
Combination of immune checkpoint inhibitors with multi-targeted tyrosine kinase inhibitors for second- or later-line therapy of non-small cell lung cancer: a systematic review and meta-analysis
Highlight box
Key findings
• This study found that the combination of immune checkpoint inhibitors (ICIs) and multi-targeted tyrosine kinase inhibitors (multi-TKIs), used as second- or later-line treatment, resulted in a median progression-free survival of 5.74 months, a median overall survival of 15.41 months, an objective response rate of 26.35%, and a disease control rate of 80.73% in patients with advanced non-small cell lung cancer (NSCLC). The safety profile included hypertension, fatigue, hepatic dysfunction, urinary abnormalities, and hand-foot syndrome.
What is known and what is new?
• Combining ICIs with multi-TKIs has emerged as a chemotherapy-free option for patients with advanced NSCLC.
• The efficacy and safety of this combined treatment strategy remained unclear when used as second- or later-line for these patients.
What is the implication, and what should change now?
• The combination of ICIs and multi-TKIs offers an alternative chemotherapy-free treatment option for patients with advanced NSCLC in the second- or later-line setting.
Introduction
Lung cancer is one of the leading causes of cancer-related mortality (1). In China, it has the highest mortality in both males and females among all cancers (2). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all cases of lung cancer. With the development of immunotherapy, many advanced NSCLC patients have benefited from first-line therapy of immune checkpoint inhibitors (ICIs) (3). However, the overall prognosis for NSCLC remains poor, partly due to limited options being available after progression on first-line therapy (4).
Administration of ICIs in second-line therapy has improved 5-year overall survival (OS) from 2.6% with chemotherapy alone to 13.6% with the use of nivolumab (5). However, ICIs only benefit about one fourth of second-line patients with NSCLC, challenges remain in optimizing treatment efficacy. Combining antiangiogenic drugs with ICIs for second- or later-line therapy has demonstrated potential by inhibiting angiogenesis and improving the tumor microenvironment. This is achieved through the normalization of tumor vasculature, reduction of tumor hypoxia, and mitigation of immunosuppression (6). Monoclonal antibodies like bevacizumab have demonstrated the ability to improve progression-free survival (PFS) in NSCLC patients when used alongside ICIs (7). Additionally, multi-targeted tyrosine kinase inhibitors (multi-TKIs), which block various angiogenesis-related pathways including VEGF/VEGFR, PDGF/PDGFR, FGF/FGFR, and c-kit, have been extensively evaluated. In a notable study by Zhang et al., the combination of ICIs and anlotinib showed a significantly longer PFS compared to ICI monotherapy in the second-line treatment of advanced NSCLC (8). Oral multi-TKIs, such as Anlotinib, has been accepted as a standard third-line treatment for both NSCLC and small cell lung cancer (SCLC) in China, due to its efficacy and easy to use (9). Ongoing research continues to investigate the efficacy and safety of combining ICIs with multi-TKIs for NSCLC patients who have progressed after first-line treatment, both in real-world settings and through prospective studies.
In this systematic review, we aim to provide a comprehensive overview of the second or later line treatment of NSCLC using the combination of ICIs and multi-TKIs. By doing so, we hope to provide clinicians and researchers with evidence to support their decision-making, and highlight future directions and challenges in this evolving field of cancer treatment. We present this article in accordance with the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1204/rc) (10).
Methods
This systematic review has been registered with PROSPERO (ID: CRD42024534887, https://www.crd.york.ac.uk/prospero/).
Search strategy
We systematically searched PubMed, Medline, Embase and ClinicalTrials.gov on March 10, 2024 to identify relevant studies evaluating ICIs plus multi-TKIs as second or later line therapy for NSCLC. We constructed the search strategy for each database using the following keywords “lung neoplasms”, “immune checkpoint inhibitor” and “multi-targeted kinase inhibitor”. The detailed search strategy and its results are supplied in Table S1.
Selection criteria
Studies would be taken into consideration if they fulfill the following inclusion criteria: (I) patients were histologically diagnosed as NSCLC and had progressed on first-line treatment; (II) taking combination of ICIs and multi-TKIs (anlotinib, apatinib, fruquintinib, sorafenib, lenvatinib, lenvatinib, cediranib, vandetanib, vandetanib, nintedanib, pazopanib, axitinib) as the primary intervention for the second or later line therapy; (III) studies that provided detailed outcomes on efficacy and safety. The studies would be excluded if they: (I) included first-line treatment patients; (II) fell into other types of publications, including cellular or animal experiments, preclinical trials, case report or case series, reviews, and abstracts; we thoroughly reviewed the relevant systematic reviews to ensure comprehensive inclusion of pertinent studies; (III) failed to provide relevant outcome results for further analysis. Two reviewers (W.X. and X.L.) independently screened the titles and abstracts followed by full text reading to select the eligible studies. Conflicts were resolved by the third independent reviewer (K.W.).
Data extraction and quality assessment
Data extraction included information such as first author, publication year, country, inclusion and exclusion criteria of the underlying population, histology, age, proportion of men, therapy line, trial design, intervention and controls, and outcomes. The quality assessment was conducted using the tool developed by the Canadian Institute of Health Economics (IHE) for the case series (11). Studies scoring 14 or higher were deemed to be acceptable (12).
Outcome measures
Median PFS (mPFS) refers to the median length of time from the start of treatment until the disease progresses or the patient dies from any cause, whichever occurs first. Median overall survival (mOS) is the median length of time from the start of treatment until death from any cause. Objective response rate (ORR) is the proportion of patients who have achieved either a complete response (CR) or partial response (PR) to the treatment. Disease control rate (DCR) is the proportion of patients who have achieved CR, PR, or stable disease (SD) after the treatment. The primary objective of this study was to evaluate the efficacy of the combined treatment based on mPFS, mOS, ORR, and DCR. Additionally, we aimed to assess the safety of the combined treatment by analyzing the adverse events reported in the included studies.
Statistical analysis
We conducted a systematic review for the enrolled studies. For the outcome that provided effects and their 95% confidence intervals (CIs), we performed a single-arm meta-analysis to combine the effects among intervention groups using a random effects model due to the substantial heterogeneity across the included studies. Forest plots were used to visually display the pooled estimates. As part of sensitivity analyses, we excluded retrospective studies. The analysis was carried out using Stata 17 statistical software (Stata Corp, USA).
Results
Description of studies
Overall, we found 3,024 citations, 301 of which were discarded as duplicates. From the remaining records, we identified 155 potentially relevant citations. Thirteen were excluded as they primarily focused on SCLC. Out of the 142 citations subjected to full-text screening, 69 were excluded due to their publication types, such as conference abstracts, letters, and reviews; 23 were excluded for incomplete data or mixed with first-line therapy; 30 were excluded for duplicate data. In the end, our review included 20 studies meeting the eligibility criteria (Figure 1).
Nineteen studies were conducted in China, with the remaining one carried out in Russia (13) (Table 1). All 20 studies focused on NSCLC patients who had experienced progression following initial therapy, which included either platinum-based chemotherapy or EGFR/ALK/ROS1 TKIs. Among these, four studies specifically included patients with EGFR/ALK/ROS1 common mutations (14-17), one with EGFR rare mutation (19), two exclusively involved lung adenocarcinoma patients (20,21), and one solely included those with squamous lung cancer (22). 11 of the studies limited participant inclusion to second-line therapy, while the remaining nine also encompassed later-line therapies. Thirteen studies provided reports on programmed cell death-ligand 1 (PD-L1) expression levels.
Table 1
| Study | Country | Main inclusion and/or exclusion criteria | Age (yrs) | Male (%) | Patient counts | Therapy line | Study type |
|---|---|---|---|---|---|---|---|
| Zhang, 2023 (8) | China | Inclusion: NSCLC with wild-type EGFR/ALK; received at least one line of systemic therapy or could not tolerate chemotherapy | 60.1 | 69.1 | 101 | 2nd | Prospective, two-arm |
| Exclusion: previous line of treatment containing anlotinib or ICIs | |||||||
| Galffy, 2023 (13) | Russia | Inclusion: NSCLC received at least one prior chemotherapy | 64 | 73.2 | 41 | ≥2nd | Prospective, single-arm |
| Exclusion: EGFR\ALK/ROS1 mutation; prior ICI therapy | |||||||
| Gao, 2022 (14) | China | Inclusion: NSCLC harboring EGFR/ALK mutation with disease progression after at least one prior platinum-based chemotherapy | 55 | 58.1 | 43 | ≥2nd | Prospective, single-arm |
| Exclusion: autoimmune disease; use of immunosuppressive agents; prior treatment with immunotherapy | |||||||
| Zhang, 2023 (15) | China | Inclusion: EGFR-mutant NSCLC with disease progression after first-line treatment | 51 | 31.6 | 19 | ≥2nd | Prospective, two-arm |
| Exclusion: autoimmune disease history; prior immunotherapy | |||||||
| Yu, 2023 (16) | China | Inclusion: lung adenocarcinoma harbored sensitive EGFR mutation and had disease progression after EGFR-TKI treatment | 63 | 47.4 | 80 | 2nd | Retrospective, two-arm |
| Chen, 2021 (17) | China | Inclusion: NSCLC with EGFR mutation progressed after EGFR-TKI and chemotherapy | 62 | 55.8 | 86 | ≥2nd | Retrospective, two-arm |
| Yu, 2022 (18) | China | Inclusion: local advanced NSCLC with disease progression after standard treatment | 62 | 61.4 | 57 | ≥2nd | Retrospective, single-arm |
| Chen, 2023 (19) | China | Inclusion: treatment-experienced advanced NSCLC with uncommon EGFR mutation | 61 | 66.7 | 21 | ≥2nd | Prospective, single-arm |
| Exclusion: components of small cell carcinoma, central nervous system metastases, risk of hemorrhage, prior immunotherapy | |||||||
| Yao, 2023 (20) | China | Inclusion: lung adenocarcinoma with relapse or failure to 1st-line chemotherapy; negative mutation in EGFR/ALK/ROS1 | 56 | 44.8 | 29 | ≥2nd | Prospective, single-arm |
| Exclusion: brain or meningeal metastases; not suitable for ICIs due to prior drug history | |||||||
| Yu, 2023 (21) | China | Inclusion: mutation negative lung adenocarcinoma with disease progression after prior standard therapy | 63 | 71.8 | 134 | ≥2nd | Retrospective, two-arm |
| Exclusion: prior usage of ICIs | |||||||
| Gao, 2022 (22) | China | Inclusion: non-central squamous NSCLC with disease progression after prior first-line chemotherapy | 63 | 92 | 25 | ≥2nd | Prospective, single-arm |
| Exclusion: autoimmune disease or usage of immunosuppressive agents; previous ICIs users; major blood vessel invasion; intratumor cavitation or necrosis | |||||||
| Zhu, 2022 (23) | China | Inclusion: NSCLC with disease progression after at least 2-line treatment | 65.1 | 65.9 | 82 | ≥3rd | Prospective, single-arm |
| Exclusion: combined with other types of tumors | |||||||
| Zhang, 2021 (24) | China | Inclusion: relapsed NSCLC who received second- or later-line treatment of anlotinib and/or PD-1 inhibitor | 59 | 67.7 | 103 | ≥2nd | Retrospective, two-arm |
| Zhang, 2021 (25) | China | Inclusion: NSCLC aged 18-85 | 69 | 59 | 139 | 3rd | Retrospective, two-arm |
| Exclusion: SCLC; follow-up duration less than 4 weeks, uncontrollable adverse reactions or toxicity | |||||||
| Yang, 2020 (26) | China | Inclusion: NSCLC patients treated with two-line platinum containing dual drug chemotherapy before | 59 | 58.4 | 101 | 3rd | Retrospective, single-arm |
| Exclusion: with targeted mutation | |||||||
| Zhai, 2020 (27) | China | Inclusion: NSCLC, taken ICIs plus TKIs for 3rd-line or further lines; normal organ function | 65 | 63.6 | 22 | ≥3rd | Prospective, single-arm |
| Exclusion: history of autoimmune diseases or taken immunosuppressive drugs; not squamous-NSCLC | |||||||
| Wang, 2021 (28) | China | Inclusion: NSCLC experienced progression after 1 systemic treatment | 60 | 70 | 67 | ≥2nd | Retrospective, single-arm |
| Zhou, 2021 (29) | China | Inclusion: NSCLC with disease progression after at least 1-line treatment | 62 | 72.5 | 45 | ≥2nd | Prospective, single-arm |
| Exclusion: SCLC, hemoptysis, symptomatic brain metastasis; central cavity of squamous NSCLC; active primary immunodeficiency | |||||||
| Zhou, 2021 (30) | China | Inclusion: NSCLC with disease progression after at least one prior platinum-based chemotherapy | 58 | 75.2 | 105 | ≥2nd | Prospective, single-arm |
| Exclusion: EGFR/ALK mutation; prior treatment with immunotherapy | |||||||
| Yuan, 2023 (31) | China | Inclusion: NSCLC received ICIs plus TKIs as 2nd-line treatment | 51 | 30 | 40 | ≥2nd | Retrospective, single-arm |
| Exclusion: mixed tumors of small cell; history of hemoptysis; prior usage of multi-TKIs or ICIs |
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; ICIs, immune checkpoint inhibitors; NSCLC, non-small cell lung cancer; PD-1, programmed death receptor-1; ROS1, ROS proto-oncogene 1; SCLC, small cell lung cancer.
Ten studies were prospectively designed, of which two were randomized controlled trials (RCTs) (8,24). The remaining 10 studies were retrospective cohorts. In 18 out of the 20 studies, anti-PD-1 antibodies were utilized as ICIs for combination therapy, while the remaining two study employed anti-PD-L1 antibodies (TQB2450, avelumab). Anlotinib emerged as the most frequently utilized multi-TKI, featuring in the combination strategy in 14 studies, followed by apatinib in five studies. Only one study utilized axitinib as the anti-angiogenic drug (13). Comparison was established in only seven studies, which encompassed ICI monotherapy in four studies (8,17,20,24), anlotinib or apatinib monotherapy in two studies (23,25) and chemotherapy in one study (16).
Quality assessment and risk of bias in included studies
Using the IHE quality assessing tool (Table 2), two studies scored 14 (24), one RCT scored 19 (8), with the remaining all higher than 14. Data were collected from multi-center in six studies. No study reported mPFS of the previous treat-line. Nine studies excluded patients with prior usage of ICIs. Only four studies reported co-interventions. Blindness was performed only in one study. Eight studies reported follow-up loss. Ten studies used cox regression or stratification to take into consideration of confounding.
Table 2
| Study | Items | Score | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Q17 | Q18 | Q19 | Q20 | ||
| S. Yang, 2020 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 15 |
| C. Chai, 2020 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 17 |
| Y. Chen, 2021 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 15 |
| P. Wang, 2021 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 15 |
| X. Zhang, 2021 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 14 |
| W. Zhang, 2021 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 15 |
| N. Zhou, 2021 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| C. Zhou, 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 |
| Y. Zhu, 2022 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 15 |
| G. Gao, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 |
| C. Yu, 2022 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 14 |
| G. Gao, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 |
| G. Galffy, 2023 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 17 |
| S. Yuan, 2023 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 15 |
| K. Chen, 2023 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 16 |
| S. Zhang, 2023 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 |
| L. Yu, 2023 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
| Y. Yao, 2023 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 18 |
| W. Zhang, 2023 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 19 |
| L. Yu, 2023 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
Q1: Was the hypothesis/aim/objective of the study clearly stated? Q2: Was the study conducted prospectively? Q3: Were the cases collected in more than one center? Q4: Were patients recruited consecutively? Q5: Were the characteristics of the patients included in the study described? Q6: Were the eligibility criteria (i.e. inclusion and exclusion criteria) for entry into the study clearly stated? Q7: Did patients enter the study at a similar point in the disease? Q8: Was the intervention of interest clearly described? Q9: Were additional interventions (co-interventions) clearly described? Q10: Were relevant outcome measures established a priori? Q11: Were outcome assessors blinded to the intervention that patients received? Q12: Were the relevant outcomes measured using appropriate objective/subjective methods? Q13: Were the relevant outcome measures made before and after the intervention? Q14: Were the statistical tests used to assess the relevant outcomes appropriate? Q15: Was follow-up long enough for important events and outcomes to occur? Q16: Were losses to follow-up reported? Q17: Did the study provide estimates of random variability in the data analysis of relevant outcomes? Q18: Were the adverse events reported? Q19: Were the conclusions of the study supported by the results? Q20: Were both competing interests and sources of support for the study reported? 1 point for a “yes” answer, 0 for an “unclear” or “no” answer. The checklist was cited from Institute of Health Economics (IHE). Quality Appraisal of Case Series Studies Checklist. Edmonton (AB): Institute of Health Economics; 2014. Available from: http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about (11).
Efficacy of the combined therapy
mPFS
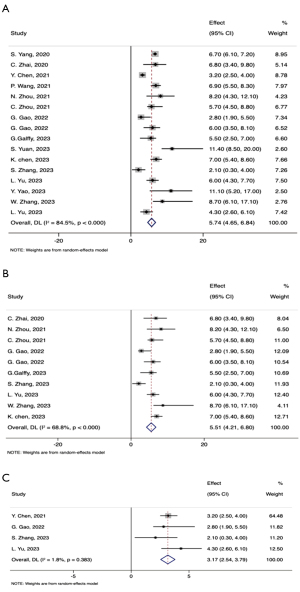
The mPFS of the combined therapy ranged from 2.10 to 11.10 months across studies. Among the 20 included studies, 16 reported the mPFS with its 95% CI (Table 3). Combining data from these 16 studies, the pooled mPFS for combination therapy was 5.74 months (95% CI: 4.65–6.84) (Figure 2A). When retrospective studies were excluded, the pooled mPFS slightly decreased to 5.51 months (95% CI: 4.21–6.80) (Figure 2B). Among the four studies specifically targeting patients with EGFR/ALK/ROS1 mutations, the pooled mPFS was 3.17 months (95% CI: 2.54–3.79) (Figure 2C). Three retrospective studies found that the mPFS for the combination therapy group demonstrated an absolute improvement in mPFS of 1.70 to 6.00 months compared to the monotherapy groups, with a reduction of hazard ratio (HR) approximately 34.0%. In Zhang’s RCT study, which made a direct comparison between ICI plus multi-TKI therapy and ICI monotherapy, the combined therapy demonstrated a significant improvement in mPFS, increasing it by nearly 6.00 months (8.70 months in combined therapy vs. 2.80 months in the ICI monotherapy).
Table 3
| Study | Histology | Treatment | Comparison | PD-L1 levels | EGFR/ALK+ (%) | mOS (months, 95% CI) | mPFS (months, 95% CI) | ORR (%) | DCR (%) | SAE |
|---|---|---|---|---|---|---|---|---|---|---|
| S. Yang, 2020 | NSCLC | Anlotinib + ICI (pembrolizumab, sintilimab, nivolumab, tislelizumab) | None | NA | 0 | NR | 6.7 (6.13–7.24) | 18.80 | 79.2 | 27/101 |
| C. Zhai, 2020 | non-sq-NSCLC | Anlotinib + ICI (nivolumab, pembrolizumab, sintilimab, toripalimab, camrelizumab) | None | High: 9.1%; Neg: 31.9%; unknown: 18.2% | 13.6 | 17.3 (16.1–18.5) | 6.8 (3.4–9.8) | 36.40 | 90.90 | 4/22 |
| Y. Chen, 2021 | EGFR/ALK+-NSCLC | Anlotinib+ pembrolizumab | Pembrolizumab | High: 30.2%; Neg: 19.8%; unknown: 12.8% | 100 | 12.28 (9.02–15.54) vs. 7.41 (P=0.02) |
3.24 (2.46–4.02) vs. 1.5 | 21.4 vs. 3.1 | NA | NA |
| P. Wang, 2021 | NSCLC | Anlotinib + ICI (pembrolizumab, nivolumab, camrelizumab, torpalimab, sintinimab, tislelizumab) | None | Pos: 6%; Neg: 7%; not reported: 87% | 13 | 14.5 (10.9–18.1) | 6.9 (5.5–8.3) | 28.40 | 86.6 | 27/67 |
| X. Zhang, 2021 | NSCLC | Anlotinib + ICI (pembrolizumab, toripalimab) | Pembrolizumab, toripalimab | Pos: 8.1% vs. 7.3%; Neg: 8.1% vs. 7.3%; unknown: 72.6% vs. 78.0% | 16.1 | NA | 8 vs. 2 (P=0.00) | 19.3 vs. 2.4 (P=0.013) | 85.5 vs. 58.5 (P=0.15) | NA |
| W. Zhang, 2021 | NSCLC | Anlotinib + ICI (pembrolizumab, sintilimab, toripalimab, camrelizumab) | Anlotinib | NA | 19.4 | 10.5 vs. 8.7 (P=0.033) | 5.8 vs. 4.2 [HR 0.68 (0.68–0.97)] | 20.5 vs. 18.2 | 84.9 vs. 71.2 | 13/73 vs. 10/66 |
| N. Zhou, 2021 | NSCLC | Anlotinib + camrelizumab | None | Pos: 25.5%; Neg: 9.8%; unknown: 64.7% | NA | 12.7 (10.2–15.1) | 8.2 (4.3–12.1) | 13.3 (95% CI: 3–23.7) | 82.2 (95% CI: 70.6–93.8) | 1/45 |
| C. Zhou, 2021 | NSCLC | Apatinib + camrelizumab | None | Pos: 2.9%; Neg: 62.9%; unknown: 13.3% | 0 | 15.5 (10.9–24.5) | 5.7 (4.5–8.8) | 30.9 (95% CI: 21.7–41.2) | 81.9 (95% CI: 72.6–89.1) | 73/104 |
| Y. Zhu, 2022 | NSCLC | Apatinib + sintilimab | Apatinib | NA | NA | NA | NA | 41.46 vs. 19.51, P=0.03 | 85.37 vs. 58.54, P=0.007 | NA |
| G. Gao, 2022 | EGFR/ALK+-NSCLC | Apatinib + camrelizumab | None | Pos: 51.2%; Neg: 30.2%; unknown: 18.6% | 100 | NR (7.3–NR) | 2.8 (1.9–5.5) | 18.6 (95% CI: 8.4–33.4) | NA | 28/43 |
| C. Yu, 2022 | NSCLC | Anlotinib + ICI (tislelizumab; toripalimab) | None | High: 23.2% | 14 | 14 | 10 | 50.9% | 87.7 | NA |
| G. Gao, 2022 | Sq-NSCLC | Apatinib + camrelizumab | None | Pos: 52%; Neg: 44%; unknown: 4% | NA | 13.3 (6.4–18.8) | 6.0 (3.5–8.1) | 32 (95% CI: 14.9–53.5) | 84 (95% CI: 63.9–95.5) | 21/25 |
| G. Galffy, 2023 | NSCLC | Axitinib + ICI (avelumab) | None | Pos: 19.5%; Neg: 58.5%; unknown: 22% | 0 | 21.3 (14.9–24.6) | 5.5 (2.5–7.0) | 31.7 (95% CI: 18.1–48.1) | 7.5 (95% CI: 3.7–15.5) | 24/41 |
| S. Yuan, 2023 | NSCLC | Anlotinib + ICI | None | NA | 32.5 | 27.0 (12–NR) | 11.4 (8.5–NR) | 40 (16/40) | 82.5 (33/40) | 1/40 |
| K. Chen, 2023 | EGFR+-NSCLC | Anlotinib + sintilimab | None | Pos: 33.3%; Neg: 38.1%; unknown: 28.6 % | 100 | 20.2 (15.6–24.4) | 7.0 (5.4–8.6) | 38.1 (95% CI: 18.1–61.6) | 85.7 (95% CI: 63.7–97) | 6/21 |
| S. Zhang, 2023 | EGFR+-NSCLC | Anlotinib + toripalimab | None | NA | 100 | NA | 2.1 (0.25–3.95) | 0 | 57.9 | 2/19 |
| L. Yu, 2023 | Ad-NSCLC | Anlotinib + ICI (pembrolizumab, nivolumab) | ICI (pembrolizumab, nivolumab) | High: 25.4%; Neg: 17.5%; unknown: 25.4% | 0 | 16.13 (10.48–21.79) vs. 11.88 (8.88–14.88), P=0.046 | 6 (4.34–7.66) vs. 3.41 (2.16–4.67), P<0.001 | 7 (5/71) vs. 3.2 (2/63), P=0.45 | 81.7 (58/71) vs. 57.1 (36/63), P=0.002 | NA |
| Y. Yao, 2023 | Ad-NSCLC | Apatinib + camrelizumab + chemotherapy | None | NA | 0 | NR | 11.1 (5.2–17) | 37.90 | 86.30 | 7/29 |
| W. Zhang, 2023 | NSCLC | TQB2450 + anlotinib | TQB2450 | Pos: 22.5% vs. 21.2%; Neg: 7.4% vs. 15.2%; unknown: 36.8% vs. 27.3% | 0 | NA | 8.7 (6.1–17.1) vs. 2.8 (1.4–4.7) | 30.9 (95% CI: 20.2–43.3) vs. 3.0 (95% CI: 36.4–71.9) | 73.5 (95% CI: 61.4–83.5) vs. 64.5 (95% CI: 36.4–71.9) |
41/68 vs. 3/33 |
| L. Yu, 2023 | Ad-NSCLC | Anlotinib + ICI (pembrolizumab, nivolumab) | Chemotherapy | NA | 100 | 14.17 (10.17–18.17) vs. 9.00 (6.92–11.08) | 4.33 (2.62–6.05) vs. 3.60 (2.48–4.73) | 92.10 | 18.40 | NA |
ALK, anaplastic lymphoma kinase; Ad-NSCLC, adenocarcinoma non-small cell lung cancer; CI, confidence interval; EGFR, epidermal growth factor receptor; ICIs, immune checkpoint inhibitors; NA, not available; NR, not reached; sq-NSCLC, squamous non-small cell lung cancer.
mOS
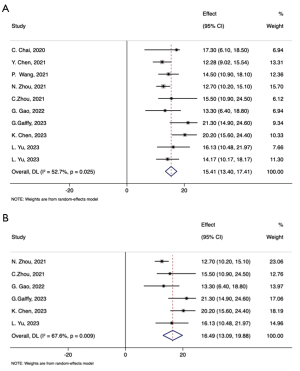
16 studies provided OS among patients who received the combined therapy as second or late-line therapy (Table 3). Among these, 12 studies presented mOS (95% CI), which ranged from 12.30 to 27.00 months. Pooling the mOS data from these 12 studies resulted in a combined mOS of 15.41 months (95% CI: 13.40–17.41) (Figure 3A). When considering only prospective studies, the pooled mOS was slightly higher at 16.49 months (95% CI: 13.09–19.88) (Figure 3B). Additionally, three studies reported the 1-year OS rate, which varied from 40.4% to 81.8%. For patients who progressed on EGFR/ALK TKI and one platinum-based chemotherapy, the mOS ranged from 12.30 to 20.20 months.
ORR and DCR
All studies included in the analysis reported ORR of the combined therapy, which ranged from 0 to 41.5% (Table 3). Notably, Zhang’s study (15), focusing on combined therapy for patients with EGFR-mutant tumors, found that none of the 19 patients achieved an ORR. When considering five studies that provided ORR along with its 95% CI, the combined ORR was calculated to be 26.35% (95% CI: 19.52–33.18%) (Figure 4A).
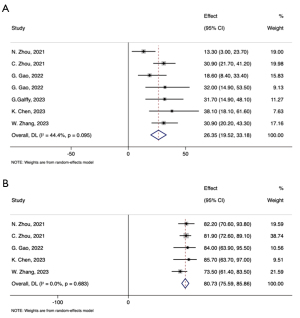
DCR data of the combined therapy were available from 18 studies, with the lowest DCR observed in Zhang’s study (15) at 57.9% for EGFR-mutant patients. DCR in the other 17 studies ranged from 73.5% to 92.1%. From the subset of five studies providing DCR with corresponding 95% CIs, the pooled DCR was determined to be 80.73% (95% CI: 75.59–85.86%) (Figure 4B).
Two RCTs facilitated direct comparisons between combined therapy and monotherapy. Y. Zhu’s study showed that combined therapy achieved an ORR of 41.5% and a DCR of 85.4%, whereas apatinib monotherapy yielded lower rates of ORR and DCR at 19.5% and 58.54%, respectively. In another RCT by Zhang (8), combined therapy was compared to ICI monotherapy, revealing higher ORR (30.9%) and DCR (73.5%) compared to monotherapy’s rates of 3.0% and 64.5%, respectively.
Adverse events of the combined therapy
The proportion of adverse events with grade 3 severe adverse events (SAE) or higher was reported by 16 studies (Table 4). Hypertension was reported in 12 out of the 16 studies. The prevalence ranged from 2.5% in Yuan’s study (31) to 44.0% in Gao’s study (21). The other top four common SAEs include Fatigue (2.4–5.9%), hepatic dysfunction (3.4–9.5%), urine abnormal (2.0–8.0%) and hand-foot syndrome (1.4–10.0%). Other less common SAEs included rash, pneumonitis, diarrhea, bone marrow suppression, mouth ulceration, cerebral infarction, hypothyroidism, changed appetite and palmar-plantar erythrodysesthesia syndrome. No treatment-related death was reported in the studies.
Table 4
| Adverse events | S. Yang, 2020 | C. Zhai, 2020 | P. Wang, 2021 | W. Zhang, 2021 | N. Zhou, 2021 | C. Zhou, 2021 | Y. Zhu, 2022 | G. Gao, 2022 | G. Gao, 2022 | G. Galffy, 2023 | S. Yuan, 2023 | K. Chen, 2023 | S. Zhang, 2023 | L. Yu, 2023 | Y. Yao, 2023 | W. Zhang, 2023 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension | 9 (8.9) | 2 (9.1) | 12 (18) | 3 (4.1) | 1 (3.7) | 19 (18.1) | 7 (16.5) | 11 (44) | 7 (17.1) | 1 (2.5) | 1 (5) | 12 (19.1) | ||||
| Fatigue | 4 (5.5) | 1 (2.4) | 2 (4.9) | 1 (4.8) | 1 (3.4) | 4 (5.9) | ||||||||||
| Hepatic dysfunction | 10 (9.5) | 2 (4.7) | 2 (8) | 2 (4.9) | 1 (3.4) | 6 (8.8) | ||||||||||
| Urine abnormal | 2 (2.0) | 8 (7.6) | 5 (11.6) | 2 (8) | 1 (3.4) | |||||||||||
| Hand-foot syndrome | 10 (10) | 3 (4.0) | 1 (1.4) | 2 (9.5) | 3 (4.4) | |||||||||||
| Rash | 1 (4.6) | 3 (4.1) | 2 (4.7) | 2 (6.9) | ||||||||||||
| Pneumonitis | 2 (2.0) | 1 (4.6) | 1 (4.8) | 3 (4.2) | ||||||||||||
| Diarrhea | 1 (4.6) | 4 (6.0) | 2 (4.9) | |||||||||||||
| Bone marrow suppression | 1 (1.4) | 1 (2.4) | 4 (16) | |||||||||||||
| Mouth ulceration | 2 (9.1) | |||||||||||||||
| Cerebral infarction | 1 (4.8) | (1.4) | ||||||||||||||
| Hypothyroidism | 4 (6.0) | 1 (3.4) | ||||||||||||||
| Changed appetite | 2 (2.0) | 1 (2.4) | 2 (4.9) | 3 (4.4) | ||||||||||||
| Palmar-plantar erythrodysesthesia syndrome | 14 (13.3) | 4 (9.3) | 4 (16) |
Data are presented as n (%).
Discussion
In this systematic review, we summarize the efficacy and safety of combining ICIs with multi-TKIs as a second- or later-line therapy for NSCLC. Evidence from 20 prospective or retrospective cohort studies suggests that the pooled mPFS and mOS for this combination therapy are approximately 5.7 and 15.4 months, respectively. The ORR reached 26.5%, while the DCR was about 80%. Common SAEs included hypertension, fatigue, abnormal hepatic function, urinary abnormalities, and hand-foot syndrome.
Second- or later-line treatments for NSCLC are highly individualized. Previous studies involving chemotherapy combined with anti-angiogenic drugs as second-line therapy reported mPFS of 3.40–4.50 months and mOS of 10.50–12.60 months (32-34). To minimize chemotherapy-related side effects, ICIs combined with anti-angiogenic drugs offer a promising alternative. A previous systematic review showed that a combination of ICIs and monoclonal anti-vascular antibodies or multi-TKIs achieved a PFS of 5.20 months and an OS of 14.09 months, suggesting comparable efficacy to chemotherapy-based regimens (35). This approach may be particularly suitable for older patients, those with poor physical status, or those unwilling to undergo chemotherapy.
Multi-TKIs, such as anlotinib and apatinib, have emerged as viable options for later-line NSCLC therapy due to their convenience in oral administration and high safety profiles (36). Anlotinib, noted for its potent anti-angiogenic properties, was the most commonly used multi-TKI in the studies reviewed, surpassing other agents like sunitinib, sorafenib, and nintedanib (37). Clinical trials have explored anlotinib for both second- and first-line therapy in advanced NSCLC patients (34,38). Our analysis revealed that the efficacy of multi-TKI plus ICI regimens in terms of mPFS, mOS, ORR, and DCR was comparable to that of ICI plus angiogenetic monoclonal antibodies, such as ramucirumab and bevacizumab. Multi-TKIs offer several advantages in second- or later-line therapy. First, they have a synergistic effect with ICIs by promoting vascular normalization and altering the tumor microenvironment (32,39). Second, multi-TKIs may benefit patients with primary resistance due to SCLC transformation, as they have shown efficacy in treating SCLC (40). Third, their oral administration facilitates easier dose management. Thus, the combination of ICIs and multi-TKIs presents a promising chemotherapy-free option for later-line NSCLC treatment.
Challenges remain in treating NSCLC patients with positive driver gene mutations, particularly those resistant to EGFR inhibitors (41). ICI monotherapy has demonstrated limited efficacy in extending OS for these patients, as shown in pooled analyses from trials like Checkmate-057, Keynote-010, and OAK (42,43). While combining platinum-based chemotherapy with ICIs and anti-angiogenesis agents has extended mPFS in this group, this comes at the cost of increased treatment-related adverse events (7,44). Thus, efforts have focused on chemotherapy-free strategies for second-line therapy. However, in patients with driver mutations, ICI and multi-TKI combinations yielded a pooled mPFS of only 3.7 months, with the lowest ORR reported in studies including patients with EGFR mutations who had progressed after EGFR-TKI therapy (15). These findings underscore the need for further optimization of treatment strategies for this subgroup.
Combining chemotherapy with ICIs is associated with a high risk of treatment-related complications. In contrast, the combination of ICIs and multi-TKIs, being chemotherapy-free, offers fewer side effects. While this review was not primarily focused on side effect analysis, the included studies offer valuable insights into the adverse events associated with the combination therapy. Most severe adverse events (e.g., hypertension, fatigue, hepatic or renal dysfunction, hand-foot syndrome) were manageable with dose adjustments (45). Importantly, the addition of multi-TKIs did not exacerbate the toxicity commonly associated with ICIs, such as pneumonitis, rash, and hypothyroidism. These findings highlight the favorable safety profile of this combined regimen for later-line NSCLC patients.
Several limitations should be noted. First, most studies included in this review were conducted in China, and studies using other multi-TKIs, such as nintedanib and lenvatinib, were excluded due to premature data (abstract only) (46,47). A well-designed RCT directly comparing multi-TKI plus ICIs with ICI monotherapy across different ethnicities is still required before recommending this treatment strategy. Second, few studies directly compared combination therapy with monotherapy, making it difficult to determine whether adding ICIs to multi-TKIs is superior to multi-TKI therapy alone, especially in patients with prior ICI treatment. Third, the inclusion of retrospective studies may introduce selection bias, although these studies provide valuable insights before RCTs are available. Notably, only one RCT implemented blinding for radiological assessments, and only 10 studies used statistical adjustments to control for confounding factors. Fourth, none of the studies evaluated the efficacy of prior immunotherapy or included data on post-progression PD-L1 levels, limiting our ability to assess the impact of prior ICI use on treatment efficacy. In this situation, future studies with suitable controls and satisfied study design are needed, even in the form of retrospective research.
Conclusions
Our review highlights the potential of ICIs combined with multi-TKIs as an alternative chemotherapy-free regimen for second- and later-line NSCLC treatment. Further investigation is needed, particularly in patients with prior ICI or EGFR inhibitor treatment, to optimize this therapeutic strategy.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1204/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1204/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1204/coif). All authors report that this work was supported by Shanghai Science and Technology Innovation Action Plan (22Y11901100), and The Top-level Clinical Discipline Project of Shanghai Pudong (PWYgf2021-05). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen C, Zhang S, Yang T, et al. Associations between environmental heavy metals exposure and preserved ratio impaired spirometry in the U.S. adults. Environ Sci Pollut Res Int 2023;30:108274-87. [Crossref] [PubMed]
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Bourreau C, Treps L, Faure S, et al. Therapeutic strategies for non-small cell lung cancer: Experimental models and emerging biomarkers to monitor drug efficacies. Pharmacol Ther 2023;242:108347. [Crossref] [PubMed]
- Auclin E, Benitez-Montanez J, Tagliamento M, et al. Second-line treatment outcomes after progression from first-line chemotherapy plus immunotherapy in patients with advanced non-small cell lung cancer. Lung Cancer 2023;178:116-22. [Crossref] [PubMed]
- Borghaei H, Gettinger S, Vokes EE, et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021;39:723-33. [Crossref] [PubMed]
- Song Y, Fu Y, Xie Q, et al. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol 2020;11:1956. [Crossref] [PubMed]
- Nogami N, Barlesi F, Socinski MA, et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups With EGFR Mutations or Metastases in the Liver or Brain. J Thorac Oncol 2022;17:309-23.
- Zhang W, Wang J, Wang Q, et al. A randomized double-blind trial of TQB2450 with or without anlotinib in pretreated driver-negative non-small cell lung cancer. Lung Cancer 2023;184:107353. [Crossref] [PubMed]
- Zhou M, Chen X, Zhang H, et al. China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond) 2019;39:36. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Institute of Health Economics (IHE). Quality Appraisal of Case Series Studies Checklist. Edmonton (AB): Institute of Health Economics; 2014. Available online: http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about
- Yao H, Chen X, Tan X. Efficacy and safety of apatinib in the treatment of osteosarcoma: a single-arm meta-analysis among Chinese patients. BMC Cancer 2021;21:449. [Crossref] [PubMed]
- Galffy G, Lugowska I, Poddubskaya EV, et al. A phase II open-label trial of avelumab plus axitinib in previously treated non-small-cell lung cancer or treatment-naïve, cisplatin-ineligible urothelial cancer. ESMO Open 2023;8:101173. [Crossref] [PubMed]
- Gao G, Ni J, Wang Y, et al. Efficacy and safety of camrelizumab plus apatinib in previously treated patients with advanced non-small cell lung cancer harboring EGFR or ALK genetic aberration. Transl Lung Cancer Res 2022;11:964-74. [Crossref] [PubMed]
- Zhang S, Yang L, Yang Y, et al. The efficacy and safety of chemo-free therapy in epidermal growth factor receptor tyrosine kinase inhibitor-resistant advanced non-small cell lung cancer: A single-arm, phase II study. Cancer Med 2023;12:19438-48. [Crossref] [PubMed]
- Yu L, Hu Y, Xu J, et al. Multi-target angiogenesis inhibitor combined with PD-1 inhibitors may benefit advanced non-small cell lung cancer patients in late line after failure of EGFR-TKI therapy. Int J Cancer 2023;153:635-43. [Crossref] [PubMed]
- Chen Y, Yang Z, Wang Y, et al. Pembrolizumab Plus Chemotherapy or Anlotinib vs. Pembrolizumab Alone in Patients With Previously Treated EGFR-Mutant NSCLC. Front Oncol 2021;11:671228. [Crossref] [PubMed]
- Yu C, Jiang L, Yang D, et al. Anlotinib Hydrochloride and PD-1 Blockade as a Salvage Second-Line Treatment in Patients with Progress of Local Advanced Non-Small Cell Lung Cancer in Half a Year After Standard Treatment. Onco Targets Ther 2022;15:1221-8. [Crossref] [PubMed]
- Chen K, Xu Y, Huang Z, et al. Sintilimab plus anlotinib as second- or third-line therapy in metastatic non-small cell lung cancer with uncommon epidermal growth factor receptor mutations: A prospective, single-arm, phase II trial. Cancer Med 2023;12:19460-70. [Crossref] [PubMed]
- Yao Y, Wang Y, Du Y, et al. Efficacy and safety of second-line camrelizumab combined with apatinib and chemotherapy in patients with advanced lung adenocarcinoma: A prospective, open-label, multicentric study. Int Immunopharmacol 2023;125:111147. [Crossref] [PubMed]
- Yu L, Xu J, Qiao R, et al. Comparative efficacy and safety of multitarget angiogenesis inhibitor combined with immune checkpoint inhibitor and nivolumab monotherapy as second-line or beyond for advanced lung adenocarcinoma in driver-negative patients: a retrospective comparative cohort study. Transl Lung Cancer Res 2023;12:1108-21. [Crossref] [PubMed]
- Gao G, Zhao J, Ren S, et al. Efficacy and safety of camrelizumab plus apatinib as second-line treatment for advanced squamous non-small cell lung cancer. Ann Transl Med 2022;10:441. [Crossref] [PubMed]
- Zhu Y, Mei D, Qian S, et al. Efficacy of sintilimab combined with apatinib in treatment of advanced non-small cell lung cancer. Cancer Research and Clinic 2022;34:898-902.
- Zhang X, Zeng L, Li Y, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother 2021;70:2517-28. [Crossref] [PubMed]
- Zhang W, Zhang C, Yang S, et al. Immune checkpoint inhibitors plus anlotinib versus anlotinib alone as third-line treatment in advanced non-small-cell lung cancer: a retrospective study. Future Oncol 2021;17:4091-9. [Crossref] [PubMed]
- Yang S, Zhang W, Chen Q, et al. Clinical Investigation of the Efficacy and Safety of Anlotinib with Immunotherapy in Advanced Non-Small Cell Lung Cancer as Third-Line Therapy: A Retrospective Study. Cancer Manag Res 2020;12:10333-40. [Crossref] [PubMed]
- Zhai C, Zhang X, Ren L, et al. The Efficacy and Safety of Anlotinib Combined With PD-1 Antibody for Third-Line or Further-Line Treatment of Patients With Advanced Non-Small-Cell Lung Cancer. Front Oncol 2020;10:619010. [Crossref] [PubMed]
- Wang P, Fang X, Yin T, et al. Efficacy and Safety of Anti-PD-1 Plus Anlotinib in Patients With Advanced Non-Small-Cell Lung Cancer After Previous Systemic Treatment Failure-A Retrospective Study. Front Oncol 2021;11:628124. [Crossref] [PubMed]
- Zhou N, Jiang M, Li T, et al. Anlotinib combined with anti-PD-1 antibody, camrelizumab for advanced NSCLCs after multiple lines treatment: An open-label, dose escalation and expansion study. Lung Cancer 2021;160:111-7. [Crossref] [PubMed]
- Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. [Crossref] [PubMed]
- Yuan S, Peng L, Liu Y, et al. Low-dose anlotinib confers improved survival in combination with immune checkpoint inhibitor in advanced non-small cell lung cancer patients. Cancer Immunol Immunother 2023;72:437-48. [Crossref] [PubMed]
- Remon J, Lacas B, Herbst R, et al. ANtiangiogenic Second-line Lung cancer Meta-Analysis on individual patient data in non-small cell lung cancer: ANSELMA. Eur J Cancer 2022;166:112-25. [Crossref] [PubMed]
- Takeda M, Yamanaka T, Seto T, et al. Bevacizumab beyond disease progression after first-line treatment with bevacizumab plus chemotherapy in advanced nonsquamous non-small cell lung cancer (West Japan Oncology Group 5910L): An open-label, randomized, phase 2 trial. Cancer 2016;122:1050-9. [Crossref] [PubMed]
- Pu X, Xiao Z, Li J, et al. Anlotinib plus docetaxel vs. docetaxel alone for advanced non-small-cell lung cancer patients who failed first-line treatment: A multicenter, randomized phase II trial. Lung Cancer 2024;191:107538. [Crossref] [PubMed]
- Chen S, Mo W, Jiang W, et al. The benefit and risk of PD-1/PD-L1 inhibitors plus anti-angiogenic agents as second or later-line treatment for patients with advanced non-small-cell lung cancer: a systematic review and single-arm meta-analysis of prospective clinical trials. Front Immunol 2023;14:1218258. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Yang Q, Li Q, Fan H. Antitumor activity of anlotinib in malignant melanoma: modulation of angiogenesis and vasculogenic mimicry. Arch Dermatol Res 2024;316:447. [Crossref] [PubMed]
- Chu T, Zhong R, Zhong H, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol 2021;16:643-52. [Crossref] [PubMed]
- Lee WS, Yang H, Chon HJ, et al. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475-85. [Crossref] [PubMed]
- Nabipur L, Mouawad M, Venketaraman V. Therapeutic Applications of Programmed Death Ligand 1 Inhibitors in Small Cell Lung Cancer. Biomedicines 2025;13:401. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J Thorac Oncol 2021;16:1718-32. [Crossref] [PubMed]
- Mazieres J, Rittmeyer A, Gadgeel S, et al. Atezolizumab Versus Docetaxel in Pretreated Patients With NSCLC: Final Results From the Randomized Phase 2 POPLAR and Phase 3 OAK Clinical Trials. J Thorac Oncol 2021;16:140-50. [Crossref] [PubMed]
- Lu S, Wu L, Jian H, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med 2023;11:624-36. [Crossref] [PubMed]
- Zheng Y, Dong H, Yu Y, et al. Treatment-related adverse events of immune checkpoint inhibitors combined with angiogenesis inhibitors in advanced lung cancer: A systematic review and meta-analysis. Int Immunopharmacol 2023;123:110785. [Crossref] [PubMed]
- Leighl N, Paz-Ares L, Abreu DR, et al. 65O Phase III LEAP-008 study of lenvatinib plus pembrolizumab versus docetaxel for metastatic non-small cell lung cancer (NSCLC) that progressed on a PD-(L)1 inhibitor and platinum-containing chemotherapy. Immuno-Oncology and Technology. 2023;20:100537.
- Sebastian M, Sadjadian P, Waller C, et al. Nintedanib in combination with nivolumab in pretreated patients with advanced adenocarcinoma of the lung (AIO-TRK-0117 Phase Ib/II trial). Oncol Res Treat 2022;45:164.



