
Radial endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) enhances diagnostic yield in pulmonary nodule biopsy
Highlight box
Key findings
• Adding transbronchial needle aspiration (TBNA) to conventional radial endobronchial ultrasound (rEBUS) guided transbronchial lung biopsy (TBLB) enhances diagnostic yield for unfavorable bronchus signs.
What is known and what is new?
• The diagnostic yield of rEBUS TBLB is higher for pulmonary lesions >2 cm with Type Ia/Ib bronchus signs and a concentric rEBUS view. However, accuracy drops to <60% for nodules <2 cm with unfavorable bronchus signs and adjacent or eccentric rEBUS views.
• Adding TBNA to conventional rEBUS-TBLB may improve the diagnostic yield (66.7%) for pulmonary nodules with adjacent or tunnel bronchi. It could be the ancillary method to improve diagnostic yields.
What is the implication, and what should change now?
• Combining rEBUS with TBLB-TBNA presents a practical approach to enhancing the diagnostic yield for specific pulmonary nodules when robotic assisted bronchoscopy is not readily available.
Introduction
Early diagnosis of lung cancer is fundamental for achieving curative outcomes and reducing the risk of recurrence or disease progression. However, identifying early stage lung cancers remains challenging because these lesions are often smaller and located peripherally. Over the past few decades, various advanced diagnostic tools have been developed, including radial endobronchial ultrasound (rEBUS)-guided transbronchial lung biopsy (TBLB), transbronchial needle aspiration (TBNA), electromagnetic navigation bronchoscopy (ENB), cryobiopsy, and robot-assisted bronchoscopy.
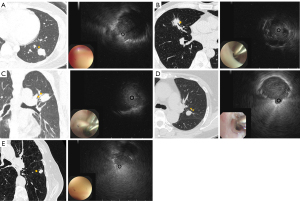
A 2010 meta-analysis by Steinfort et al. reported that the diagnostic yield of rEBUS-guided TBLB was 56.3% for lung lesions ≤2 cm and 77.7% for lesions >2 cm (1). Similarly, the AQuIRE Registry later demonstrated a diagnostic yield of 57.0% for rEBUS-guided TBLB (2). Key factors associated with improved diagnostic yield using rEBUS-guided biopsy include lesion size >2 cm, the presence of a direct bronchus sign on computed tomography (CT), and a concentric rEBUS view (2-4). Cryobiopsy may be particularly valuable for lesions with eccentric or adjacent rEBUS views (5-7). In addition to the traditional classification of within- or adjacent-bronchus signs, Imabayashi et al. introduced a more sophisticated classification of bronchus signs and showed that the diagnostic performance differed depending on the CT-bronchus subclassification (8). As shown in Figure 1, bronchial patterns were classified based on their interaction with the target lesion. Type Ia, Ib, and Ic referred to bronchi that directly reached the lesion and were obstructed, narrowed, or penetrating, respectively. Type IIa exhibited a compressed bronchus at the lesion’s edge, while Type IIb presented an untraceable bronchus, with the pulmonary artery leading to the target lesion. Lastly, in Type IIc, there was no leading bronchus or pulmonary artery to the target lesion. According to this classification, lesions adjacent (IIa) to or surrounding the bronchus (Ic) were associated with relatively lower diagnostic yields. This limitation is primarily due to the inability of forceps biopsy to penetrate the bronchus wall and adequately reach the target lesion, resulting in insufficient tissue acquisition.
To address the diagnostic challenges of lesions located near the bronchus, as indicated in previous studies, TBNA can be adopted to access these target areas (9,10). For this purpose, a newly introduced 21 g needle PeriView FLEX needle (Olympus, Tokyo, Japan, 2018), designed with a spiral-cut flexible tip and a 1.5 mm outer diameter, was employed. This needle demonstrated seamless compatibility with thin or ultrathin bronchoscopes, with or without the use of Olympus guide sheaths. Previous studies have identified the 21 g PeriView FLEX needle as a valuable ancillary diagnostic tool for lung lesions (9-11), highlighting its potential to enhance diagnostic outcomes. However, most studies have presented more information on its clinical availability and did not focus on specific cases in which the lesions were located outside the bronchus.
In this study, we investigated the clinical effectiveness of combining rEBUS-guided TBNA and TBLB as an alternative diagnostic strategy, particularly in challenging cases where forceps biopsy is unsuitable, and evaluated the safety profile of this combined approach. We present this article in accordance with the STARD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-68/rc).
Methods
Patients and data collection
We retrospectively reviewed data from all patients who underwent combined rEBUS-guided TBNA and TBLB at the Samsung Medical Center over a one-year period, from September 2023 to August 2024. The Samsung Medical Center, a 1,979-bed tertiary care referral hospital in Seoul, Korea, is the largest and most active center for bronchoscopic interventions in Korea.
As part of the procedure, chest CT scans were first analyzed to map the route from the trachea to the target lesion. If the bronchus leading to the target lesion either penetrated the nodule or was adjacent to it, we performed rEBUS-guided TBNA followed by TBLB to optimize diagnostic success. Additionally, TBNA was performed when the radial probe was located within the target lesion but no infiltrative endobronchial lesion was visible, suggesting a lower likelihood of diagnostic success.
After completing the procedures, we collected baseline data including patient demographics, tumor characteristics based on CT and bronchoscopy findings, procedural details, and clinical outcomes. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Review Board of the Samsung Medical Center (No. 2024-12-101), and the requirement for informed consent was waived because of its retrospective nature.
Bronchoscopic procedure
All patients underwent contrast-enhanced chest CT to evaluate lung lesions and identify the presence of the bronchus sign. Airway mapping was performed to determine the optimal pathway for the target lesion (12). Patients were admitted one day prior to the procedure for risk assessment and optimization. The procedure was conducted under conscious sedation with midazolam and fentanyl, with supplemental oxygen provided throughout the procedure. A flexible bronchoscope (BF-P260F/BF-P290, Olympus, Japan) was used in conjunction with an rEBUS probe (UM-S20-17), with or without a guide sheath (SG-200C), to locate the target lesion. TBNA was performed using a PeriView Flex aspiration needle (21G NA-403D), followed by TBLB with forceps (FB-233D) through the needle puncture site. The rEBUS probe was reintroduced between the TBNA and the TBLB to confirm that the guide sheath was correctly positioned at the target lesion. No ancillary tools, such as fluoroscopic guidance, navigation bronchoscopy, or MDCT, were used in our cohort.
Definitions of diagnostic yields
A strict diagnostic yield criterion was applied to assess the performance of rEBUS-guided TBLB and TBNA based on the Delphi Consensus Definition of Diagnostic Yield (13). Procedure success was determined based on predefined pathological classifications following the final pathology reports. Diagnostic success included positive identification of malignant cells in smears or confirmation of specific malignancies in tissue samples. Additionally, specific benign pathologies, such as granuloma, organizing pneumonia, fungal infections, or other infectious pathologies consistent with the clinical findings, were considered diagnostic. Nondiagnostic TBNA results were defined as cases in which aspiration smears or core samples showed only atypical cells, inflammatory cells, or blood cells, without definitive pathological findings.
Comparison for the diagnostic yields between TBLB alone vs. TBNA plus TBLB
To better evaluate the improved performance of TBNA followed by TBLB compared with TBLB alone, we employed propensity score matching to minimize selection bias. rEBUS-guided TBNA is typically performed in selected cases where TBLB alone is less likely to succeed. These cases often involve lesions adjacent to or surrounding the bronchus, and decisions are made at the physician’s discretion. We utilized our rEBUS registry, established in 2020, which included 736 patients as the control cohort. To minimize selection bias, we matched cases based on nodule size, CT bronchus sign, and rEBUS probe position within the lesion in a 1:3 ratio.
Statistical analysis
Results are presented as mean ± standard deviation (SD) for normally distributed variables and as median and interquartile range (IQR) for non-normally distributed variables. Categorical data were expressed as absolute numbers and percentages and compared using Pearson’s chi-square test or Fisher’s exact test. Propensity scores, representing the probability of undergoing TBNA given the observed covariates, were estimated using logistic regression. The TBNA-TBLB and TBLB-only groups were matched using 1:3 nearest-neighbor matching without replacement. The balance of covariates was assessed using standardized mean differences (SMD), with values below 0.25 indicating acceptable balance. Statistical significance was set at P<0.05. Statistical analyses were performed using R software (version 4.3.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
During the study period, 33 patients underwent combined rEBUS-guided TBNA and TBLB. The median age of the cohort was 68 years, with a male-to-female ratio of 1.2:1.0. Approximately 65% of the lesions were located in the outer two-thirds of the thorax with a median size of 16.75 mm. Most lesions were solid (81.8%), followed by consolidation (9.1%), part-solid (6.1%) and cavitary lesions (3%). In our cohort, 21.2% had type Ia and 15.2% had type Ib, classified as favorable bronchial patterns on CT. However, approximately two-thirds had unfavorable bronchial patterns, including type Ic (12.1%), IIa (45.5%), and IIb (6.1%). Representative cases of CT bronchus subclassification are shown in Figure 1. Nearly 52% of lung lesions exhibited an adjacent rEBUS view after probing with rEBUS. The median procedure times for rEBUS-guided TBNA and TBLB were 15 min (IQR, 10–18 min) and 7.0 min (IQR, 5–12 min), respectively. The number of needle passes was two in 42.4% and once in 24.2%. Eighty-two subjects who underwent TBLB alone were matched to the combined TBNA-TBLB cohort using propensity scoring at a 3:1 ratio. The matched TBLB-alone cohort had similar baseline characteristics to the combined TBNA-TBLB cohort. The median age was 65 years, with a female-to-male ratio of 0.9:1. Approximate 70% of the lesions were located in the outer two-thirds of the thorax, 79% were solid, and the median lesion size was 18.0 mm. Additionally, 39.4% had favorable bronchial types (Ia and Ib), while 60.6% had unfavorable bronchial types (Ic, IIa, and IIb). Detailed demographic data, pulmonary nodule characteristics, and procedural parameters are summarized in Tables 1,2.
Table 1
| Characteristic | Value |
|---|---|
| Pulmonary nodule characteristic | |
| Age, years | 68.0 (61.0–76.0) |
| Gender | |
| Male | 18 (54.5) |
| Female | 15 (45.5) |
| Pulmonary nodule location | |
| Left upper lobe | 11 (33.3) |
| Right lower lobe | 10 (30.3) |
| Right upper lobe | 6 (18.2) |
| Left lower lobe | 3 (9.1) |
| Right middle lobe | 3 (9.1) |
| Nodule location in thorax† | |
| Central | 12 (36.4) |
| Intermediate | 15 (45.5) |
| Peripheral | 6 (18.2) |
| Nodule size, mm | 16.75 (13.00–23.88) |
| Short axis | 14.0 (9.0–18.0) |
| Long axis | 19.0 (16.0–26.0) |
| Distance from pleural, mm | 14.0 (5.0–34.0) |
| CT characteristic of nodule | |
| Solid | 27 (81.8) |
| Consolidation | 3 (9.1) |
| Part solid | 2 (6.1) |
| Cavity | 1 (3.0) |
| CT bronchus sign | |
| Adjacent | 16 (48.5) |
| Within | 15 (45.5) |
| Invisible | 2 (6.1) |
| CT classification of bronchus type | |
| 1a | 7 (21.2) |
| 1b | 5 (15.2) |
| 1c | 4 (12.1) |
| 2a | 15 (45.5) |
| 2b | 2 (6.1) |
| Procedure related factors | |
| EBUS-TBLB procedural duration, minutes | 7.0 (5.0–12.0) |
| EBUS-TBNA procedural duration, minutes | 15.0 (10.0–18.0) |
| Sedation dose | |
| Midazolam, mg | 5.0 (4.0–6.0) |
| Fentanyl, mcg | 50.0 (40.0–70.0) |
| Radial EBUS sonographic classification | |
| Adjacent | 17 (51.5) |
| Within | 16 (48.5) |
| Number of needle pass | |
| 1 | 8 (24.2) |
| 2 | 14 (42.4) |
| 3 | 7 (21.2) |
| 4 | 3 (9.1) |
| 5 | 1 (3.0) |
Data are presented as n (%) or median (IQR). †, central: inner one-third of the lung from the hilum, intermediate: middle two-third of the lung from hilum, peripheral: outer one-third of the lung from the hilum. CT, computed tomography; EBUS, endobronchial ultrasound; IQR, interquartile range; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration.
Table 2
| Characteristics/outcome | TBLB (n=82)* | TBNA-TBLB (n=33) | SMD | P value |
|---|---|---|---|---|
| Age, years | 65.18±11.56 | 67.55±12.26 | 0.198 | 0.33 |
| Gender, male | 42.2 (51.5) | 18.0 (54.5) | 0.061 | 0.92 |
| Procedure time, mins | 14.47±8.29 | 25.33±9.40 | 1.226 | <0.001 |
| Midazolam, mg | 4.89±6.80 | 4.97±1.85 | 0.016 | 0.92 |
| Fentanyl, mcg | 62.73±26.84 | 51.21±18.16 | 0.503 | 0.02 |
| Nodule size, mm | 18.0 (12.50–25.50) | 16.75 (13.00–23.88) | 0.058 | 0.75 |
| Lobe | 0.742 | 0.02 | ||
| LUL | 18.6 (22.7) | 11.0 (33.3) | ||
| LLL | 14.9 (18.2) | 3.0 (9.1) | ||
| RUL | 29.0 (35.4) | 6.0 (18.2) | ||
| RML | 12.4 (15.2) | 3.0 (9.1) | ||
| RLL | 7.0 (8.6) | 10.0 (30.3) | ||
| CT location† | 0.207 | 0.62 | ||
| Central | 25.3 (30.8) | 12.0 (36.4) | ||
| Intermediate | 45.6 (55.6) | 15.0 (45.5) | ||
| Peripheral | 11.2 (13.6) | 6.0 (18.2) | ||
| Characteristic of nodule | 0.442 | 0.34 | ||
| Solid | 65.0 (79.3) | 27.0 (81.8) | ||
| Part-solid | 12.8 (15.7) | 2.0 (6.1) | ||
| Pure GGO | 0.8 (1.0) | 0.0 (0.0) | ||
| Consolidation | 2.5 (3.0) | 3.0 (9.1) | ||
| Cavity | 0.8 (1.0) | 1.0 (3.0) | ||
| CT bronchus | 0.216 | 0.68 | ||
| Ia | 14.9 (18.2) | 7.0 (21.2) | ||
| Ib | 17.4 (21.2) | 5.0 (15.2) | ||
| Ic | 9.1 (11.1) | 4.0 (12.1) | ||
| IIa | 38.1 (46.5) | 15.0 (45.5) | ||
| IIb | 2.5 (3.0) | 2.0 (6.1) | ||
| rEBUS, “within” | 38.1 (46.5) | 16.0 (48.5) | 0.04 | 0.57 |
| Outcome, non-diagnostic | 42.2 (51.5) | 11.0 (33.3) | 0.374 | 0.10 |
Data are presented as n‡ (%), mean ± SD or median (IQR). ‡, in the weighted analysis for propensity score matching, the effective number of matched subjects (1:3) could be expressed as fractional values. *, propensity score-matched cohort. †, central: inner one-third of the lung from the hilum, intermediate: middle two-third of the lung from hilum, peripheral: outer one-third of the lung from the hilum. CT, computed tomography; GGO, ground glass opacity; IQR, interquartile range; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; rEBUS, radial endobronchial ultrasound; SMD, standardized mean differences; SD, standard deviation; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration.
Clinical outcomes of combined TBNA-TBLB cohort (33 subjects)
When analyzed separately in this cohort, the diagnostic yield was 57.6% for TBNA and 51.5% for TBLB. However, when combined, the yield increased to 66.7%, suggesting that TBNA enhances diagnostic accuracy when performed together with TBLB in the same setting (Figures 2,3). The highest pooled diagnostic yield was observed for CT bronchi types 1a and 1b (83.3%), whereas lower yields (57.1%) were observed for CT bronchi types 1c, 2a, and 2b (adjacent to or outside the bronchus). A detailed summary of the diagnostic yields across the CT bronchus types is provided in Table 3. Most lung nodules were diagnosed as primary lung adenocarcinomas (48.5%), followed by squamous cell carcinomas (9.1%). Approximately 24% of the cases showed only blood or inflammatory cells, providing no definitive diagnostic clues for lung nodules. No major periprocedural or postprocedural complications were observed, except for one patient (3.0%) who developed a postprocedural pneumothorax.
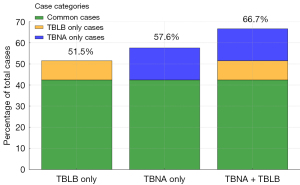
Table 3
| CT bronchus classification | Diagnostic yield, n (%) | P value |
|---|---|---|
| Type | 0.39 | |
| 1a | 5/7 (71.4) | |
| 1b | 5/5 (100.0) | |
| 1c | 3/4 (75.0) | |
| 2a | 8/15 (53.3) | |
| 2b | 1/2 (50.0) | |
| Group | 0.25 | |
| 1a+1b | 10/12 (83.3) | |
| 1c+2a+2b | 12/21 (57.1) |
CT, computed tomography; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration.
Comparison for the diagnostic yields between TBLB alone vs. TBNA plus TBLB
Table 2 presents the comparative results of the TBNA-TBLB cohort (33 patients) and the matched control cohort (82 patients). Considering nodule size and CT bronchus sign as critical predictive factors for diagnostic success as well as probe position within the lesion, these variables showed no significant differences between the two cohorts after matching (SMD <0.25). In the matched cohort, the diagnostic success rate of combined TBNA-TBLB (66.7%) was higher than that of TBLB alone (48.5%), although this difference was not statistically significant (P=0.10, SMD =0.374).
Discussion
Lee et al. and Sumi et al. conducted similar retrospective studies to evaluate the diagnostic yield of combined TBNA-TBLB for pulmonary lesions (9,10). The median age of the patients was 68 and 75 years, respectively, with a predominance of males (68.9% and 58.8%, respectively) (9,10). The median lesion size in our cohort (16.75 mm) was significantly smaller than those in the other two cohorts (24 and 23 mm, respectively) (9,10). Additionally, our cohort included 51.5% of lesions with adjacent rEBUS views, which was comparable to the Japanese cohort (54.9%), but substantially higher than that of Lee et al.’s cohort (24.3%) (9,10).
While the diagnostic tools used in our study were similar to those employed by Lee et al., their protocol involved performing TBLB first, followed by TBNA for concentric rEBUS view lesions, and reversing this sequence for adjacent rEBUS view lesions (10). In contrast, we performed TBNA first, followed by TBLB for all patients. In the Japanese cohort, the lesions were approached using ultrathin bronchoscopy (9). Our cohort had smaller nodules compared to both cohorts, a higher proportion of lesions with an adjacent rEBUS view compared to Lee et al.’s cohort, and no use of ultrathin bronchoscopy compared to the Japanese cohort. These findings suggest that the TBNA-TBLB approach, even without ultrathin bronchoscopy, can achieve a good diagnostic yield for nodules <20 mm with an adjacent rEBUS view. A retrospective study demonstrated that the PeriView Flex device had a sensitivity of 70% and a specificity of 100% for diagnosing lung malignancy using the TBNA method (14). Regarding the sequence of TBNA-first vs. TBLB-first, a recent prospective study reported no difference in diagnostic yields between the two groups (63.9% vs. 70.0%). However, the authors still favored initiating TBNA, particularly in eccentric-view cases, despite a lack of statistical significance (15).
A larger lesion size and a high proportion (75.7%) of lesions with concentric rEBUS views were likely key factors contributing to the superior diagnostic yield of combined TBLB-TBNA (70.3%) in Lee et al.’s cohort (3,10,16). Similarly, the significantly higher diagnostic yield (86.3%) of the Japanese cohort could be attributed to larger lesion sizes (3,9,16). Additionally, the use of ultrathin bronchoscopy in a Japanese cohort allowed deeper access to the smaller subsegmental bronchi, enabling proximity to the target lesion and improving diagnostic outcomes (16,17).
In contrast, the diagnostic yield in our cohort (66.7%) was slightly lower than that of the comparison cohort. This could result from the smaller median lesion size and the predominance of lesions with adjacent rEBUS views or those surrounding the bronchus, which may appear as if they were within the lesion on rEBUS imaging. However, our findings demonstrated that TBNA combined with TBLB improved the diagnostic yield for lesions with CT bronchus types Ic, IIa, and IIb (75%, 53.3%, and 50%, respectively). These results outperformed those of TBLB alone, as reported by Imabayashi et al., in which the diagnostic yields were 59% for type 1c, 52.8% for type 2a, and 46.3% for type 2b (8). This improvement may be attributed to the initial TBNA step, which potentially creates an optimal pathway by puncturing the bronchus, thereby disrupting the bronchial mucosa near the target lesion, converting an eccentric or adjacent rEBUS view to a concentric view, and enhancing the diagnostic yield.
CT-guided transthoracic biopsy was once considered the preferred method for diagnosing peripheral pulmonary lesions (PPLs) before the introduction of rEBUS. Its diagnostic yield has been reported to range from 82% to 93% (18,19). However, the complication rate is high, with a pneumothorax risk of 9–43% and a bleeding risk of 2.3–3.2% (18). Consequently, there has been a paradigm shift from CT-guided biopsy to an endobronchial approach for PPL diagnosis. Our cohort clearly demonstrated that the endobronchial approach with TBNA-TBLB offers a lower complication rate while maintaining a promising diagnostic yield.
Previous studies have suggested that cryobiopsy can serve as an adjunct tool to further enhance the diagnostic yield of adjacent or eccentric lesions (7,20,21). A multicenter randomized controlled trial (RCT) reported that rEBUS-guided transbronchial cryobiopsy (TbCb) for PPLs achieved a diagnostic yield of 85%, with a 5.2% risk of pneumothorax and a 5% risk of bleeding (22). Another multicenter retrospective cohort reported a high diagnostic yield of 91% for TbCb of PPLs, with a 3.5% risk of significant bleeding and a 6.3% rate of pneumothorax requiring intervention (23). The introduction of a 1.1 mm cryoprobe has significantly reduced both pneumothorax and bleeding rates to 4% (22). Hopefully, the ongoing FROSTBITE-2 trial will provide more evidence on the safety profile of the 1.1 mm cryoprobe. Additionally, TbCb allows for the retrieval of larger specimens, which is essential for molecular genetic analyses and immunohistochemical staining in non-small cell lung cancer. Robotic-assisted bronchoscopy has significantly improved the diagnostic yield for PPLs, ranging from 77% to 96% (24). A systematic review and meta-analysis reported a pooled diagnostic yield of 78.0% for lesions ≤20 mm and 88.4% for lesions >20 mm. The addition of cone beam CT further improved the pooled diagnostic yield to 80.6%, while TbCb significantly increased it to 90.0% (25). The overall pooled complication rate was 3.0%, with pneumothorax (1.8%) being the most common complication (25). However, the combined TBNA-TBLB approach presents a promising strategy for diagnosing smaller lung lesions, particularly those with unfavorable CT bronchus signs and adjacent rEBUS views (26,27). While our cohort’s diagnostic yield is lower than that of navigation bronchoscopy combined with cone beam CT and cryobiopsy, it offers a lower bleeding risk while maintaining a similar pneumothorax risk. A diagnostic yield of 66.7% in our cohort using rEBUS-guided TBNA-TBLB alone, without ancillary technologies such as navigation bronchoscopy or cryobiopsy, is promising. Thus, the TBNA-TBLB approach could serve as a viable alternative for improving the diagnostic yield of PPLs ≤20 mm in institutions where robotic assisted bronchoscopy is not readily available due to cost, a lack of trained operators, or limited procedure time. Rapid on-site evaluation (ROSE) was not available in this cohort due to due to the lack of available pathologists and limited time. The diagnostic yield could potentially improve if ROSE were readily available.
This study had several limitations. First, the generalizability of the findings is restricted due to their retrospective nature, single-center setting, and relatively small sample size. Larger prospective studies are necessary to further validate these findings. Second, the procedure was performed by an experienced bronchoscopist, which may introduce variability when comparing the outcomes among operators with different levels of experience. The bronchoscopists engaged in interventional pulmonology since 2017. Third, patient selection bias was inherent because this was not a randomized trial.
Conclusions
In conclusion, rEBUS-guided TBNA followed by TBLB is safe and effective for the diagnosis of pulmonary lesions. This combined approach has the potential to improve the diagnostic yield compared to conventional rEBUS-guided TBLB, particularly in cases involving specific bronchus types that are typically associated with lower diagnostic success and when robotic assisted bronchoscopy is not readily available.
Acknowledgments
We acknowledge the Institutional Review Board of Samsung Medical Center for approving this study. We also acknowledge the bronchoscopy staff for their assistance during the procedures.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-68/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-68/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-68/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-68/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board of Samsung Medical Center (No. 2024-12-101). The requirement for informed consent was waived because of its retrospective nature. Some patients were not being followed up, and personal information that could be used to infer individual patients was not contained. However, we emphasize that we strictly adhered to ethical principles and ensured patient confidentiality throughout the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Ali MS, Ghori UK, Wayne MT, et al. Diagnostic Performance and Safety Profile of Robotic-assisted Bronchoscopy: A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2023;20:1801-12. [Crossref] [PubMed]
- Nakai T, Matsumoto Y, Suzuk F, et al. Predictive factors for a successful diagnostic bronchoscopy of ground-glass nodules. Ann Thorac Med 2017;12:171-6. [Crossref] [PubMed]
- Schuhmann M, Bostanci K, Bugalho A, et al. Endobronchial ultrasound-guided cryobiopsies in peripheral pulmonary lesions: a feasibility study. Eur Respir J 2014;43:233-9. [Crossref] [PubMed]
- Matsumoto Y, Nakai T, Tanaka M, et al. Diagnostic Outcomes and Safety of Cryobiopsy Added to Conventional Sampling Methods: An Observational Study. Chest 2021;160:1890-901. [Crossref] [PubMed]
- Kho SS, Chan SK, Yong MC, et al. Performance of transbronchial cryobiopsy in eccentrically and adjacently orientated radial endobronchial ultrasound lesions. ERJ Open Res 2019;5:00135-2019. [Crossref] [PubMed]
- Imabayashi T, Matsumoto Y, Uchimura K, et al. Computed Tomography Bronchus Sign Subclassification during Radial Endobronchial Ultrasound-Guided Transbronchial Biopsy: A Retrospective Analysis. Diagnostics (Basel) 2023;13:1064. [Crossref] [PubMed]
- Sumi T, Shijubou N, Sawai T, et al. Transbronchial needle aspiration with endobronchial ultrasonography and ultrathin bronchoscopy for peripheral pulmonary lesions. Respir Investig 2021;59:766-71. [Crossref] [PubMed]
- Lee D, Chae G, Kim JH, et al. Diagnostic utility of adding needle aspiration (using PeriView FLEX needle) to radial endobronchial ultrasound guide sheath transbronchial lung biopsy: a single center retrospective study. J Thorac Dis 2024;16:3818-27. [Crossref] [PubMed]
- Ito T, Nishida K, Iwano S, et al. Diagnostic Value and Safety of Addition of Transbronchial Needle Aspiration to Transbronchial Biopsy Through Endobronchial Ultrasonography Using a Guide Sheath Under Virtual Bronchoscopic Navigation for the Diagnosis of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2024;31:e0984. [Crossref] [PubMed]
- Kurimoto N, Morita K. Bronchial Branch Tracing. Bronchial Branch Tracing. Springer Singapore; 2020. 2-161 p.
- Gonzalez AV, Silvestri GA, Korevaar DA, et al. Assessment of Advanced Diagnostic Bronchoscopy Outcomes for Peripheral Lung Lesions: A Delphi Consensus Definition of Diagnostic Yield and Recommendations for Patient-centered Study Designs. An Official American Thoracic Society/American College of Chest Physicians Research Statement. Am J Respir Crit Care Med 2024;209:634-46. [Crossref] [PubMed]
- Naso J, Bras J, Villamil C, et al. Cytologic features and diagnostic value of PeriView FLEX transbronchial needle aspiration targeting pulmonary nodules. Cancer Cytopathol 2020;128:333-40. [Crossref] [PubMed]
- Olive GN, Leong SC, Marshall HM, et al. Transbronchial Needle Aspiration via Ultrathin Bronchoscope Improves Diagnostic Yield for Peripheral Lung Lesions: Randomized Sequencing Trial. J Bronchology Interv Pulmonol 2024;32:e0996. [Crossref] [PubMed]
- Kim SH, Kim J, Pak K, et al. Ultrathin Bronchoscopy for the Diagnosis of Peripheral Pulmonary Lesions: A Meta-Analysis. Respiration 2023;102:34-45. [Crossref] [PubMed]
- Oki M, Saka H. Diagnostic value of ultrathin bronchoscopy in peripheral pulmonary lesions: a narrative review. J Thorac Dis 2020;12:7675-82. [Crossref] [PubMed]
- Nakamura K, Matsumoto K, Inoue C, et al. Computed Tomography-guided Lung Biopsy: A Review of Techniques for Reducing the Incidence of Complications. Interv Radiol (Higashimatsuyama) 2021;6:83-92. [Crossref] [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [Crossref] [PubMed]
- Oberg CL, Lau RP, Folch EE, et al. Novel Robotic-Assisted Cryobiopsy for Peripheral Pulmonary Lesions. Lung 2022;200:737-45. [Crossref] [PubMed]
- Kim SH, Mok J, Jo EJ, et al. The Additive Impact of Transbronchial Cryobiopsy Using a 1.1-mm Diameter Cryoprobe on Conventional Biopsy for Peripheral Lung Nodules. Cancer Res Treat 2023;55:506-12. [Crossref] [PubMed]
- Yong SS, Kapp CM. The rising role of cryobiopsy in diagnosis of pulmonary disorders: a narrative review. Curr Chall Thorac Surg 2024;6:17.
- Herth FJ, Mayer M, Thiboutot J, et al. Safety and Performance of Transbronchial Cryobiopsy for Parenchymal Lung Lesions. Chest 2021;160:1512-9. [Crossref] [PubMed]
- Fernandez-Bussy S, Chandra NC, Koratala A, et al. Robotic-assisted bronchoscopy: a narrative review of systems. J Thorac Dis 2024;16:5422-34. [Crossref] [PubMed]
- Zhang C, Xie F, Li R, et al. Robotic-assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Thorac Cancer 2024;15:505-12. [Crossref] [PubMed]
- Song N, Yang L, Wang H, et al. Radial endobronchial ultrasound-assisted transbronchial needle aspiration for pulmonary peripheral lesions in the segmental bronchi adjacent to the central airway. Transl Lung Cancer Res 2021;10:2625-32. [Crossref] [PubMed]
- Ma D, Zhang J, Zeng Q, et al. Diagnostic efficacy and safety of radial probe endobronchial ultrasound-guided transbronchial needle aspiration for adjacent lesions in segmental or subsegmental bronchi: a single-center retrospective study. BMC Pulm Med 2023;23:485. [Crossref] [PubMed]


