
Chinese expert consensus on shape-sensing robotic-assisted bronchoscopy (ssRAB) in the management of peripheral pulmonary lesions
Highlight box
Key recommendations
• Create multiple pathways and review the critical anatomy borders reconstructed by the Planning Station and draw additional ones when needed.
• Perform the procedure under general anesthesia and neuromuscular blockade with endotracheal intubation and mechanical ventilation, and implement a dedicated anesthesia protocol to mitigate atelectasis and reduce unnecessary respiratory motion.
• Use radial endobronchial ultrasound (rEBUS) and the rEBUS articulation guide function of shape-sensing robotic-assisted bronchoscopy (ssRAB).
• Use fluoroscopy and/or cone-beam computed tomography (CT) with ssRAB.
• Solve CT-to-body divergence using the preview path mode, adjust the anesthesia parameters, or use advanced imaging technologies.
• Use compatible sampling tools with ssRAB.
• Use cloud biopsy.
• Use rapid on-site evaluation with ssRAB.
What was recommended and what is new?
• There is no prior consensus on ssRAB, and all recommendations in the consensus are the first time they have been recommended.
What is the implication, and what should change now?
• This consensus establishes standardized procedural guidelines for ssRAB implementation, offering valuable guidance for operators, particularly clinicians new to the technology. As the technical execution of ssRAB differs significantly from traditional bronchoscopy, clinicians require dedicated training to master this advanced methodology.
Introduction
Lung cancer is the leading cause of cancer and cancer-related death in China. It counts for 1.06 million new cancer cases and 0.73 million deaths in 2022 (1). The survival rate of lung cancer is related to the cancer stage at the time of diagnosis. A study in Dalian, where a comprehensive lung cancer screening program was implemented, demonstrated an increased proportion of stage I lung cancer and improved stage-specific survival among early-stage lung cancer patients (2). Efforts in early detection and treatment of lung cancer have resulted in a big number of lung lesions requiring further diagnosis, as well as the need to treat early-stage lung cancer minimally invasively. Robotic-assisted bronchoscopy (RAB) has emerged as an advanced technology for early lung cancer diagnosis and treatment (3). It provides improved maneuverability, reachability, stability and navigational capability. To date, three RAB systems have been cleared by regulatory bodies in the United States. Two of them are approved by China National Medical Products Administration (NMPA).
The Ion Endoluminal System was developed by Intuitive Surgical (Sunnyvale, CA, USA). It was approved by the United States Food and Drug Administration (FDA) in February 2019, and by the NMPA in March 2024. It is the only existing bronchoscopic platform that utilizes shape-sensing technology. The optic fiber embedded along the entire length of catheter provides accurate position information of each point of the catheter and is immune to the electromagnetic interference in bronchoscopy suite. The catheter is controlled by a robotic arm, with a removable video scope inserted into its working channel for direct visualization during navigation. Other components of Ion System include a Planning Station, a robot cart with a display screen, and a controller.
Another RAB platform, the Monarch Robotic Endoscopy System, was developed by Auris Robotics (now Johnson & Johnson) in Redwood City, CA, USA. It was approved by FDA in March 2018 and then by NMPA in September 2023. Monarch system is comprised of a bronchoscope system, cart, and tower. It employs an articulating bronchoscope (4.2 mm outer diameter, 2.1 mm working channel) within an articulating sheath (6 mm outer diameter), each controlled by an independent robotic arm. Monarch utilizes electromagnetic navigation (EMN) technology for positioning, which is the same as last generation of navigational bronchoscopy modality. The third RAB platform, the Galaxy System was developed by Noah Medical in San Carlos, CA, USA. It received FDA approval in March 2023, but has not approved by NMPA in China yet. Similar to Monarch, the Galaxy System relies on EMN technology for positioning but with additional digital tomosynthesis feature. It employs a disposable, single-use bronchoscope with 4 mm outer diameter, 2.1 mm working channel and integrated visualization.
This consensus aims to standardize the clinical application of the shape-sensing RAB (ssRAB) platform in the management of pulmonary lesions. Since 2021, ssRAB has been utilized in China both in clinical studies and clinical practice. In contrast, other guiding technologies like electromagnetic navigational bronchoscopy (ENB) and augmented reality optical lung navigation have been used for quite a few years and consensuses have been published, independent of robotic assistance (4,5). The current consensus is developed using Delphi method through two phases: in phase 1, a panel of nine experts identified key topics, reviewed published evidence and clinical experiences, and generated a list of eight consensus statements; in phase 2, an external panel of 39 physicians responded to a questionnaire, providing agreement or disagreement opinion on each item of the eight consensus statements. The final consensus document was formed after analyzing the responses of the external panel. Ion Endoluminal System is the only ssRAB platform available worldwide so far. This consensus is subject to update once more evidence becomes available or other ssRAB platform is developed.
Operational norms of ssRAB
Indications and contraindications of ssRAB
Indications of ssRAB technology include: (I) peripheral pulmonary lesions (PPLs) that require pathology or etiological diagnosis; (II) PPLs that are diagnosed but require reassessment during disease treatment.
Especially, ssRAB technology can have a significant impact on the diagnostic yield of the PPLs that are challenging for other bronchoscopic technologies, hence favoring its use in such cases. For example:
- PPL with a diameter <2 cm. A subgroup analysis of meta-analysis by Balasubramanian et al. showed that RAB is the only transbronchial platform that can achieve comparable diagnostic yield in the PPLs with a diameter <2 cm as to the computed tomography-guided transthoracic biopsy or needle aspiration (CT-TBNA) (6). Specifically for ssRAB, Ost et al. reported the results from 115 subjects with a mean nodule diameter of 17.0±5.5 mm, showing a diagnostic yield of 82% in the nodules ≤2 cm (7). Similar results were reported by Xie et al. and Abia-Trujillo et al. showing diagnostic yields of 85.7% and 85.4% in the nodules ≤2 cm, respectively (8,9). The constantly improved diagnostic yield of ssRAB guarantees its favored use in the smaller lesions.
- PPL without bronchus sign. A meta-analysis by Zhang et al. showed that the diagnostic yield for such lesions was 71.9% by RAB, largely improved compared to the diagnostic yield of 39.2% by prior techniques (10). Specifically for ssRAB, a diagnostic yield of 72.7% was seen in the nodules without bronchus sign (8). In the absence of bronchus sign, it’s most likely that a transparenchymal pathway needs to be created by puncturing the wall of a nearby airway. The precise maneuverability of ssRAB allows the proceduralist to tent against the airway, and the stability of ssRAB can keep it steady during repeated puncturing and biopsy tool insertion. It is likely for these reasons that in comparison to other guided bronchoscopy modalities, RAB does significantly better for PPL (11).
- PPLs accessible only via a sharply angulated transbronchial trajectory. Such lesions can only be reached using a modality with improved flexibility, precise maneuverability and stability, which can be seen in ssRAB.
- PPLs for which biopsy and/or treatment procedure that requires steady and precise positioning of the catheter for a relatively long duration. For example, ablation procedure with a cryoablation probe. Such procedures can be done through conventional bronchoscope with or without EMN guidance, but requires prolonged hand holding or additional holding device for bronchoscope. Meanwhile, the catheter of RAB can remain stationary without manual handling.
The contraindication of ssRAB is the same as conventional bronchoscopy procedures. Please refer to the guidelines for the application of diagnostic flexible bronchoscopy in adults (2019 edition) by the Interventional Respiratory Group of the Chinese Medical Association of Chinese Thoracic Society (12). It’s worth noting that there are no precautions or contraindications for patients with implanted cardiac devices when using ssRAB, as the optic fiber used for shape-sensing does not produce electromagnetic signals and therefore poses no risk of interference. In contrast, close cardiac monitoring for such patients is necessary during EMN procedures due to the potential (albeit low) risk of electromagnetic interference (13).
System, instruments and accessories
The Ion Endoluminal System consists of a system cart, a controller and a planning workstation, and instruments including an ultrathin flexible catheter, and a vision probe for real-time visualization during registration and navigation (Figure 1).
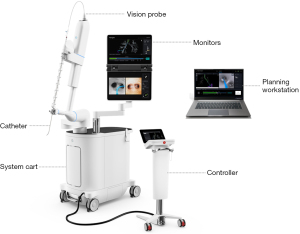
The planning workstation provides detailed procedure planning by generating a virtual three-dimensional (3D) map of the patient’s airways and pathways to the target lesion from an existing thin-slice computed tomography (CT) scan. Movement of the flexible catheter is enabled by the bronchoscopist’s manipulation of the controller under direct visualization provided by the vision probe during registration and navigation. The monitors on the system cart display navigational information such as live video from the vision probe, navigation view, and lesion target information during biopsy.
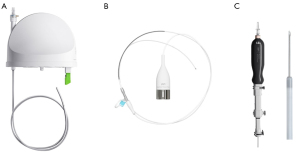
The Ion Endoluminal System uses shape-sensing technology for navigation guidance. The optic fiber shape sensor embedded throughout the entire length of the catheter provides the shape and position of the entire catheter on the system cart monitors. The shape-sensing catheter can articulate 180 degrees and has a 3.5 mm outer diameter, and a 2 mm working channel that allows for insertion of compatible tools (Figure 2). Once the target lesion is reached, the vision probe can be removed to allow the insertion of radial endobronchial ultrasound (rEBUS) probe for navigation confirmation, and a flexible needle (Flexision, Intuitive Surgical) and/or biopsy forceps, cytology brush, or cryoprobe for tissue acquisition.
Bronchoscopy suite personnel configuration
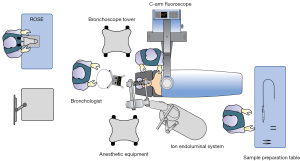
Only bronchoscopists and assistants with adequate ssRAB system trainings and experience with bronchoscopic techniques should perform procedures with the Ion Endoluminal System. Since ssRAB procedures are performed under general anesthesia, presence of anesthesiologist is needed during procedure. Figure 3 shows the schematic room setup for a typical ssRAB procedure.
Biopsy procedures
Preoperative preparation and evaluation
Patients
Patients should be informed of the procedure purpose, potential risks and benefits associated with ssRAB, as well as the alternative approaches available. Written informed consent should be obtained prior to the procedure. The preoperative examinations are the same as those for conventional bronchoscopy, evaluation of patient’s current medical condition, medical history, treatment history, allergy history, hematological tests, cardiopulmonary function and imaging examinations should be completed to identify contraindications related to bronchoscopy. Pregnancy test should be completed when necessary.
Chest CT
It is recommended that patients should have a recent thin-slice contrast-enhanced chest CT scan with 0.5 to 1.0 mm slice thickness, 0.5 to 0.8 mm slice interval, and an image resolution of 512×512 pixels in the Digital Imaging and Communications in Medicine (DICOM) standard format.
Planning
The chest CT data are uploaded to the planning workstation and segmented automatically by the software; manual segmentation is also possible in case of non-typical anatomy. The planning workstation creates a 3D virtual image of the lung with important anatomy borders, where one or multiple lesions can be identified as the target. For each target, an optimal pathway is automatically generated, and additional pathways can be added by the user. The user can also manually update the automatically generated pathway when needed. Plans should be carefully reviewed and exported from the workstation and loaded onto the system through the controller. The user can update the biopsy target to any desired point during navigation to correct potential deviations during the real-time navigation.
Intraoperative workflows
Anesthesia
ssRAB procedure should be performed under general anesthesia and neuromuscular blockade with endotracheal intubation and mechanical ventilation. Preoperative anesthesia assessment of individual situation along with lesion location enables the selection of the most appropriate anesthesia parameters and airway management methods.
Preparation and patient docking
Follow the step-by-step instructions provided on the monitors to perform system tests, attach instruments and accessories, load the patient plan to controller, and dock system cart arm to the patient for procedure. For all instruments (such as rEBUS probe and sampling tools planned to be used during procedure) passing the working channel except the vision probe and Flexision needles that are part of the ssRAB system, they should be inserted into the catheter in advance, with their distal ends brought into the appropriate position and marked the proximal end. For patient placement, it is recommended that the patient be placed in the supine position, with the head at the edge of the bed and with the neck slightly extended to expose the glottic plane. Prior to patient docking, routine white light bronchoscopy should be performed. When docking the system arm to the patient, always stabilize the endotracheal tube and swivel connector while bringing the arm magnetic mount to the swivel connector.
Routine bronchoscopy
After system setup and patient anesthesia, prior to docking, routine white light bronchoscopy should be performed to examine the airway lumen and mucosa, and to remove secretions.
Registration
After routine bronchoscopic survey of airway, place the lubricated catheter into the endotracheal tube and extend the catheter guide. Advance the catheter to the main carina, and match live view to navigation view in centering, depth and rotation, and confirm the main carina match. Drive into each main bronchus until the right and left indicators are illuminated, and then into each of the four lung quadrants until the indicator for each quadrant illuminates. Complete registration if the live view and navigation view match well enough, in case of visual mismatch or significant patient movement, consider a redo registration. In case of specific anatomy (e.g., missing one lobe), consider accepting partial registration.
Navigation
The ssRAB system provides a virtual 3D bronchial tree image with anatomy borders, a virtual bronchoscopy image to track and observe the entire catheter in relation to the target lesion, the distance from the catheter tip to the near and far ends of the target, as well as the drive force displaying the estimation of force measured at the back end of the catheter. After registration is completed, the first planned destination (target and path) automatically appears on-screen. The bronchoscopist should then follow the planned pathway to the target, while watching the live view. Once the target is reached (approximately within 30 mm of striking distance of the needle), aim the catheter towards the center. Remove the vision probe from the catheter for rEBUS confirmation and subsequent biopsy.
Obtaining rEBUS images
When the catheter reaches the target lesion and the vision probe is removed, insert the rEBUS probe into the catheter at the retained position to confirm lesion location. Adjust the rEBUS-catheter position slightly back and forth according to the ultrasonic characteristics (concentric, eccentric, or no signal) to obtain the optimal rEBUS images. Fluoroscopy can be used to confirm the position of rEBUS probe. Once the optimal position is reached, remove the rEBUS probe from the catheter, the catheter is kept at the optimal position and is ready for sampling tool insertion.
Adjunctive imaging modalities
Perform rEBUS confirmation and biopsy under fluoroscopy. Use the optimal angle reference provided by ssRAB to guide the fluoroscopy. If available, use a cone-beam CT (CBCT) system with 3D imaging to verify the placement of the tool related to the lesion. When available, use the direct integration of mobile CBCT to update the target, adjust the catheter direction and confirm the reach of the tool.
Biopsy sampling and specimen collection
During the biopsy process, Flexision biopsy needle, compatible biopsy forceps, cytology brush and/or cryoprobe can be used per evaluation of the target lesion condition, under fluoroscopy guidance.
Undocking system
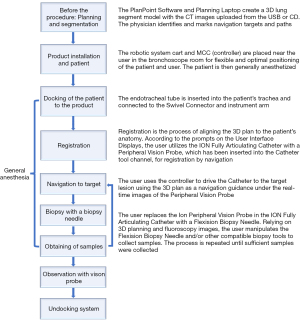
After biopsy and before concluding the procedure, insert the vision probe back to observe if any bleeding and use fluoroscopy to check for pneumothorax. Once all conditions are cleared, retract the catheter guide, remove the catheter from the endotracheal tube, and undock the swivel connector from the system arm. The ssRAB biopsy procedure workflow is summarized in Figure 4.
Management of intraoperative complications
Compared to conventional transthoracic biopsy, ssRAB has demonstrated a higher safety profile with lower complication rates (14). Usually complications are not related to ssRAB technology itself, but rather a common situation relating to peripheral lesion localization, diagnosis and treatment.
Pneumothorax is a major complication of interest to PPL biopsy procedures in general. A previous study compared conventional transthoracic biopsy with ssRAB biopsy, and the incidence of pneumothorax requiring admission for observation or chest tube placement in transthoracic and ssRAB biopsy is 16.1% (18/112) and 3.5% (4/113), respectively (14). This is comparable to the pneumothorax reported in other ssRAB publications, ranging from 0% to 5.8% (11,15). In case of pneumothorax, the management should follow the established guidelines (16), where chest tube insertion or needle aspiration for drainage is required in large pneumothorax, while observation is recommended for small pneumothorax.
Airway bleeding is another common complication in patients undergoing biopsy. The previously reported airway bleeding incidence requiring intervention ranges from 0% to 0.8% (11,15). Treatment is based on the severity of bleeding and should follow the recommendations in relevant guidelines (17,18). Suction can be applied for mild bleeding; catheter wedging, or cold saline instillation for moderate bleeding; and in more severe cases, selective intubation, balloon/bronchial blocker, interruption of procedure and blood transfusion might be needed. Specifically, after all biopsy attempts are completed, the catheter can be retained for approximately 1 minute to prevent bleeding. The vision probe should be re-inserted into the catheter to survey the airway for any sign of bleeding or other complications.
Hypoxemia, chest pain, pulmonary infection, and pleural effusion, etc. are also common complications associated with PPL biopsy procedures but not specific to ssRAB technology, and management should follow relevant guidelines (19).
Post-operative follow-up and management
Patients should follow standard of care post-procedure. For patients with pathology results indicating malignancy and specific benign diagnoses, follow-up treatment should be provided accordingly. For patients with non-specific benign or negative pathological results, clinical and imaging evidence should be considered, and standard empirical treatment and follow-up should be advised. In case of inadequate specimen or non-diagnostic pathology, subsequent biopsy, surgery or routine follow-up is necessary to establish diagnosis.
Methods
The Delphi is a well-established method of investigation, taking an iterative approach to collect and evaluate the opinions of a group of experts through several rounds of questionnaires and discussions, aiming to bring together the most agreed opinions into a consensus (20-22). By adopting the Delphi approach, the development of this consensus was done through two phases (Figure 5).
In phase 1, a panel consisting of a group of nine experts, who had extensive experience in ssRAB technology, was formed. The group reviewed the current landscape and identified key topics in the use of ssRAB in September 2024, which were as follows: (I) best practices of using ssRAB; (II) tools and technologies that should be used in combination with ssRAB; (III) measures to maximize the clinical value of ssRAB.
In the first phase, after a careful review of the scientific literature and sharing of clinical experiences, a first version of a questionnaire with 10 consensus statements was created. The nine experts gathered in an online meeting to discuss the statements. Comments on the statements were collected and analyzed. If consensus was not reached, the discussion continues through both online and offline meetings. In the end, eight consensus statements were generated.
In the second phase, the statements were put into a questionnaire and sent to an external panel of 39 physicians in February 2025. The panel members were invited to express their agreement or disagreement on each statement.
After all the responses were collected, the percentage of responses and the percentage of agreement on each statement were calculated. The consensus was defined as achieved with an agreement percentage threshold of 80% or above. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Consent was obtained from all participants.
Results
Phase 1 results
A total of eight consensus statements were agreed upon by the panel, as shown below.
Consensus statement 1: recommendations for path planning
- It’s recommended to create multiple pathways. The maneuverability of ssRAB enables the proceduralist to reach the lesion from different routes.
- It’s recommended to review the critical anatomy borders reconstructed by Planning Station and draw additional ones when needed.
If the lesion can’t be reached along one route, the proceduralist can switch to another route for biopsy during the operation. Therefore, it is necessary to create multiple pathways. In the PlanPoint software of ssRAB, critical anatomy borders like the pleura are shown as a curved surface of purple color. The distance to the border is also indicated. This will provide important information when the proceduralist sets the needle insertion distance, which should be shorter than the distance to the border to prevent complication like pneumothorax.
Consensus statement 2: recommendations for anesthesia
- It’s recommended to perform the procedure under general anesthesia and neuromuscular blockade with endotracheal intubation and mechanical ventilation, and to implement a dedicated anesthesia protocol to mitigate atelectasis and reduce unnecessary respiratory motion. Positive end-expiratory pressure (PEEP) and increased tidal volumes can help maintain optimal lung inflation and prevent atelectasis, and breath-hold is required sometimes to reduce motion artifact during intraprocedural imaging.
- It’s recommended to adjust PEEP (usually 8–10 cmH2O), tidal volume (usually about 8 mL/kg body weight), and FiO2 (usually 0.6 to 0.8, maintained at the lowest tolerable level for the entire procedure) according to the patient’s situation. If the patient is obese or the lesion is located in the lower lobe, PEEP can be higher in the case of patient tolerance. It’s recommended to perform manual pulmonary re-expansion when intraoperative atelectasis occurs during the operation.
Several studies reported that optimal PEEP settings could open small distal airways to avoid atelectasis and more closely approximate the conditions of full inspiratory breath-hold in CT imaging, thus potentially overcoming CT-to-body divergence and facilitating the advancement of the scope without air insufflation (23-26). A lower tidal volume of 6–8 mL/kg body weight can maintain optimal lung inflation and minimize airway motion during navigation (24,25). The use of 100% oxygen during induction and anesthesia maintenance is a major cause of atelectasis. An FiO2 of 0.6 to 0.8 can avoid absorption atelectasis and lung injury while maintaining adequate procedural oxygenation for the patient (23).
Consensus statement 3: recommendations for the use of rEBUS
- It’s recommended to use rEBUS that is compatible with the 2 mm working channel of the ssRAB system to confirm the lesion location, rEBUS view (concentric, eccentric, or no signal) and show the internal structure of the lesion prior to and during biopsy.
- It’s recommended to use the rEBUS articulation guide function of ssRAB to provide direction for the adjustment of the rEBUS probe. For the cases in which initial rEBUS examination resulted in an eccentric view, a concentric view can be achieved after fine-adjustment of catheter position and/or needle passes.
rEBUS is easy to operate, cost-effective, and does not involve radiation. Examples of compatible rEBUS include UM-S20-17S and UM-S20-20R (Olympus, Tokyo, Japan). It can clearly identify the optimal bronchus for the lesion that should be subjected to biopsy. A study published by Xie et al. and the meta-analysis of RAB, showed that rEBUS confirmation is associated with increased diagnostic yield of ssRAB biopsy procedure (8,11). Meanwhile, it permits the visualization of the internal structure of PPL that can suggest the benign and malignant nature of the lesion (27,28). The articulation guide function can help the user to localize a lesion with rEBUS by indicating whether the catheter is articulated against an airway wall as the operator evaluates the strongest rEBUS signal (25). For poor initial rEBUS signal, a concentric view can be achieved after fine-adjustment of catheter position and/or needle pass (29,30). The disadvantage of rEBUS is that it performs poorly in visualizing ground-glass nodule (GGN), distinguishing atelectasis, and heavily relies on the experiences of the operator (31).
Consensus statement 4: the use of fluoroscopy and/or CBCT with ssRAB
- It’s recommended to perform rEBUS confirmation and biopsy under fluoroscopy to ensure the accuracy and safety.
- If conditions permit, a CBCT system with 3D imaging is recommended to correct CT-to-body divergence, verify tool-in-lesion (TIL) placement, and visualize lesions, particularly for GGN.
- It’s recommended that the combined use of the ssRAB system and mobile CBCT be employed for intraoperative target updating.
Ion Endoluminal System provides guidance about the optimal angle of fluoroscopy; the fluoroscopy reference function of ssRAB can be used to track the movement of the catheter. A study showed that ≥2 spins of CBCT during ssRAB procedure increased TIL rate and decreased the distance between the tool and the center of the lesion, demonstrating that CBCT provides incremental contribution to ssRAB procedures (32). The augmented fluoroscopy function in the CBCT system helps to locate PPL intraoperatively, especially for lesions that are invisible under fluoroscopy (33). Currently, the Ion Endoluminal System supports the direct integration with mobile CBCT (Cios Spin, Siemens Healthineers, Erlangen, Germany), providing direct access and processing CBCT data for target update. When integrated with Cios Spin, ssRAB can guide the adjustment of catheter direction and confirm the reach of the tool. When using Cios Spin, perform orbital scans at the discretion of the operator and under a breath-hold using imaging protocols defined by the manufacturer. Reformatted axial, sagittal and coronal projection images should be reviewed intraoperatively. Per operator assessment, redirect the ssRAB catheter to augment tool-lesion approximation. This process can be repeated when needed, and additional spins can be acquired to achieve optimal TIL status.
Results from a prospective multicenter study evaluating the integration of Ion and Cios Spin in the PPLs smaller than 20 mm (median diameter 14 mm) were reported, showing a TIL rate of 99.4%, a strict diagnostic yield of 89% (138/155) and an intermediate diagnostic yield of 91% (141/155) after 12-month follow-up. Notably, the intermediate diagnostic yield was 91% in the small nodules of 5 to 13.9 mm. There were no pneumothoraces of any kind, and intraprocedural bleeding (Nashville ≥3) was 1.3% (2/155) (34).
Consensus statement 5: recommendation for solving CT-to-body divergence
In case of CT-to-body divergence, the following approaches are recommended to correct and/or prevent divergence: (I) use the preview path mode to match the real-time images to the virtual navigation and update the virtual navigation when necessary; (II) adjust the anesthesia parameters such as PEEP and peak inspiratory pressure (PIP); (III) use advanced imaging technologies such as CBCT/fluoroscopy or rEBUS to locate the target.
The preview path allows the operator to preview the virtual path by scrolling distally on the virtual pathway. The operator can advance the catheter step by step and realign the virtual image with the live image at each branch point following the planned pathway. Therefore, it can serve as a guiding method to overcome CT-to-body divergence (25). As mentioned in consensus statement 2, optimal anesthesia parameters can maintain optimal lung inflation, open small distal airways to avoid atelectasis and more closely approximate the conditions of full inspiratory breath-hold in CT imaging, thus potentially overcoming CT-to-body divergence. The advanced imaging technologies can provide guidance for locating and thus moving the target to correct CT-to-body divergence.
Consensus statement 6: recommendations for the use of sampling tools with ssRAB
- Biopsy sampling tools include 19G/21G/23G Flexision biopsy needles (Intuitive Surgical), and compatible biopsy forceps (Model G53006, Cook Medical, Bloomington, IN, USA), cytology brush as well as cryoprobe. The compatible sampling tools should have a maximum diameter of 2 mm and a minimum length of 100 cm. Theoretically, any tools that meet this specification can be used as well.
- If the tools are not listed in the ssRAB user manual as validated compatible, it is recommended to test whether the tool can pass the turning radius of the ssRAB catheter before using it in an ssRAB procedure.
- The choice and sequence of biopsy tools (needle, forceps, brush) used are at the physician’s discretion. For extraluminal lesions, unless the target lesion is not suitable for needle puncture, it’s recommended to perform needle biopsy first. Needle puncture can create a path directly leading to the lesion for obtaining a concentric view of rEBUS, further biopsy, facilitating the acquisition of diagnostic specimens.
- It’s recommended to use a 1.1 mm cryoprobe when appropriate. Cryobiopsy accesses tissue in a 360-degree manner, obtains a larger amount of tissue and preserves tissue pattern, which is especially beneficial for the diagnosis of GGNs and benign diseases.
- When performing a biopsy attempt, it’s recommended to consider the minimum distance to target as provided by the navigation guidance, sheath extension measurement, and fluoroscopy before choosing a needle extension length; the length to which brush, forceps and/or cryoprobe are inserted into the working channel should follow the mark, which is manually labeled on the proximal end.
- After all biopsy attempts are completed, it’s recommended to keep the catheter in place for approximately 1 minute to prevent bleeding, then remove the catheter and flush with normal saline to collect samples to be sent for histology, cytology and/or microbiology examination.
The robotic-controlled catheter can remain in place stably when exchanging biopsy tools, allowing for multiple biopsy attempts from the same position precisely. Oberg et al. reported a retrospective analysis of 112 patients with 120 PPLs biopsied via ssRAB using needle aspirate, forceps and cryobiopsy with 1.1 mm cryoprobe (35). The overall diagnostic yield was 90% and nearly 18% of diagnoses were made exclusively from the cryobiopsy sample. Molecular analysis was adequate on all cryobiopsy samples obtained. Digital imaging software confirmed an increase in quantity and quality of samples taken via cryobiopsy compared to needle aspiration and traditional forceps biopsy. When using a 1.1 mm cryoprobe with a freezing duration of 3 to 6 seconds, the cryoprobe with acquired specimen can be directly retrieved through the 2 mm working channel of the ssRAB catheter. Repeated biopsy can be achieved without removing the catheter. If using a thick cryoprobe, the catheter needs to be withdrawn together with the cryoprobe. If another biopsy is required, the catheter needs to be re-inserted and re-navigated to the lesion. This may increase the risk of bleeding.
Consensus statement 7: recommendations for cloud biopsy
It’s recommended to perform cloud biopsy during an ssRAB procedure, during which the catheter tip can be fine-tuned to biopsy multiple points within a lesion, thus increasing the chance of acquiring diagnostic specimens.
The orientation guide of ssRAB can provide direction for catheter tip articulation. The biopsy marker feature can indicate the location of biopsy attempts. The operator is able to perform cloud biopsy due to the ability of ssRAB to make micro-adjustments of the catheter tip (11,29,30). It can be modified based on the rapid on-site evaluation (ROSE) evaluation feedback when available (30).
Consensus statement 8: recommendations for the use of ROSE with ssRAB
- After obtaining a specimen, ROSE is recommended to assess quality, provide preliminary results, and guide subsequent biopsy steps, particularly when it is difficult to determine whether a biopsy is performed within the lesion and when using the cloud biopsy technique.
- Combining ROSE with ssRAB is recommended to integrate diagnostic and therapeutic procedures (e.g., ablation or resection) within a single anesthesia procedure.
ROSE is a useful technique for the diagnosis of PPLs. It has proven advantageous in decreasing the number of biopsy attempts for adequate samples, providing real-time feedback to the operator (36,37). The operator can change the biopsy location based on the results of ROSE, thus potentially improving the diagnostic yield. The development of guided bronchoscopy has made the one-stop diagnosis and treatment possible. ROSE can provide intraoperative diagnosis and thus help the operator make subsequent medical decisions.
Phase 2 results
All 39 (100%) panel physicians responded to the questionnaire. A total of 6 statements received 100% (39/39) agreement and 2 other statements received 97.4% (38/39) agreement. One physician suggested that it’s recommended to perform the procedure under general anesthesia and neuromuscular blockade with endotracheal intubation and mechanical ventilation. This should be added to consensus statement 2. Another disagreement is about the consensus statement 6. One physician suggested that any cryoprobe with a diameter of <2 mm could be used instead of only the 1.1 mm cryoprobe.
Discussion
Positive consensus was reached for all eight proposed statements, providing a list of standard practices prior to, during and post ssRAB procedure. This lays a foundation for better use of ssRAB in the biopsy and diagnosis of PPLs.
The first clinical study of ssRAB was conducted in Australia by Fielding et al. and published in 2019 (38). In this feasibility study, an overall diagnostic yield of 79.3% and a sensitivity for malignancy of 88% were reported with a mean lesion size of 12.2±4.2 mm in the axial plane. No instance of pneumothorax or excessive bleeding was observed during the procedure. This study was the very first piece of clinical evidence that demonstrated the promising performance and safety of ssRAB. Soon after FDA approval, a multi-center clinical study was initiated in the United States to investigate the clinical performance and safety of Ion System in sampling PPLs in a large sample size of real-world scenario (PRECIsE Study, NCT03893539). A couple of publications are now available from this study (29,30).
Use of ssRAB in the Chinese population was validated in a single-arm, multi-center study (8). PPLs with a diameter of 8 to 30 mm were biopsied using ssRAB in 90 subjects. Tissue samples were successfully obtained in all cases and a diagnostic yield of 87.8% was achieved. The sensitivity for malignancy was 87.7%. Only one case of procedure-related pneumothorax was reported and solved without intervention. No hemorrhage occurred during or after the procedure. This study demonstrated the encouraging clinical performance and safety of ssRAB in Chinese patients.
The diagnostic performance of ssRAB has also been evaluated in recent meta-analyses. According to Ali et al., the pooled diagnostic yield of ssRAB across 13 studies involving 1,479 lesions was 85.2% [95% confidence interval (CI): 81.6–88.6%] (11). Zhang et al. reported a pooled diagnostic yield of 82.5% (95% CI: 76.5–88.5%) for ssRAB across six studies involving 507 lesions (10). Similarly, another meta-analysis by Pyarali et al. found that ssRAB achieved a pooled diagnostic yield of 83.5% (95% CI: 72.7–92.1%) across six studies involving 557 lesions (15).
Taken together, ssRAB is an innovative technology in managing PPLs with promising diagnostic performance. It shows promising efficacy and safety in challenging cases like GGN and small nodules with a diameter ≤2 cm. It opens opportunities in diagnosing and treating lung cancer in the early stage with minimum invasiveness.
Localization of PPLs with dye marking is possible using the ssRAB platform, due to its improved reachability, stability and precision. Several studies have reported the use of methylene blue or indocyanine green for lesion localization using ssRAB. The localization time was around 10–15 minutes (39), the injection success rate was 83.3% (40), and low overall complications with no major incidents were reported (39,40). Once the lesion is diagnosed and localized, the operator has the option to proceed with resection during a single anesthesia; however, consensus between pathologist, operator and anesthesiologist should be achieved prior to subsequent resection. When comparing ssRAB localized group vs. non-localized group, ssRAB localized group demonstrated larger negative margins in the subsequent resection with no re-resection needed (39). The mean chest tube duration and length of stay for patients with biopsy and resection during a single anesthetic event were 2.4 and 3.4 days, respectively (41). With the above feasibility and safety proven, combining diagnosis, localization and therapeutic surgical resection into a single-setting anesthesia procedure has the potential to improve patient experiences, decrease costs, and reduce delays in cancer care (42).
ssRAB provides an approach to access PPLs with improved reachability, stability and safety, warranting its use in facilitating delivery of therapeutic treatments like localized ablation and intratumoral drug injection (43-48). Ablation methods being investigated include microwave, pulsed electric field (PEF), radiofrequency (RF), cryoablation, photodynamic therapy, and laser ablation (49).
In addition to the existing indications, shape-sensing robotic-assisted technology may be used in more scenarios, which should be further explored: the robotic-controlling function of ssRAB may provide an approach to remote interventional pulmonology procedures, addressing the unequal distribution of medical resources in different regions. Its capability of navigating through natural orifice with improved precision and stability also makes it a good option for various minimally invasive procedures through endoluminal routes like the digestive tract and urological tract.
Conclusions
Shape-sensing technology, in combination with robotic-controlled feature, provides an innovative approach in diagnosing and treating PPLs. Various studies and reports indicate that ssRAB favors the biopsy and diagnosis of challenging PPLs like small PPLs with a diameter <2 cm, PPL without bronchus sign, GGN, multiple nodules, and those nodules that can only be reached through sharply curved endoluminal trajectory; as well as procedures in which prolonged holding of catheter that requires stability and precision is needed. Based on the existing clinical experiences and studies, a list of consensus statements was achieved by using the Delphi method, providing a standard practice for using ssRAB in the management of PPLs.
With the emerging evidence, the use of ssRAB in PPL localization and lung cancer treatment like transbronchial ablation and intratumoral drug injection is warranted. The improved reachability, stability, and preciseness will make ssRAB a preferred option for transbronchial treatment of PPLs.
Acknowledgments
The authors thank Intuitive Surgical, Inc. for providing the figures.
Footnote
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-400/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-400/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4:47-53. [Crossref] [PubMed]
- Fu R, Sun K, Wang X, et al. Survival differences between the USA and an urban population from China for all cancer types and 20 individual cancers: a population-based study. Lancet Reg Health West Pac 2023;37:100799. [Crossref] [PubMed]
- Fernandez-Bussy S, Chandra NC, Koratala A, et al. Robotic-assisted bronchoscopy: a narrative review of systems. J Thorac Dis 2024;16:5422-34. [Crossref] [PubMed]
- Xie F, Yang H, Huang R, et al. Chinese expert consensus on technical specifications of electromagnetic navigation bronchoscopy in diagnosing peripheral pulmonary lesions. J Thorac Dis 2021;13:2087-98. [Crossref] [PubMed]
- Interventional Pulmonology Group of the Chinese Thoracic Society. Experts consensus on transbronchial diagnosis, localization and treatment of peripheral pulmonary nodules guided by the augmented reality optical lung navigation. Zhonghua Yi Xue Za Zhi 2024;104:1371-80. [Crossref] [PubMed]
- Balasubramanian P, Abia-Trujillo D, Barrios-Ruiz A, et al. Diagnostic yield and safety of diagnostic techniques for pulmonary lesions: systematic review, meta-analysis and network meta-analysis. Eur Respir Rev 2024;33:240046. [Crossref] [PubMed]
- Ost D, Prichett M, Reisenauer J, et al. Prospective multicenter analysis of shape-sensing robotic-assisted bronchoscopy for the biopsy of pulmonary nodules: results from the precise study. Chest 2021;160:A2531-A2533.
- Xie F, Zhang Q, Mu C, et al. Shape-sensing Robotic-assisted Bronchoscopy (SS-RAB) in Sampling Peripheral Pulmonary Nodules: A Prospective, Multicenter Clinical Feasibility Study in China. J Bronchology Interv Pulmonol 2024;31:e0981. [Crossref] [PubMed]
- Abia-Trujillo D, Folch EE, Yu Lee-Mateus A, et al. Mobile cone-beam computed tomography complementing shape-sensing robotic-assisted bronchoscopy in the small pulmonary nodule sampling: A multicentre experience. Respirology 2024;29:324-32. [Crossref] [PubMed]
- Zhang C, Xie F, Li R, et al. Robotic-assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Thorac Cancer 2024;15:505-12. [Crossref] [PubMed]
- Ali MS, Ghori UK, Wayne MT, et al. Diagnostic Performance and Safety Profile of Robotic-assisted Bronchoscopy: A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2023;20:1801-12. [Crossref] [PubMed]
- Interventional pulmonology group of the Chinese Thoracic Society, Chinese Medical Association. Guideline for diagnostic flexible bronchoscopy in adults (2019). Zhonghua Jie He He Hu Xi Za Zhi 2019;42:573-90. [Crossref] [PubMed]
- Magnani A, Balbo P, Facchini E, et al. Lack of interference of electromagnetic navigation bronchoscopy to implanted cardioverter-defibrillator: in-vivo study. Europace 2014;16:1767-71. [Crossref] [PubMed]
- Yu Lee-Mateus A, Reisenauer J, Garcia-Saucedo JC, et al. Robotic-assisted bronchoscopy versus CT-guided transthoracic biopsy for diagnosis of pulmonary nodules. Respirology 2023;28:66-73. [Crossref] [PubMed]
- Pyarali FF, Hakami-Majd N, Sabbahi W, et al. Robotic-assisted Navigation Bronchoscopy: A Meta-Analysis of Diagnostic Yield and Complications. J Bronchology Interv Pulmonol 2024;31:70-81. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Folch EE, Mahajan AK, Oberg CL, et al. Standardized Definitions of Bleeding After Transbronchial Lung Biopsy: A Delphi Consensus Statement From the Nashville Working Group. Chest 2020;158:393-400. [Crossref] [PubMed]
- Bernasconi M, Koegelenberg CFN, Koutsokera A, et al. Iatrogenic bleeding during flexible bronchoscopy: risk factors, prophylactic measures and management. ERJ Open Res 2017;3:00084-2016. [Crossref] [PubMed]
- Lee P, Mehta AC, Mathur PN. Management of complications from diagnostic and interventional bronchoscopy. Respirology 2009;14:940-53. [Crossref] [PubMed]
- Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum 2011;41:95-105. [Crossref] [PubMed]
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008-15.
- Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67:401-9. [Crossref] [PubMed]
- Pritchett MA, Lau K, Skibo S, et al. Anesthesia considerations to reduce motion and atelectasis during advanced guided bronchoscopy. BMC Pulm Med 2021;21:240. [Crossref] [PubMed]
- Ortiz-Jaimes G, Reisenauer J. Real-World Impact of Robotic-Assisted Bronchoscopy on the Staging and Diagnosis of Lung Cancer: The Shape of Current and Potential Opportunities. Pragmat Obs Res 2023;14:75-94. [Crossref] [PubMed]
- Ho E, Hedstrom G, Murgu S. Robotic bronchoscopy in diagnosing lung cancer-the evidence, tips and tricks: a clinical practice review. Ann Transl Med 2023;11:359. [Crossref] [PubMed]
- Skouras VS, Gkiozos I, Charpidou AG, et al. Robotic Bronchoscopy in Lung Cancer Diagnosis. Cancers (Basel) 2024;16:1179. [Crossref] [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest 2002;122:1887-94. [Crossref] [PubMed]
- Zheng X, Wang L, Chen J, et al. Diagnostic value of radial endobronchial ultrasonographic features in predominant solid peripheral pulmonary lesions. J Thorac Dis 2020;12:7656-65. [Crossref] [PubMed]
- Simoff MJ, Pritchett MA, Reisenauer JS, et al. Shape-sensing robotic-assisted bronchoscopy for pulmonary nodules: initial multicenter experience using the Ion™ Endoluminal System. BMC Pulm Med 2021;21:322. [Crossref] [PubMed]
- Reisenauer J, Simoff MJ, Pritchett MA, et al. Ion: Technology and Techniques for Shape-sensing Robotic-assisted Bronchoscopy. Ann Thorac Surg 2022;113:308-15. [Crossref] [PubMed]
- Sagar AS, Sabath BF, Eapen GA, et al. Incidence and Location of Atelectasis Developed During Bronchoscopy Under General Anesthesia: The I-LOCATE Trial. Chest 2020;158:2658-66. [Crossref] [PubMed]
- Husta BC, Menon A, Bergemann R, et al. The incremental contribution of mobile cone-beam computed tomography to the tool-lesion relationship during shape-sensing robotic-assisted bronchoscopy. ERJ Open Res 2024;10:00993-2023. [Crossref] [PubMed]
- Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. [Crossref] [PubMed]
- Husta B, Batra H, Cheng G, et al. A prospective multicenter evaluation of shape-sensing robotic-assisted bronchoscopy with integrated mobile cone-beam computed tomography: interim results from the CONFIRM study. Abstract presented at: Annual Conference of American Association for Bronchology and Interventional Pulmonology; August 22, 2024; Charlotte, NC. Session 0430.
- Oberg CL, Lau RP, Folch EE, et al. Novel Robotic-Assisted Cryobiopsy for Peripheral Pulmonary Lesions. Lung 2022;200:737-45. [Crossref] [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Utility of rapid on-site evaluation of transbronchial needle aspirates. Respiration 2005;72:182-8. [Crossref] [PubMed]
- Chen CH, Cheng WC, Wu BR, et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J Thorac Dis 2015;7:S418-25. [Crossref] [PubMed]
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019;98:142-50. [Crossref] [PubMed]
- Dolan DP, Lee DN, Bharat A, et al. Chemical Localization With Robotic Bronchoscopy: Can It Aid Resection of Subsolid Lung Nodules? J Surg Res 2024;296:93-7. [Crossref] [PubMed]
- Shahoud J, Weksler B, Ghosh S, et al. Robot-Assisted Bronchoscopy for Identification of Lung Nodules During Minimally Invasive Pulmonary Resection. Innovations (Phila) 2024;19:263-7. [Crossref] [PubMed]
- Brownlee AR, Watson JJJ, Akhmerov A, et al. Robotic navigational bronchoscopy in a thoracic surgery practice: Leveraging technology in the management of pulmonary nodules. JTCVS Open 2023;16:1-6. [Crossref] [PubMed]
- Patel PP, Duong DK, Mahajan AK, et al. Single Setting Robotic Lung Nodule Diagnosis and Resection. Thorac Surg Clin 2023;33:233-44. [Crossref] [PubMed]
- Agrawal A, Hogarth DK, Murgu S. Robotic bronchoscopy for pulmonary lesions: a review of existing technologies and clinical data. J Thorac Dis 2020;12:3279-86. [Crossref] [PubMed]
- Prado RMG, Cicenia J, Almeida FA. Robotic-Assisted Bronchoscopy: A Comprehensive Review of System Functions and Analysis of Outcome Data. Diagnostics (Basel) 2024;14:399. [Crossref] [PubMed]
- Diddams MJ, Lee HJ. Robotic Bronchoscopy: Review of Three Systems. Life (Basel) 2023;13:354. [Crossref] [PubMed]
- Sabath BF, Casal RF. Bronchoscopic ablation of peripheral lung tumors. J Thorac Dis 2019;11:2628-38. [Crossref] [PubMed]
- Xie F, Wagh A, Wu R, et al. Robotic-assisted bronchoscopy in the diagnosis of peripheral pulmonary lesions. Chin Med J Pulm Crit Care Med 2023;1:30-5. [Crossref] [PubMed]
- Chan JWY, Siu ICH, Chang ATC, et al. Review on endobronchial therapies-current status and future. Ann Transl Med 2024;12:75. [Crossref] [PubMed]
- Sun T, Ge Y, Chen Z, et al. Ion robotic bronchoscopy laser ablation and Da Vinci robotic segmentectomy for bilateral pulmonary nodules: a case report. Transl Lung Cancer Res 2025;14:619-24. [Crossref] [PubMed]



