15). The initial SBRT plan was carefully evaluated to ensure the appropriateness of the treatment plan before planning the second SBRT. The second SBRT followed the methodology of the initial SBRT, with slight adjustments in dose-fractionation schedules. The BodyFIX system (Medical Intelligence, Schwabmuenchen, Germany) or a thermoplastic cast (Hip-Fix, Med-Tec, Orange City, IA, USA) was used for immobilization of patients and minimizing the respiratory movements of targets. The gross tumor volume (GTV) was visible lesions, identified with FDG-PET, and the clinical target volume (CTV) was equal to the GTV in order to minimize the irradiation of normal tissues. The CTV was delineated in three breathing phases, and the delineations were summed up. The final delineation was defined as the internal target volume (ITV). Anisotropic margins of 5 mm in the lateral and anteroposterior directions and 10 mm in the craniocaudal directions were added to the ITV to make planning target volume (PTV). The second SBRT was administered via a linear accelerator (CLINAC 23EX, Varian Medical Systems, Palo Alto, CA, USA, or Novalis image-guided system, BrainLAB, Feldkirchen, Germany), similar to the initial SBRT. Planned doses were prescribed with a photon beam of 6 MV. A combination of coplanar and noncoplanar static fields was used, with total 7 to 10 fields. The majority of patients were treated with different dose regimens in 4 or 8 fractions, in accordance with the initial SBRT protocols. Table 2 shows dose-fractionation schedules for the initial SBRT as well as the schedules for the second SBRT. The median prescription dose was 48 Gy in 4 fractions (range, 48–60 Gy) and 60 Gy in 8 fractions (range, 48–66 Gy) prescribed to the median isodose of 50% for the initial and second SBRT, respectively. The dose constraints for PTV were as follows: (I) D95% (Gy) ≥95% of the prescribed dose; (II) D50% (Gy) =100% of the prescribed dose; (III) D2% (Gy) ≤110% of the prescribed dose. The 4- and 8-fraction schedules were applied in 13 and 20 patients, respectively. Two patients underwent treatment with 10 or 20 fractions. Treatment information of SBRT is summarized in Table 2. The dose calculation algorithm was pencil beam convolution with Batho power law correction until November 2008 and January 2011 for CLINAC 23EX and Novalis treatment, respectively. Subsequently, the analytical anisotropic algorithm was applied.
Table 2
| Parameter | Initial SBRT | Second SBRT | Total dose |
|---|---|---|---|
| Dose (Gy/fr) | 48/4:50/4:52/4 =9:15:3 | 48/4:50/4:52/4 =4:4:5 | NA |
| 56/8:60/8 =2:6 | 48/8:56/8:60/8 =1:4:15 | ||
| 66/10:56/20 =1:1 | |||
| PTV (cm3) | 39.2 (6.4–107.6) | 60.6 (13.5–149) | NA |
| PTV D98 (%) | 93 (9–97) | 93.9 (86.9–100) | NA |
| PTV D95 (%) | 94.6 (80.5–99) | 96 (86.9–100) | NA |
| PTV D50 (%) | 100.2 (96.2–103.4) | 100.6 (94.8–105.3) | NA |
| PTV D2 (%) | 104.4 (101.6–112) | 106.6 (100–113) | NA |
| PTV D50 (BED10) (Gy) | 112.5 (95.2–119.6) | 105 (71.7–119.6) | 217.5 (176.7–232.1) |
| Lung V20Gy (%) | 5.1 (1.9–11.2) | 6.4 (2.4–14.5) | 12.4 (4.9–19.7) |
| Dmax (EQD2, α/β =3) (Gy) | |||
| Proximal bronchial tree | 87.2 (0.4–160.7) | 131.8 (0.8–173.4) | 209 (1.7–322.3) |
| Esophagus | 5.9 (0.3–44.4) | 6.9 (0.3–136.5) | 15 (0.8–146.8) |
| Great vessels | 31 (3.2–140.2) | 37.2 (4.9–149.5) | 108.1 (9.3–247.8) |
Data are presented as median (range) or number. BED, biologically effective dose; EQD2, equivalent dose in 2 Gy; NA, not applicable; PTV, planning target volume; SBRT, stereotactic body radiotherapy.
Assessment and statistical analyses
After the second SBRT, follow-up evaluations, including chest CT, were conducted every 2 months for the first 6 months, and subsequently every 2 to 4 months. FDG-PET and brain magnetic resonance imaging were performed as needed. The diagnosis of local re-recurrence was conducted using a combination of serial CT and FDG-PET (SUVmax ≥5), and a biopsy for suspected re-recurrence was not conducted. Toxicity evaluations were conducted according to the Common Terminology Criteria for Adverse Events version 5.0. The Kaplan-Meier method was used to calculate the rates of overall survival (OS), LC within the irradiated region, and progression-free survival (PFS) from the start of the second SBRT. Univariate analyses were conducted using the Cox proportional hazards model to identify factors for OS, LC, and PFS. P value of <0.05 was considered to indicate a significant difference. All statistical analyses were conducted using open-source software, R Version 4.4.0 (The R Foundation for Statistical Computing, Vienna, Austria). The following formula was used for the biologically equivalent dose in 2 Gy (EQD2): EQD2 = D × [d + (α/β)]/[2 Gy + (α/β)]. The following formula was used for biologically effective dose (BED): BED = D × [1 + d/(α/β)]. The D and d in the formula denote the total dose and per dose, respectively. The α/β-values for the target were 10 and for the other organs at risk 3.
Results
Outcomes
The median time interval between the first and second SBRT was 24 months (range, 6–81 months). Table 2 shows the treatment information for SBRT. The median PTV was 39.2 cm3 (range, 6.4–107.6 cm3) and 60.6 cm3 (range, 13.5–149 cm3), and the median BED10 (Gy) was 112.5 Gy (range, 95.2–119.6 Gy) and 105 Gy (range, 71.7–119.6 Gy) for the initial and second SBRT, respectively. The median follow-up period was 29 months (range, 3–124 months). Of the 35 patients, 17 (49%) died. Of these, 12 patients died from cause-specific deaths and five died from other diseases. Four patients demonstrated no further recurrence for more than five years after the second SBRT. Figure 1 shows the OS, LC, and PFS curves for all 35 patients. The three-year OS, PFS, and LC rates were 50%, 38%, and 54%, respectively. The prognostic factors on univariate analyses for OS, LC, and PFS are summarized in Table 3. The univariate analyses revealed the histological type as a significant prognostic factor [adenocarcinoma vs. squamous cell carcinoma: OS: hazard ratio (HR), 3.50, 95% confidence interval (CI): 1.07–11.5, P=0.04; LC: HR, 5.71, 95% CI: 1.45–22.5, P=0.01; PFS: HR, 4.62, 95% CI: 1.67–12.8, P=0.003]. The three-year OS, LC, and PFS rates were 72% vs. 21%, 85% vs. 34%, and 62% vs. 9% in the adenocarcinoma and squamous cell carcinoma groups, respectively.
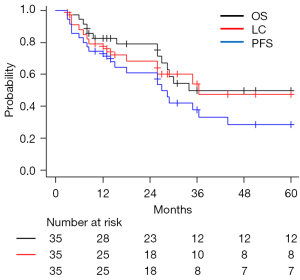
Table 3
| Variable | OS | LC | PFS | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (vs. <75 years) | ||||||||
| ≥75 years | 1.74 (0.55–5.50) | 0.30 | 1.19 (0.39–3.57) | 0.75 | 1.84 (0.71–4.74) | 0.21 | ||
| Treatment interval (vs. <24 months) | ||||||||
| ≥24 months | 0.76 (0.27–2.10) | 0.59 | 0.62 (0.21–1.80) | 0.38 | 0.88 (0.37–2.10) | 0.78 | ||
| Histological type (vs. adenocarcinoma) | ||||||||
| Squamous cell carcinoma | 3.50 (1.07–11.5) | 0.04 | 5.71 (1.45–22.5) | 0.01 | 4.62 (1.67–12.8) | 0.003 | ||
| Maximum diameter (vs. <30 mm) | ||||||||
| ≥30 mm | 1.75 (0.61–5.01) | 0.30 | 1.65 (0.57–4.81) | 0.35 | 1.96 (0.81–4.76) | 0.14 | ||
| PTV at second SBRT (vs. <70 cm3) | ||||||||
| ≥70 cm3 | 0.89 (0.30–2.64) | 0.84 | 0.84 (0.26–2.70) | 0.77 | 0.91 (0.36–2.28) | 0.85 | ||
| BED10 at second SBRT (vs. <105 Gy) | ||||||||
| ≥105 Gy | 0.58 (0.18–1.86) | 0.36 | 0.99 (0.34–2.91) | 0.99 | 0.82 (0.34–1.96) | 0.67 | ||
| BED10 sum of SBRT (vs. <215 Gy) | ||||||||
| ≥215 Gy | 0.87 (0.31–2.41) | 0.79 | 0.57 (0.20–1.63) | 0.29 | 0.69 (0.30–1.61) | 0.40 | ||
BED, biological effective dose; CI, confidence interval; HR, hazard ratio; LC, local control; OS, overall survival; PFS, progression-free survival; PTV, planning target volume; SBRT, stereotactic body radiotherapy.
Toxicities
Grade ≥3 toxicity after the second SBRT did not occur. Radiation pneumonitis was classified as grades 0 and 1 in 32, and grade 2 in three patients. Grade 2 radiation pneumonitis occurred in all three cases with peripheral lesions. Grade 2 rib fractures occurred in nine patients, with only three of them developing fractures after the initial SBRT. Other toxicities, including the trachea, bronchus, esophagus, and great vessels, did not occur.
Case presentation
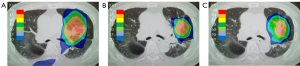
A 79-year-old man was diagnosed with clinical stage T1cN0M0 adenocarcinoma of the left upper lobe and received SBRT of 50 Gy in 4 fractions to the lesion in May 2015. An in-field recurrence based on PET-CT (SUVmax 10.9) was observed 15 months after the initial SBRT. He received a second SBRT of 50 Gy in 4 fractions to the recurrence lesion. Figure 2 shows the dose distributions of the initial and second SBRT. Summing the doses of the initial and second SBRT, the Dmax was 62 Gy for the left main bronchus, and 35 Gy for the aorta. The V20Gy, V40Gy, V60Gy, V80Gy, and V100Gy of the lung were 11.5%, 4.3%, 2.2%, 0.8%, and 0.1%, respectively. This patient developed grade 1 radiation pneumonitis after the initial SBRT, and subsequently no grade ≥2 toxicity after the second SBRT was observed.
Discussion
The salvage treatment of recurrence of NSCLC in patients who have received initial SBRT is challenging. In this study, we present the retrospective data focusing on the salvage second SBRT for patients with in-field local relapse after initial SBRT for NSCLC, with a favorable median OS of 36 months compared to the median OS of 20–30 months reported in most published studies (18-22). Regarding LC, the 3-year LC of 47% in our study was similar compared to most published 3-year LC data with a range of 51–67% (22,23). Studies of re-irradiation with SBRT for in-field recurrence after initial SBRT are summarized in Table 4 (22-27). In the study of salvage surgery for patients with local recurrence after definitive SBRT, the median survival after surgery was 13.6 months and 3-year survival was 43.1% (28). Comparison of treatment outcomes should be performed with caution; however, salvage SBRT is considered to have favorable outcomes compared to those of salvage surgery. One concern for the second SBRT is that not all patients are candidates and the criteria are unclear. The suitability should be determined by comprehensively assessing the patient’s health condition, the effect of initial treatment, and the characteristics of the recurrent site. In general, recurrent tumors may be radioresistant, because they have not been cured with the initial SBRT. Acquired radioresistance after radiotherapy for lung cancer has been identified as an important cause of local recurrence (29,30). Therefore, not controlling recurrent tumors with similar SBRT is a concern. However, this was not necessarily the case in our data. Our study revealed that the LC rate was better in the adenocarcinoma group than in the squamous cell carcinoma group. The impact of different histological types of lung cancer on the therapeutic efficacy of SBRT has been reported (31). This indicates that differences in histological type may also be important criteria for selecting suitable patients for a second SBRT. Some studies have reported that squamous cell carcinoma histology is associated with poorer clinical outcomes following SBRT compared to adenocarcinoma in early-stage NSCLC (32,33). However, the biological mechanisms underlying this disparity remain unclear. Lung squamous cell carcinoma often contains extensive hypoxic regions, and this hypoxic environment contributes to its resistance to radiotherapy (34). Some studies have demonstrated that EGFR mutation-positive adenocarcinoma exhibits increased radiosensitivity (35).
Table 4
| Author | n | Age (years) | OS (2-year) (%) | LC (2-year) (%) | Cumulative BED10 (Gy) |
|---|---|---|---|---|---|
| John C (22) | 27 | 71 (34–88) | 67.5 | 51.1 | N/A |
| Caivano D (23) | 22 | 70 (47–82) | 63 | 54 | 212.5 (87–271.2) |
| Valakh V (24) | 9 | 74 (59–83) | 68.6 | 75 | 264 (120–360) |
| Meijneke TR (25) | 20 | 71 (50–80) | 33 | 50 | 216 (67–300) |
| Peulen H (26) | 29 | 65 (18–87) | 43 | N/A | 150 (100–225) |
| Wang HH (27) | 17 | 73 (54–87) | 46.8 | 85.6 | 270 (181.6–360) |
| Present study | 35 | 77 (52–95) | 79.1 | 68.2 | 217.5 (176.7–232.1) |
Data are presented as median (range). BED, biological effective dose; LC, local control; NA, not applicable; OS, overall survival; SBRT, stereotactic body radiotherapy.
One of the main concerns of re-irradiation with SBRT is life-threatening treatment-related toxicity, including fatal bleeding in central lesions (36). The re-irradiation with SBRT with doses of 50–60 Gy in 3–5 fractions was feasible in systematic reviews (27). Further, our data demonstrated a similar dose, and no severe adverse events were observed. However, caution is warranted for centrally-located tumors due to the potential risk of severe toxicity. Our study revealed that centrally-located tumors were only six (17%) patients and ultra centrally-located tumors were not included. We provide dosimetric considerations to clinicians who attempt re-irradiation with SBRT for these patients. Establishing safety dose-volume parameters for a second SBRT is challenging, as mentioned in other studies. One example of cumulative dose constraints in re-irradiation (EQD2) is as follows: esophagus maximum dose of <100 Gy (37); bronchial tree of <100 Gy (37); lung V20Gy of <15% (38); major blood vessels maximum dose of <120 Gy (37). The incidence of grade ≥2 radiation pneumonitis and rib fractures after initial SBRT was reported to be 15.3% and 8.0%, respectively (39,40). Compared to these results, the incidence of rib fractures (25.7%) was high, but the incidence of radiation pneumonia (8.5%) was similar. The composite plan V20Gy >30% was reported to be correlated with an increased risk of radiation pneumonitis (41). The recovery of lung damage after initial SBRT over time remains unclear. However, murine and clinical data indicate the recovery of normal lung tissue based on the re-irradiation interval (42-44). The median interval between initial and second SBRT was 24 months in our study, which could have contributed to the low lung toxicity rates. Serum biomarkers for predicting radiation pneumonitis have been reported (45), and may also be applicable to patients undergoing re-irradiation with SBRT. Radioprotective agents for preventing radiation pneumonitis have also been reported in mice models (46). Re-irradiation with SBRT may be considered in selected patients, especially in those with peripheral recurrence lesions. It was reported that patients received SBRT for in-field recurrences experienced lower rates of severe toxicities than those received SBRT for out-field recurrences (47). These factors could be the reasons there were low rates of severe adverse events in the present study. Various biological studies aimed at enhancing the antitumor effects of radiotherapy are advancing, and their application to SBRT for lung cancer is also a possibility (48-51). This study has several limitations. First, this was a retrospective study with a potential bias in patient characteristics. Second, the number of evaluated patients was small, and thus a larger sample size is needed for a more detailed analysis. Third, treatment outcomes may be falsely elevated because 51% of patients had no histological confirmation of recurrence at the second SBRT. Fourth, the recruitment period of 17 years was very long.
Conclusions
This study indicated that re-irradiation with SBRT is safe and can be a salvage treatment option for in-field local recurrence of NSCLC after definitive SBRT. A prospective study is warranted to establish the optimal treatment method, including dose escalation and alternative fractionation schedules.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-89/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-89/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-89/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-89/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study has been conducted in compliance with the guidelines of the Helsinki Declaration and its subsequent amendments. This study was performed after approval by the institutional review board of Nagoya City University Hospital (No. 60-23-0059). Written informed consent was waived due to the retrospective nature of this study, its content was disclosed in the form of an opt-out available on the website.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol 2015;10:960-4. [Crossref] [PubMed]
- Miyakawa A, Shibamoto Y, Baba F, et al. Stereotactic body radiotherapy for stage I non-small-cell lung cancer using higher doses for larger tumors: results of the second study. Radiat Oncol 2017;12:152. [Crossref] [PubMed]
- Kita N, Tomita N, Takaoka T, et al. Comparison of Recurrence Patterns between Adenocarcinoma and Squamous Cell Carcinoma after Stereotactic Body Radiotherapy for Early-Stage Lung Cancer. Cancers (Basel) 2023;15:887. [Crossref] [PubMed]
- Kumar SS, McGarry RC. Management of local recurrences and regional failure in early stage non-small cell lung cancer after stereotactic body radiation therapy. Transl Lung Cancer Res 2019;8:S213-21. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Treatment and Prognosis of Isolated Local Relapse after Stereotactic Body Radiotherapy for Clinical Stage I Non-Small-Cell Lung Cancer: Importance of Salvage Surgery. J Thorac Oncol 2015;10:1616-24. [Crossref] [PubMed]
- Verstegen NE, Maat AP, Lagerwaard FJ, et al. Salvage surgery for local failures after stereotactic ablative radiotherapy for early stage non-small cell lung cancer. Radiat Oncol 2016;11:131. [Crossref] [PubMed]
- Antonoff MB, Correa AM, Sepesi B, et al. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J Thorac Cardiovasc Surg 2017;154:689-99. [Crossref] [PubMed]
- Milton DT, Miller VA. Advances in cytotoxic chemotherapy for the treatment of metastatic or recurrent non-small cell lung cancer. Semin Oncol 2005;32:299-314. [Crossref] [PubMed]
- Andratschke N, Willmann J, Appelt AL, et al. European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus on re-irradiation: definition, reporting, and clinical decision making. Lancet Oncol 2022;23:e469-78. [Crossref] [PubMed]
- Ward MC, Oh SC, Pham YD, et al. Isolated Nodal Failure after Stereotactic Body Radiotherapy for Lung Cancer: The Role for Salvage Mediastinal Radiotherapy. J Thorac Oncol 2016;11:1558-64. [Crossref] [PubMed]
- Brooks ED, Sun B, Feng L, et al. Association of Long-term Outcomes and Survival With Multidisciplinary Salvage Treatment for Local and Regional Recurrence After Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer. JAMA Netw Open 2018;1:e181390. [Crossref] [PubMed]
- Kennedy WR, Gabani P, Nikitas J, et al. Repeat stereotactic body radiation therapy (SBRT) for salvage of isolated local recurrence after definitive lung SBRT. Radiother Oncol 2020;142:230-5. [Crossref] [PubMed]
- Milano MT, Mihai A, Kong FS. Review of thoracic reirradiation with stereotactic body radiation therapy: A focus on toxicity risks. Pract Radiat Oncol 2018;8:251-65. [Crossref] [PubMed]
- Ricco A, Barlow S, Feng J, et al. Repeat Thoracic Stereotactic Body Radiation Therapy (SBRT) for Nonsmall Cell Lung Cancer: Long-Term Outcomes, Toxicity, and Dosimetric Considerations. Adv Radiat Oncol 2020;5:984-93. [Crossref] [PubMed]
- Ogawa Y, Shibamoto Y, Hashizume C, et al. Repeat stereotactic body radiotherapy (SBRT) for local recurrence of non-small cell lung cancer and lung metastasis after first SBRT. Radiat Oncol 2018;13:136. [Crossref] [PubMed]
- Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer 2012;118:2078-84. [Crossref] [PubMed]
- Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015;89:50-6. [Crossref] [PubMed]
- Evans JD, Gomez DR, Amini A, et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother Oncol 2013;106:327-32. [Crossref] [PubMed]
- Kilburn JM, Kuremsky JG, Blackstock AW, et al. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol 2014;110:505-10. [Crossref] [PubMed]
- Patel NR, Lanciano R, Sura K, et al. Stereotactic body radiotherapy for re-irradiation of lung cancer recurrence with lower biological effective doses. J Radiat Oncol 2015;4:65-70. [Crossref] [PubMed]
- Horne ZD, Dohopolski MJ, Clump DA, et al. Thoracic reirradiation with SBRT for residual/recurrent and new primary NSCLC within or immediately adjacent to a prior high-dose radiation field. Pract Radiat Oncol 2018;8:e117-23. [Crossref] [PubMed]
- John C, Dal Bello R, Andratschke N, et al. In-field stereotactic body radiotherapy (SBRT) reirradiation for pulmonary malignancies as a multicentre analysis of the German Society of Radiation Oncology (DEGRO). Sci Rep 2021;11:4590. [Crossref] [PubMed]
- Caivano D, Valeriani M, De Matteis S, et al. Re-irradiation in lung disease by SBRT: a retrospective, single institutional study. Radiat Oncol 2018;13:87. [Crossref] [PubMed]
- Valakh V, Miyamoto C, Micaily B, et al. Repeat stereotactic body radiation therapy for patients with pulmonary malignancies who had previously received SBRT to the same or an adjacent tumor site. J Cancer Res Ther 2013;9:680-5. [Crossref] [PubMed]
- Meijneke TR, Petit SF, Wentzler D, et al. Reirradiation and stereotactic radiotherapy for tumors in the lung: dose summation and toxicity. Radiother Oncol 2013;107:423-7. [Crossref] [PubMed]
- Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol 2011;101:260-6. [Crossref] [PubMed]
- Wang HH, Chen Y, Liu X, et al. Reirradiation with stereotactic body radiotherapy for primary or secondary lung malignancies: Tumor control probability and safety analyses. Radiother Oncol 2023;187:109817. [Crossref] [PubMed]
- Eisenberg M, Deboever N, Antonoff MB. Salvage surgery in lung cancer following definitive therapies. J Surg Oncol 2023;127:319-28. [Crossref] [PubMed]
- Li J, Zong Y, Tuo Z, et al. The role of RASA2 in predicting radioresistance in lung cancer through regulation of p53. Transl Lung Cancer Res 2024;13:587-602. [Crossref] [PubMed]
- Wang M, Yi J, Gao H, et al. Radiation-induced YAP/TEAD4 binding confers non-small cell lung cancer radioresistance via promoting NRP1 transcription. Cell Death Dis 2024;15:619. [Crossref] [PubMed]
- Allen AJ, Labella DA, Kowalchuk RO, et al. Effect of histology on stereotactic body radiotherapy for non-small cell lung cancer oligometastatic pulmonary lesions. Transl Lung Cancer Res 2023;12:66-78. [Crossref] [PubMed]
- Hörner-Rieber J, Bernhardt D, Dern J, et al. Histology of non-small cell lung cancer predicts the response to stereotactic body radiotherapy. Radiother Oncol 2017;125:317-24. [Crossref] [PubMed]
- Abel S, Hasan S, White R, et al. Stereotactic ablative radiotherapy (SABR) in early stage non-small cell lung cancer: Comparing survival outcomes in adenocarcinoma and squamous cell carcinoma. Lung Cancer 2019;128:127-33. [Crossref] [PubMed]
- Xu P, Hu K, Zhang P, et al. Hypoxia-mediated YTHDF2 overexpression promotes lung squamous cell carcinoma progression by activation of the mTOR/AKT axis. Cancer Cell Int 2022;22:13. [Crossref] [PubMed]
- Tanaka H, Karita M, Ueda K, et al. Differences in Radiosensitivity According to EGFR Mutation Status in Non-Small Cell Lung Cancer: A Clinical and In Vitro Study. J Pers Med 2023;14:25. [Crossref] [PubMed]
- Grambozov B, Stana M, Kaiser B, et al. High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC. Cancers (Basel) 2022;14:573. [Crossref] [PubMed]
- Schröder C, Stiefel I, Tanadini-Lang S, et al. Re-irradiation in the thorax - An analysis of efficacy and safety based on accumulated EQD2 doses. Radiother Oncol 2020;152:56-62. [Crossref] [PubMed]
- Reyngold M, Wu AJ, McLane A, et al. Toxicity and outcomes of thoracic re-irradiation using stereotactic body radiation therapy (SBRT). Radiat Oncol 2013;8:99. [Crossref] [PubMed]
- Kita N, Tomita N, Takaoka T, et al. Clinical and dosimetric factors for symptomatic radiation pneumonitis after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Clin Transl Radiat Oncol 2023;41:100648. [Crossref] [PubMed]
- Kita N, Tomita N, Takaoka T, et al. Symptomatic radiation-induced rib fractures after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Clin Transl Radiat Oncol 2023;43:100683. [Crossref] [PubMed]
- Liu H, Zhang X, Vinogradskiy YY, et al. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:1017-23. [Crossref] [PubMed]
- Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, et al. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol 2007;17:89-98. [Crossref] [PubMed]
- Beach TA, Groves AM, Williams JP, et al. Modeling radiation-induced lung injury: lessons learned from whole thorax irradiation. Int J Radiat Biol 2020;96:129-44. [Crossref] [PubMed]
- Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, et al. Radiation-induced lung injury: current evidence. BMC Pulm Med 2021;21:9. [Crossref] [PubMed]
- Seto Y, Kaneko Y, Mouri T, et al. Changes in serum transforming growth factor-beta concentration as a predictive factor for radiation-induced lung injury onset in radiotherapy-treated patients with locally advanced lung cancer. Transl Lung Cancer Res 2022;11:1823-34. [Crossref] [PubMed]
- Kaytor MD, Serebrenik AA, Lapanowski K, et al. The radioprotectant nano-genistein enhances radiotherapy efficacy of lung tumors in mice. Transl Lung Cancer Res 2023;12:999-1010. [Crossref] [PubMed]
- Kelly P, Balter PA, Rebueno N, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys 2010;78:1387-93. [Crossref] [PubMed]
- Takaoka T, Shibamoto Y, Matsuo M, et al. Biological effects of hydrogen peroxide administered intratumorally with or without irradiation in murine tumors. Cancer Sci 2017;108:1787-92. [Crossref] [PubMed]
- Kondo T, Shibamoto Y, Kawai T, et al. Effects of a combined treatment regimen consisting of Hsp90 inhibitor DS-2248 and radiation in vitro and in a tumor mouse model. Transl Cancer Res 2021;10:2767-76. [Crossref] [PubMed]
- Yuan M, Wang C, Wu Y, et al. Targeting complement C5a to improve radiotherapy sensitivity in non-small cell lung cancer. Transl Lung Cancer Res 2023;12:1093-107. [Crossref] [PubMed]
- Manabe Y, Takahashi Y, Sugie C, et al. Biological effects of prostaglandin E2-EP4 antagonist (AAT-008) in murine colon cancer in vivo: enhancement of immune response to radiotherapy and potential as a radiosensitizer. Transl Cancer Res 2023;12:351-8. [Crossref] [PubMed]


