
Prognostic factors of liver metastases in extensive-stage small cell lung cancer receiving chemo-immunotherapy
Highlight box
Key findings
• Biomarkers predicting for the outcome after chemo-immunotherapy remain unclear in patients with extensive-stage small cell lung cancer (ES-SCLC).
• Univariate analysis identified advanced lung cancer inflammation index, pro-gastrin-releasing peptide, liver metastasis, and bone metastasis as significant predictors for the outcome after chemo-immunotherapy.
• Liver metastasis was a most useful predictor for the prognosis after chemo-immunotherapy.
What is known and what is new?
• The combination of maximal tumor diameter >30 mm and the presence of >10 metastatic lesions in liver metastases was identified as powerful predictor.
• The occurrence of immune-related adverse events and tumor response was closely associated with liver metastasis.
What is the implication, and what should change now?
• Liver metastasis is a significant predictor of outcomes after chemoimmunotherapy in patients with ES-SCLC.
Introduction
Small cell lung cancer (SCLC) is a progressive neoplasm with dismal features. The patients with extensive-stage SCLC (ES-SCLC) experience recurrence, regardless even if their initial response to systemic chemotherapy. The combination of platinum-based chemotherapy with an anti-programmed death-ligand 1 (PD-L1) antibody, also called chemoimmunotherapy, has recently been reported to significantly improve prognosis compared to platinum-based chemotherapy alone (1,2). A recent study identified the potential for long-term survival after initiation of chemoimmunotherapy (3). However, the expression level of PD-L1 within tumor tissues remains low in most patients with SCLC and little is known about the detailed mechanism underlying its low expression. Therefore, PD-L1 expression is unavailable for the prediction of immune checkpoint inhibitors (ICIs) in patients with ES-SCLC and has not yet been established as a significant predictor of promising biomarkers including tumor mutation burden or tumor-infiltrative lymphocytes (4).
Several real-world studies have reported potential prognostic biomarkers in patients with ES-SCLC following platinum-etoposide chemotherapy combined with anti-PD-L1 antibodies (5-7). Some retrospective studies using real-world data also confirmed that the combination of an anti-PD-L1 antibody with carboplatin and etoposide is both feasible and effective, with results comparable to those of prospective studies like IMpower 133 and Caspian (5-7). Several researchers have reported that consolidative palliative thoracic radiotherapy, liver metastasis, inflammatory markers, and performance status (PS) are closely associated with valuable prognostic factors (8-10). However, which risk factors can predict the outcome of chemoimmunotherapy for patients with ES-SCLC remains unclear. A previous study described that age ≥65 years old, PS, thoracic radiotherapy, and lactate dehydrogenase are positive prognostic factors for the patients with SCLC receiving platinum-based chemotherapy (11). Moreover, the distant sites of brain, bone, and liver metastases also suggested a shorter survival after platinum-based chemotherapy (11). However, it remains unclear what is most promising for the prognostic factors for ES-SCLC.
Thus, we examined the real-world data of patients with ES-SCLC who received chemo-immunotherapy and explored valuable risk factors, comparing our findings with those from previous studies. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1091/rc).
Methods
Patients
Between December 2019 and December 2023, 110 patients with pathologically confirmed ES-SCLC who received platinum-based chemotherapy, including etoposide and anti-PD-L1 antibodies, at our institution were eligible for the current study. Our study design was a retrospective study. Some of these cases have been reported previously (12,13). Clinical data were extracted from the medical records. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Ethics Committee of the International Medical Center at Saitama Medical University (Nos. 20-125 and 2021-113). The requirement for written informed consent was waived due to the retrospective nature of the study (14).
Treatment and evaluation
All patients received platinum-based regimens (carboplatin or cisplatin) combined with anti-PD-L1 antibodies (atezolizumab or durvalumab). Moreover, IMpower 133 (atezolizumab 1,200 mg, area under the concentration-time curve of 5 mg/mL per min carboplatin, and etoposide 100 mg/m2) and Caspian (durvalumab 1,500 mg, area under the concentration-time curve of 5 mg/mL per min carboplatin or cisplatin 80 mg/m2, and etoposide 100 mg/m2) regimens were administered intravenously (1,2). Complete blood counts, biochemical testing, physical examinations, and side effects were assessed based on the chief physician’s judgment. Toxicity was graded based on the Common Terminology Criteria for Adverse Events version 5.0. Additionally, tumor response was examined according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (15).
Assessment of the inflammatory and nutritional indices
Clinical and biological data (e.g., total protein, albumin, and C-reactive protein levels; white blood cell, neutrophil, platelet, and lymphocyte counts; and height and weight) were extracted from medical records and analyzed. Six indices reflecting systemic inflammatory and nutritional status based on a previous study (16) were calculated at baseline within 1 week of the first cycle of each treatment. The inflammatory indices were as follows: (I) neutrophil-to-lymphocyte ratio (NLR) (17); and (II) platelet-to-lymphocyte ratio (PLR) (17). The nutritional indices were as follows: (I) prognostic nutrition index (PNI) = 10 × albumin (g/dL) + 0.005 × lymphocyte count (16); and (II) advanced lung cancer inflammation index (ALI) = body mass index (BMI) (kg/m2) + albumin level (g/dL)/NLR (18).
Statistical analysis
Statistical significance was set at P<0.05. Fisher’s exact test was used to examine the association between two categorical variables. Progression-free survival (PFS) was defined as the time from initial treatment to disease progression or death. Overall survival (OS) was defined as the time from initial treatment to death from any cause. Responders and non-responders were categorized as those with an OS >12 months and those with an OS less than 12 months, respectively. The optimal cutoff values for smoking index, serum pro-gastrin-releasing peptide (ProGRP), NLR, PLR, PNI, and ALI were determined using receiver operating characteristic (ROC) curve analyses. Moreover, sensitivity and specificity were assessed to determine the optimal cutoff value for differentiating responders from non-responders using ROC curves. The Kaplan-Meier analysis was employed to estimate survival as a function of time, with survival differences analyzed using the log-rank test. Univariate and multivariate analyses of variables were performed using Cox regression. As different prognostic factors, age, sex, PS, smoking index, NLR, PLR, PNI, ALI, ProGRP, brain metastases, liver metastases, and bone metastases were analyzed. The median follow-up period was 338 days, ranging from 45 to 1,833 days. Our sample size was identified as 110 patients within the limits of resources. All statistical analyses were performed using GraphPad Prism (v.7.0e; GraphPad Software, San Diego, CA, USA) and JMP Pro 16.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics
Table 1 displays the characteristics of the 110 patients. The median age of the patients included in the study was 72 years (range, 50–88 years). Eighty-seven (79.1%) patients had a PS of 0 or 1, 91 (82.7%) were male, and 93 (84.5%) presented with stage M1. At diagnosis, metastases were present in the brain (32.7%), liver (25.5%), and bones (39.1%). The platinum-based chemotherapy regimens with atezolizumab and durvalumab were administered to 67.3% and 32.8% of patients, respectively. Among 104 patients with evaluable lesions for response, the objective response rate (ORR) was 63.6%, whereas the disease control rate was 88.2%.
Table 1
| Baseline characteristics | Data (n=110) |
|---|---|
| Age (years) | |
| ≤70 | 47 (42.7) |
| >70 | 63 (57.3) |
| ECOG PS | |
| 0 | 31 (28.2) |
| 1 | 56 (50.9) |
| 2 | 14 (12.7) |
| 3 | 8 (7.3) |
| 4 | 1 (0.9) |
| Sex | |
| Male | 91 (82.7) |
| Female | 19 (17.3) |
| Smoking index | |
| ≤1,040 | 49 (44.5) |
| >1,040 | 61 (55.5) |
| TNM M status | |
| Mo | 17 (15.5) |
| M1a | 16 (14.5) |
| M1b | 11 (10.0) |
| M1c | 66 (60.0) |
| ProGRP | |
| ≤6,803 | 22 (20.0) |
| >6,803 | 88 (80.0) |
| Brain metastases at diagnosis | |
| Present | 36 (32.7) |
| Absent | 74 (67.3) |
| Liver metastases at diagnosis | |
| Present | 28 (25.5) |
| Absent | 82 (74.5) |
| Bone metastases at diagnosis | |
| Present | 43 (39.1) |
| Absent | 67 (60.9) |
| Regimens | |
| CBDCA/VP-16/atezolizumab | 74 (67.3) |
| CBDCA/VP-16/durvalumab | 29 (26.4) |
| CDDP/VP-16/durvalumab | 7 (6.4) |
| Maintenance by PD-L1 antibody (cycles) | 2 [0–38] |
| Yes | 76 (69.1) |
| No | 34 (30.9) |
| Any grade irAEs | |
| Present | 18 (16.4) |
| Absent | 92 (83.6) |
| Response by RECIST | |
| CR | 2 (1.8) |
| PR | 68 (61.8) |
| SD | 21 (19.1) |
| PD | 13 (11.8) |
| NE | 6 (5.5) |
| Received second line | |
| Yes | 60 (54.5) |
| No | 50 (45.5) |
| NLR | |
| High | 75 (68.2) |
| Low | 35 (31.8) |
| PLR | |
| High | 61 (55.5) |
| Low | 49 (44.5) |
| PNI | |
| High | 29 (26.4) |
| Low | 81 (73.6) |
| ALI | |
| High | 65 (59.1) |
| Low | 45 (40.9) |
Data are presented as number (%) or median [range]. ALI, advanced lung cancer inflammation index; CBDCA, carboplatin; CDDP, cisplatin; CR, complete response; ECOG, Eastern Cooperative Oncology Group; irAE, immune-related adverse event; M, metastasis; NE, not evaluable; NLR, neutrophil-to-lymphocyte ratio; PD, progressive disease; PD-L1, programmed death-ligand 1; PNI, prognostic nutrition index; PLR, platelet-to-lymphocyte ratio; PR, partial response; ProGRP, pro-gastrin-releasing peptide; PS, performance status; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; TNM, tumor-node-metastasis; VP-16, etoposide.
The median values for the NLR, PLR, PNI, ALI, smoking index, and ProGRP before chemo-immunotherapy were 4.2 (range, 0.9–22.3), 205.8 (range, 12.3–1579.2), 42.9 (range, 22.0–57.0), 18.8 (range, 0–120.0), 1,020 (range, 0–2,640), and 600 (range, 31–95,277), respectively. The optimal cutoff values for the NLR, PLR, PNI, ALI, smoking index, and ProGRP as determined by ROC curve analyses were 3.3 (sensitivity: 41.3%, specificity: 75.0%), 197 (sensitivity: 63.0%, specificity: 50.0%), 47 (sensitivity: 47.8%, specificity: 82.8%), 14 (sensitivity: 73.9%, specificity: 43.8%), 1,040 (sensitivity: 77.8%, specificity: 31.3%), and 6,803 (sensitivity: 86.9%, specificity: 23.5%), respectively. The areas under the curve in the ROC analysis were as follows: 0.568 for NLR, 0.507 for PLR, 0.682 for PNI, 0.611 for ALI, 0.488 for smoking index, and 0.479 for ProGRP. High values for the NLR, PLR, PNI, ALI, smoking index, and ProGRP were observed in 68.2%, 55.5%, 26.4%, 59.1%, 55.5%, and 80.0% of patients, respectively.
Survival analysis based on different variables
The median PFS and OS of all the patients were 174 and 372 days, respectively. Ninety-eight patients experienced recurrence, and 82 died due to disease progression. The Kaplan-Meier survival curves for PFS and OS are displayed in Figure 1.
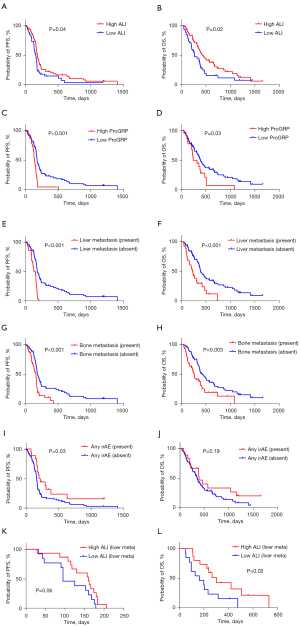
Univariate analysis identified sex, ALI, ProGRP, liver metastasis, and bone metastasis as significant predictors of PFS, while age, sex, PS, NLR, ALI, ProGRP, liver metastasis, and bone metastasis were demonstrated to be significant predictors of OS (Table 2). The application of a univariate log-rank test enabled the screening of variables with a cutoff of P<0.05 for subsequent multivariate analysis. Multivariate analysis demonstrated that liver metastasis was an independent prognostic factor for predicting poor PFS and OS, meanwhile, sex was an independent predictor of OS (Table 2).
Table 2
| Different variables | PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| Median MST (days) | P value | P value | Median MST (days) | P value | P value | ||||
| Age (≤70/>70 years) | 174/174 | 0.36 | 475/356 | 0.03* | 0.14 | ||||
| Sex (male/female) | 168/182 | 0.02* | 0.054 | 356/464 | 0.16 | 0.03* | |||
| PS (0–1/2–4) | 174/166 | 0.55 | 375/364 | 0.03* | 0.06 | ||||
| Smoking index (high/low) | 174/170 | 0.66 | 339/386 | 0.18 | |||||
| NLR (high/low) | 168/182 | 0.15 | 356/576 | 0.01* | 0.08 | ||||
| PLR (high/low) | 170/174 | 0.27 | 376/339 | 0.38 | |||||
| PNI (high/low) | 181/165 | 0.23 | 561/339 | 0.07 | |||||
| ALI (high/low) | 182/160 | 0.04* | 0.19 | 420/268 | 0.02* | 0.19 | |||
| ProGRP (high/low) | 139/179 | <0.001* | 0.39 | 238/386 | 0.03* | 0.57 | |||
| Brain metastases (yes/no) | 165/180 | 0.09 | 372/376 | 0.55 | |||||
| Liver metastases (yes/no) | 141/182 | <0.001* | 0.001* | 227/389 | <0.001* | 0.005* | |||
| Bone metastases (yes/no) | 160/182 | <0.001* | 0.18 | 262/425 | 0.003* | 0.31 | |||
*, P<0.05. ALI, advanced lung cancer inflammation index; MST, median survival time; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; ProGRP, pro-gastrin-releasing peptide; PS, performance status.
Different variables based on efficacy and outcome
Table 3 presents the patients’ characteristics according to OS >12 months, OS >24 months, induction of maintenance therapy, and response to RECIST. Female sex, high PNI, and the absence of liver metastasis were significantly associated with OS >12 months, whereas female sex and low NLR were closely correlated with OS >24 months. However, none of the variables predicted the therapeutic benefit of maintenance therapy or the achievement of complete response or partial response (PR) according to RECIST.
Table 3
| Different variables | All patients (n=110) | OS 12 months | OS 24 months | Maintenance | Response by RECIST | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| More (n=46) | Less (n=64) | P value |
More (n=16) | Less (n=94) | P value |
Yes (n=76) | No (n=34) | P value |
CR/PR (n=70) | SD/PD (n=34) | P value | |||||
| Age (≤70/>70 years) | 47/63 | 23/23 | 24/40 | 0.24 | 9/7 | 38/56 | 0.28 | 34/42 | 13/21 | 0.54 | 31/39 | 12/22 | 0.40 | |||
| Sex (male/female) | 91/19 | 33/13 | 58/6 | 0.01* | 10/6 | 81/13 | 0.03* | 64/12 | 27/7 | 0.58 | 54/16 | 31/3 | 0.10 | |||
| PS (0–1/2–4) | 87/13 | 39/7 | 48/16 | 0.24 | 15/1 | 72/22 | 0.18 | 60/16 | 27/7 | >0.99 | 60/10 | 28/6 | 0.77 | |||
| Smoking index (high/low) | 49/61 | 19/27 | 30/34 | 0.69 | 6/10 | 43/51 | 0.59 | 33/43 | 16/18 | 0.83 | 35/35 | 12/22 | 0.20 | |||
| NLR (high/low) | 75/35 | 27/19 | 48/16 | 0.09 | 7/9 | 68/26 | 0.03* | 50/26 | 25/9 | 0.50 | 48/22 | 23/11 | >0.99 | |||
| PLR (high/low) | 61/49 | 29/17 | 32/32 | 0.24 | 10/6 | 51/43 | 0.14 | 44/32 | 17/17 | 0.53 | 38/32 | 18/16 | >0.99 | |||
| PNI (high/low) | 29/81 | 19/27 | 10/54 | 0.004* | 7/9 | 22/72 | 0.12 | 21/55 | 8/26 | 0.81 | 17/53 | 10/24 | 0.63 | |||
| ALI (high/low) | 65/45 | 31/15 | 34/30 | 0.16 | 12/4 | 53/41 | 0.18 | 48/28 | 17/17 | 0.21 | 43/28 | 19/15 | 0.67 | |||
| ProGRP (high/low) | 22/88 | 7/39 | 15/49 | 0.34 | 1/15 | 21/73 | 0.18 | 14/62 | 8/26 | 0.60 | 13/57 | 8/26 | 0.60 | |||
| Brain metastases (yes/no) | 36/74 | 16/30 | 20/44 | 0.83 | 4/12 | 32/62 | 0.57 | 28/48 | 8/26 | 0.19 | 22/48 | 12/22 | 0.82 | |||
| Liver metastases (yes/no) | 28/82 | 6/40 | 22/42 | 0.01* | 1/15 | 27/67 | 0.06 | 19/57 | 9/25 | >0.99 | 13/57 | 12/22 | 0.08 | |||
| Bone metastases (yes/no) | 43/67 | 13/33 | 30/34 | 0.07 | 3/13 | 40/54 | 0.09 | 28/48 | 15/19 | 0.52 | 24/48 | 17/17 | 0.13 | |||
| Any grade irAEs (yes/no) | 18/92 | 10/36 | 8/56 | 0.29 | 4/12 | 14/80 | 0.29 | 15/61 | 3/31 | 0.17 | 11/59 | 6/28 | 0.78 | |||
| Steroid use (yes/no) | 11/99 | 4/42 | 7/57 | 0.75 | 2/14 | 9/85 | 0.66 | 7/69 | 4/30 | 0.73 | 7/63 | 4/30 | 0.74 | |||
Data are presented as number. *, P<0.05. ALI, advanced lung cancer inflammation index; irAE, immune-related adverse event; CR, complete response; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PD, progressive disease; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; PR, partial response; ProGRP, pro-gastrin-releasing peptide; PS, performance status; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable response.
Patient’s characteristics according to the presence of liver metastases
Considering the results of the survival analysis (Table 2), we investigated different variables related to liver metastases (Table 4). High ProGRP levels, bone metastasis, occurrence of immune-related adverse events (irAEs) of any grade, and a PR response were significantly associated with the presence of liver metastasis.
Table 4
| Different variables | All patients (n=110) | Liver metastases | ||
|---|---|---|---|---|
| Present (n=28) | Absent (n=82) | P value | ||
| Age (≤70/>70 years) | 47/63 | 13/15 | 34/48 | 0.66 |
| Sex (male/female) | 91/19 | 24/4 | 67/15 | 0.77 |
| PS (0–1/2–4) | 87/13 | 27/1 | 60/12 | 0.06 |
| Smoking index (high/low) | 49/61 | 14/14 | 35/47 | 0.51 |
| NLR (high/low) | 75/35 | 19/9 | 56/26 | >0.99 |
| PLR (high/low) | 61/49 | 14/14 | 47/35 | 0.51 |
| PNI (high/low) | 29/81 | 4/24 | 25/57 | 0.13 |
| ALI (high/low) | 65/45 | 15/13 | 50/32 | 0.51 |
| ProGRP (high/low) | 22/88 | 13/15 | 9/73 | <0.001* |
| Brain metastases (yes/no) | 36/74 | 7/21 | 29/53 | 0.35 |
| Bone metastases (yes/no) | 43/67 | 18/10 | 25/57 | 0.003* |
| Maintenance (yes/no) | 76/34 | 19/9 | 57/25 | >0.99 |
| Any grade irAEs (yes/no) | 18/92 | 1/27 | 17/65 | 0.03* |
| Steroid use (yes/no) | 11/99 | 2/26 | 9/73 | 0.72 |
| ICI type (Atez/Durva) | 74/36 | 17/11 | 57/25 | 0.48 |
| Response (PR/non-PR) | 47/63 | 7/21 | 40/42 | 0.04* |
Data are presented as number. *, P<0.05. ALI, advanced lung cancer inflammation index; Atez, atezolizumab; Durva, durvalumab; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; PR, partial response; ProGRP, pro-gastrin-releasing peptide; PS, performance status.
Survival analysis in 28 patients with liver metastasis
Univariate and multivariate analyses of patients with liver metastasis were performed in addition to the survival analysis of all patients (Table 5). The maximum size and number of liver metastases were incorporated into the subsequent analysis. Among the 28 patients with liver metastases, the median maximal diameter of liver metastases was 28 mm, ranging from 9 to 103 mm. Additionally, 18 (64.3%) patients had more than 10 liver metastases. The cutoff values for the maximal size and number of liver metastases were 30 and 10, respectively. Univariate analysis identified ALI, maximal size of liver metastasis, and a combination of a maximal size greater than 30 mm with more than 10 metastases as significant predictors for OS, but not PFS. The application of a univariate log-rank test enabled the screening of variables with a cutoff of P<0.05 for subsequent multivariate analysis. Multivariate analysis identified the combination of maximal size >30 mm and number of metastases >10 as independent predictors of OS in 28 patients with liver metastasis.
Table 5
| Different variables | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Univariate | Multivariate | ||||||
| Median MST (days) | P value | Median MST (days) | P value | HR (95% CI) | P value | |||
| Age (≤70/>70 years) | 125/160 | 0.11 | 223/238 | 0.36 | ||||
| Sex (male/female) | 155/127 | 0.35 | 212/248 | 0.89 | ||||
| PS (0–1/2–4) | 141/123 | 0.31 | 235/136 | 0.16 | ||||
| Smoking index (high/low) | 139/143 | 0.78 | 181/238 | 0.46 | ||||
| NLR (high/low) | 125/160 | 0.92 | 202/302 | 0.40 | ||||
| PLR (high/low) | 121/161 | 0.42 | 217/230 | 0.65 | ||||
| PNI (high/low) | 165/131 | 0.75 | 490/199 | 0.08 | ||||
| ALI (high/low) | 160/95 | 0.06 | 302/161 | 0.02* | 2.137 (0.865–5.434) | 0.09 | ||
| ProGRP (high/low) | 157/130 | 0.99 | 238/166 | 0.63 | ||||
| Brain metastases (yes/no) | 130/157 | 0.32 | 265/223 | 0.72 | ||||
| Bone metastases (yes/no) | 145/139 | 0.49 | 212/273 | 0.70 | ||||
| Maximal size of liver metastases (≤30/>30 mm) | 118/153 | 0.82 | 315/202 | 0.03* | ||||
| Numbers of liver metastases (≤10>10) | 160/121 | 0.26 | 315/199 | 0.20 | ||||
| Size >30/numbers >10 of liver metastases (yes/no) | 139/143 | 0.64 | 181/302 | 0.008* | 2.875 (1.015–8.173) | 0.04* | ||
*, P<0.05. ALI, advanced lung cancer inflammation index; CI, confidence interval; HR, hazard ratio; MST, median survival time; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; ProGRP, pro-gastrin-releasing peptide; PS, performance status.
Sequential treatment and irAEs in all patients
Of 110 patients, 45 (40.9%) were treated with amurubicin monotherapy as a second-line setting, 9 (8.2%) received a re-challenge of platinum (carboplatin or cisplatin)-based regimens with anti-PD-L1 antibodies (atezolizumab or durvalumab), and four (3.6%) were managed with carboplatin plus etoposide. Among 45 patients receiving amurubicin monotherapy, 16 achieved an ORR of 35.6% (16/45). In subsequent treatments, 10 patients received carboplatin plus paclitaxel, while one patient was treated with irinotecan monotherapy.
Moreover, irAEs were observed in 18 (16.4%) of 110 patients. Grade 1 or 2 hypothyroidism, grade 1 adrenal deficiency, grade 2 renal disorder, grade 1 pneumonitis, grade 1 arthritis, and grade 2 fatigue were observed in five, one, one, one, one, and two patients, respectively. Nine (8.2%) patients experienced grade 3 irAEs: pneumonitis for five patients, encephalitis for one, adrenal deficiency for two, and myocarditis for one. However, no patients experienced treatment-related deaths in this study.
Discussion
We attempted to identify a promising biomarker to predict outcomes following chemoimmunotherapy in patients with ES-SCLC. Although some real-world data is available regarding chemoimmunotherapy for patients with ES-SCLC, little is known about the possible risk factors, aside from consolidative palliative thoracic radiotherapy, liver metastasis, inflammatory markers, and PS (8-10). In the present study, we identified liver metastasis as a significant predictor of outcome after chemotherapy. Furthermore, liver metastasis was significantly associated with high levels of ProGRP, bone metastasis, and resistance to chemoimmunotherapy. Moreover, patients with a maximum tumor size greater than 30 mm and more than 10 liver metastases had an increased risk after chemoimmunotherapy. We believe that the maximal tumor size, in addition to multiple liver metastases, may be resistant to chemoimmunotherapy in patients with ES-SCLC. However, our study could not elucidate why liver metastasis significantly increased the risk of recurrence or death after chemotherapy.
ES-SCLC is biologically characterized by its tendency to rapidly spread to different organs and is highly responsive to chemotherapy and radiotherapy. However, therapeutic prediction of chemotherapy for ES-SCLC is still being developed worldwide. Considering the results of our study, we determined that ALI and liver metastasis are promising predictors for chemoimmunotherapy. Several researchers have reported that a low ALI is significantly associated with poor OS in patients with SCLC (19-21). Although previous reports focused on the heterogeneous population including limited-stage (LS) or ES-SCLC, and chemotherapy or chemoradiotherapy (18-20), Ürün et al. reported that low ALI was identified as an independent predictor for poor OS in 98 patients with ES-SCLC receiving chemotherapy (22). However, little is known about the relationship between ALI and the outcomes after immunotherapy for ES-SCLC. Cai et al. described the efficacy and safety of thoracic radiotherapy combined with chemoimmunotherapy in 78 patients with ES-SCLC, indicating that primary liver metastasis was a predictor of poor outcomes (23). Surveillance, Epidemiology, and End Results data analyzing 27,163 SCLC cases identified age, sex, clinical stage, presence of liver metastasis, and absence of chemotherapy as risk factors for early mortality (24). A recent meta-analysis suggested that lactate dehydrogenase and baseline liver and brain metastases may be helpful risk factors in predicting the efficacy of ICIs in patients with SCLC (25). Although metastasis is generally associated with poor prognosis, the liver microenvironment is particularly conducive to the survival of SCLC cells, and liver metastases promote an immunosuppressive environment (25-30). Therefore, the therapeutic efficacy of immunotherapy can be reduced by inhibiting the immune response to liver metastases. As a possible mechanism, the decreased immune response may be caused by a significant reduction in the CD8+ T cells at the margins of liver metastatic tumors in patients with melanoma (31). In addition, our study highlighted the prognostic relevance between poor response and the maximal diameter of liver metastasis. We discovered that patients with SCLC with a maximal tumor diameter of >30 mm and >10 liver metastases were not suitable for chemoimmunotherapy. In these patients, a new targeted therapy may be necessary to improve the efficacy of systemic treatment.
ProGRP is a common tumor marker used for the diagnosis and therapeutic monitoring of patients with SCLC. Li et al. reported that a decrease in ProGRP levels after chemotherapy could be a better predictor of response to chemotherapy in patients with SCLC with high ProGRP levels at baseline (32). However, whether high levels of ProGRP at baseline indicate a poor response to chemoimmunotherapy in ES-SCLC remains unclear. Our study identified ProGRP as a significant predictor of outcome after chemotherapy. Our previous study demonstrated that the metabolic tumor volume as measured by 2-deoxy-2-(fluorine-18)-fluoro-D-glucose (18F-FDG) uptake within SCLC cells could predict the outcome of chemoimmunotherapy in patients with ES-SCLC (13). The level of ProGRP may also correlate with total tumor volume, similar to the assessment by 18F-FDG accumulation. In our study, the presence of liver metastasis was significantly correlated with high levels of ProGRP and bone metastasis. Further investigations are required to elucidate the discovery of the close relationship between ProGRP levels and liver metastasis.
Our study had several limitations. First, the sample size was limited to elucidating the optimal conclusion by sub-analysis. The number of patients with liver metastases was 28; thus, further studies are warranted to recruit more patients with liver metastases. Secondly, liver metastasis was identified as a significantly poor outcome following chemotherapy. However, the tumor immune microenvironment in SCLC with liver metastasis, particularly in hepatic tumor specimens, remains unclear. To elucidate the mechanism underlying the poor immune response, a biopsy of hepatic tumor tissues may be required for further analysis. Finally, the follow-up period in our study was limited; thus, we could not examine possible predictors of long-term survival after initial treatment.
Conclusions
Liver metastasis was identified as a significant predictor of outcome after chemoimmunotherapy in patients with ES-SCLC. In particular, the maximal diameter and number of liver metastases may affect the immune response in patients with liver metastasis. ALI is a significant serum biomarker for predicting poor prognosis after chemoimmunotherapy. Further investigations are warranted to elucidate the immunological mechanisms underlying the poor response in patients with ES-SCLC with liver metastasis.
Acknowledgments
The authors thank Ms. Kozue Watanabe, Ms. Saki Toita, and Ms. Koko Kodaira for their assistance in preparing the manuscript. The authors also thank Editage (https://www.editage.jp/) for English language editing.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1091/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1091/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1091/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1091/coif). K.K. received a speaker honorarium from Ono Pharmaceutical Company, Chugai Pharmaceutical, and AstraZeneca and research grants from AstraZeneca, and reports that this work was supported by the JSPS Grant-in-Aid for Scientific Research C (No. 24K10292). A.M. and O.Y. received speaker honoraria from Chugai Pharmaceutical and AstraZeneca, respectively. H.K. received research grants and speaker honoraria from Ono Pharmaceutical Company, Bristol-Myers Company, Boehringer Ingelheim, MSD, Chugai Pharmaceutical, and AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Ethics Committee of the International Medical Center at Saitama Medical University (Nos. 20-125 and 2021-113). The requirement for written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022;7:100408. [Crossref] [PubMed]
- Paz-Ares L, Garassino MC, Chen Y, et al. Durvalumab ± Tremelimumab + Platinum-Etoposide in Extensive-Stage Small Cell Lung Cancer (CASPIAN): Outcomes by PD-L1 Expression and Tissue Tumor Mutational Burden. Clin Cancer Res 2024;30:824-35. [Crossref] [PubMed]
- Lee S, Shim HS, Ahn BC, et al. Efficacy and safety of atezolizumab, in combination with etoposide and carboplatin regimen, in the first-line treatment of extensive-stage small-cell lung cancer: a single-center experience. Cancer Immunol Immunother 2022;71:1093-101. [Crossref] [PubMed]
- Elegbede AA, Gibson AJ, Fung AS, et al. A Real-World Evaluation of Atezolizumab Plus Platinum-Etoposide Chemotherapy in Patients With Extensive-Stage SCLC in Canada. JTO Clin Res Rep 2021;2:100249. [Crossref] [PubMed]
- Qu J, Kalyani FS, Shen Q, et al. Efficacy and Safety of PD-L1 Inhibitors plus Chemotherapy versus Chemotherapy Alone in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer: A Retrospective Real-World Study. J Oncol 2022;2022:3645489. [Crossref] [PubMed]
- Lamy D, Mouillot P, Mariet A, et al. Real-world comparison of chemo-immunotherapy and chemotherapy alone in the treatment of extensive-stage small-cell lung cancer. Respir Med Res 2024;86:101125. [Crossref] [PubMed]
- Dang J, Xu G, Guo G, et al. Construction of a prognostic model for extensive-stage small cell lung cancer patients undergoing immune therapy in northernmost China and prediction of treatment efficacy based on response status at different time points. J Cancer Res Clin Oncol 2024;150:255. [Crossref] [PubMed]
- Xie J, Chen M, Han H, et al. Clinical impact of first-line PD-1 or PD-L1 inhibitors combined with chemotherapy in extensive-stage small cell lung cancer patients: A real-world multicenter propensity score-matched study. Thorac Cancer 2023;14:1327-38. [Crossref] [PubMed]
- He M, Chi X, Shi X, et al. Value of pretreatment serum lactate dehydrogenase as a prognostic and predictive factor for small-cell lung cancer patients treated with first-line platinum-containing chemotherapy. Thorac Cancer 2021;12:3101-9. [Crossref] [PubMed]
- Masubuchi K, Imai H, Wasamoto S, et al. Post-progression survival after atezolizumab plus carboplatin and etoposide as first-line chemotherapy in small cell lung cancer has a significant impact on overall survival. Thorac Cancer 2022;13:2776-85. [Crossref] [PubMed]
- Hashimoto K, Kaira K, Imai H, et al. Metabolic Tumor Volume as Significant Predictor for Chemotherapy Containing PD-L1 Blocker in Extensive Stage Small Cell Lung Cancer. Anticancer Res 2024;44:1541-51. [Crossref] [PubMed]
- Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol 2022;52:539-44. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Mahiat C, Bihin B, Duplaquet F, et al. Systemic Inflammation/Nutritional Status Scores Are Prognostic but Not Predictive in Metastatic Non-Small-Cell Lung Cancer Treated with First-Line Immune Checkpoint Inhibitors. Int J Mol Sci 2023;24:3618. [Crossref] [PubMed]
- Seban RD, Assié JB, Giroux-Leprieur E, et al. Prognostic value of inflammatory response biomarkers using peripheral blood and 18F-FDG PET/CT in advanced NSCLC patients treated with first-line chemo- or immunotherapy. Lung Cancer 2021;159:45-55. [Crossref] [PubMed]
- Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013;13:158. [Crossref] [PubMed]
- He X, Zhou T, Yang Y, et al. Advanced Lung Cancer Inflammation Index, a New Prognostic Score, Predicts Outcome in Patients With Small-Cell Lung Cancer. Clin Lung Cancer 2015;16:e165-71. [Crossref] [PubMed]
- Kim EY, Kim N, Kim YS, et al. Prognostic Significance of Modified Advanced Lung Cancer Inflammation Index (ALI) in Patients with Small Cell Lung Cancer_ Comparison with Original ALI. PLoS One 2016;11:e0164056. [Crossref] [PubMed]
- Seo BM, Choi J, Chang B, et al. Clinical significance of the advanced lung cancer inflammation index in patients with limited-stage small cell lung cancer treated with chemoradiotherapy. Sci Rep 2024;14:10347. [Crossref] [PubMed]
- Ürün M, Güner G, Sezgin Y, et al. Prognostic Significance of the Advanced Lung Cancer Inflammation Index in Metastatic Small Cell Lung Cancer: A Retrospective Analysis of 96 Patients. Med Sci Monit 2024;30:e945752. [Crossref] [PubMed]
- Cai Z, Gu X, Xie J, et al. Safety and efficacy of thoracic radiotherapy combined with chemo-immunotherapy in patients with extensive-stage small cell lung cancer: a multicenter retrospective analysis. Transl Lung Cancer Res 2023;12:1987-2000. [Crossref] [PubMed]
- Li Z, Pang M, Liang X, et al. Risk factors of early mortality in patients with small cell lung cancer: a retrospective study in the SEER database. J Cancer Res Clin Oncol 2023;149:11193-205. [Crossref] [PubMed]
- Li LL, Yu CF, Xie HT, et al. Biomarkers and factors in small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med 2023;12:11211-33. [Crossref] [PubMed]
- Cheng Y, Wang Q, Li K, et al. Anlotinib for patients with small cell lung cancer and baseline liver metastases: A post hoc analysis of the ALTER 1202 trial. Cancer Med 2022;11:1081-7. [Crossref] [PubMed]
- Fan Z, Huang Z, Tong Y, et al. Sites of Synchronous Distant Metastases, Prognosis, and Nomogram for Small Cell Lung Cancer Patients with Bone Metastasis: A Large Cohort Retrospective Study. J Oncol 2021;2021:9949714. [Crossref] [PubMed]
- Sun P, Snow S. On the precipice of a new generation of biomarkers for immunotherapy in small cell lung cancer. Transl Cancer Res 2025;14:24-8. [Crossref] [PubMed]
- Li R, Li H, Tang C, et al. Metastatic sites, prognosis, and a new nomogram for predicting overall survival among small cell lung cancer patients with liver metastasis: a retrospective study based on SEER. J Thorac Dis 2025;17:344-56. [Crossref] [PubMed]
- Zhang W, Zhao W, Zhang X, et al. Efficacy and safety of first-line immunotherapy-based regimens for patients with extensive-stage small cell lung cancer: a systematic review and network meta-analysis. Transl Lung Cancer Res 2025;14:163-75. [Crossref] [PubMed]
- Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res 2017;5:417-24. [Crossref] [PubMed]
- Li M, Han D, Wang W, et al. Decline in serum progastrin-releasing peptide predicts the response of patients with small cell lung cancer to chemotherapy. Oncol Lett 2020;20:301. [Crossref] [PubMed]


