
Change of bone mineral density as a prognostic marker in small cell lung cancer treated with immune checkpoint inhibitors: a multicenter retrospective study
Highlight box
Key findings
• This study demonstrates that the change in bone mineral density (ΔBMD) is significantly associated with shorter progression-free survival and overall survival (OS) in small cell lung cancer (SCLC) patients treated with immune checkpoint inhibitors (ICIs). ΔBMD was identified as an independent prognostic factor for OS (risk ratio =0.461; P<0.001). A nomogram incorporating ΔBMD and other factors achieved robust predictive accuracy, with time-dependent area under the curve (AUC) values of 0.743, 0.782, and 0.781 for OS at 9, 12, and 18 months, respectively.
What is known and what is new?
• While bone mineral density (BMD) changes are known to predict survival in various diseases, this is the first study to establish ΔBMD as a prognostic marker specifically for SCLC patients undergoing ICI therapy. The novel integration of ΔBMD into a clinical nomogram provides a practical tool for outcome prediction in this population.
What is the implication, and what should change now?
• These findings highlight the importance of monitoring BMD dynamics in SCLC patients receiving ICIs. Clinicians should consider incorporating BMD assessments into routine clinical practice to improve risk stratification and personalize treatment strategies. Further validation in larger cohorts and exploration of mechanisms linking BMD loss to ICI response are warranted.
Introduction
Lung cancer represents the foremost cause of cancer-related mortality globally, accounting for 18.0% of all cancer deaths (1), and small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancers (2), which is highly invasive, proliferates rapidly, and metastasizes early, and has the highest degree of malignancy and the worst prognosis among all lung cancer subtypes. Since SCLC often metastasizes early, radical resection is feasible in only a small proportion of patients with limited stage, and most patients are treated with chemotherapy, radiotherapy, and immunotherapy.
In recent years, immune checkpoint inhibitors (ICIs) combined with chemotherapy have made major breakthroughs and become the new standard of first-line treatment as the median overall survival (OS) of SCLC patients reaches 12 months (3). Notably, ICIs that block cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and inhibit programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathways may offer potential benefits to SCLC patients (4). Most patients with SCLC develop metastatic disease, and this, along with drug resistance, contributes to a generally poor long-term prognosis. Incorporating ICIs into first-line chemotherapy regimens has only modestly improved median survival. The 5-year survival rates range from 15–30% for patients with limited-stage disease and less than 1% for those with extensive-stage disease (1,5). Consequently, clinical benefit has been limited, with slight improvements in OS and only a minority of patients benefiting from these immune-based therapies (6-8). In order to determine which patients are candidates for ICI treatment, accurate predictive biomarkers are desperately needed.
Body composition analysis is increasingly recognized for its prognostic significance in oncology, as specific metrics such as muscle mass, adiposity, and bone mineral density (BMD) can provide valuable insights into patient outcomes and treatment responses across various cancers (9-11). Among these indicators, BMD reflects not only skeletal health but also broader systemic factors that may impact disease progression (12). Notably, lower BMD has been associated with poorer outcomes in cancer patients, suggesting that BMD may serve as a useful biomarker for assessing prognosis (11).
Recent advancements in the study of BMD have also highlighted its relevance in the context of ICIs, where changes in BMD may reflect modifications within the bone microenvironment and shifts in immune response associated with ICI therapy (13). Techniques for measuring BMD, including dual-energy X-ray absorptiometry (DXA), quantitative computed tomography (QCT), and computed tomography (CT) plain scan, offer reliable and reproducible assessments, enhancing the potential for BMD to guide treatment planning and risk stratification. The gold standard for diagnosing osteoporosis is the T-score obtained using DXA (14). Research indicates that there is a moderate to good positive association between the average pixel density [Hounsfield unit (HU) values] on chest CT and BMD and trabecular bone score (TBS). Additionally, HU values of CT show good performance in discriminating between people with osteopenia/osteoporosis and those without it (15). Since lung cancer patients routinely undergo chest CT scans, baseline, and post-immunotherapy BMD can be noninvasively assessed using HU values at the first lumbar vertebral level (16). While the prognostic role of BMD has been well-documented in non-small cell lung cancer (NSCLC) (17), where it correlates with patient outcomes, its significance in SCLC remains less clear, particularly given the distinct biological characteristics and limited response to immunotherapy in SCLC. Therefore, in patients with SCLC, dynamic quantification of BMD may be a novel and promising prognostic factor as a surrogate for bone loss, aiming to fill a crucial gap in understanding the role of BMD in this aggressive cancer type. The aim of this study was to analyze the prognostic value of BMD in SCLC. We present this article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1125/rc).
Methods
This retrospective cohort study has been authorized by the institutional review board of the following three hospitals and the local ethics committees: Wuhan Union Hospital (Institutional Review Board No. 0464), Xiangyang No.1 People’s Hospital (Institutional Review Board No. XYYYE20240076), The 900th Hospital of the Joint Logistics Team, Fujian Medical University (Institutional Review Board No. 2023-097). And they waived the obligation for informed consent. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Clinical data were evaluated retrospectively and anonymously.
Study design and patient selection
This retrospective analysis was conducted on patients diagnosed with SCLC and treated with ICIs from January 2016 to May 2024 at three hospitals: Wuhan Union Hospital, Xiangyang No.1 People’s Hospital, and The 900th Hospital of the Joint Logistics Team. We gathered and examined CT scans both before and after the course of treatment. Before starting therapy, CT scans were regularly carried out, and 6 months later, they were routinely followed up on. The inclusion criteria were as follows: (I) non-enhanced chest CT scans before and after ICI or other anti-tumor treatments; (II) histologically or cytologically confirmed SCLC; (III) age >18 years old; and (IV) receipt of ICIs and other anti-tumor treatments or receipt of ICIs only for at least four consecutive cycles. Exclusion criteria were as follows: (I) combination of other primary malignancies; (II) surgically treated SCLC; (III) subsequent treatment with ICI (not at the start of treatment); and (IV) incomplete medical records.
Procedure
Covariates of interest were gathered retrospectively, such as the following: patient demographics [age, gender, body mass index, Eastern Cooperative Oncology Group performance status (ECOG performance status), hypertension, smoking, hyperlipidemia, alcohol consumption], biochemical data [alkaline phosphatase, lactate dehydrogenase, Ca, blood urea nitrogen, hemoglobin, albumin to globulin ratio (A/G ratio), neuronal enolase, carcinoembryonic antigen], tumor-related information (pathology type, stage) and further disease-specific information (ICI type, previous radiotherapy treatment). All baseline data were derived from the patient’s first admission and discharge medical records.
BMD measurement and assessment
Baseline and pretreatment CT scans were obtained using one of the following commercially available multidetector computed tomography (MDCT) scanners at the Wuhan Union Hospital: 64-, 128-, 256-section (Siemens, Erlangen, Germany; Philips, Amsterdam, Netherlands; Toshiba, Tokyo, Japan; GE, Chicago, USA). Baseline and pretreatment CT scans were obtained using one of the following commercially available MDCT scanners at the Xiangyang No.1 People’s Hospital: 64-, 128-, 320-section (Siemens; Toshiba; GE). Baseline and pretreatment CT scans were obtained using one of the following commercially available MDCT scanners at The 900th Hospital of the Joint Logistics Team, Fujian Medical University: 64-, 128-section (Siemens; Philips). Detailed information regarding the CT parameters is summarized in Table S1. Without access to the clinical information, two independent radiologists (Lingli Li and Qianqian Fan with 11 and 12 years of thoracic imaging experience, respectively) independently evaluated the pictures and determined the BMD on a Phillips Intelli Space Portal workstation (version 10.1, Best, The Netherlands). The study involved CT plain scans of intervertebral discs at the L1 level. The region of interest (ROI) was defined as the largest rounded portion of the disc in the L1 plane, while avoiding areas with high CT values caused by tumor-induced bone metastasis. Two observers independently outlined the ROI on CT lung images obtained before and after immunotherapy. Each observer performed two measurements per CT image, with a 2-week interval between measurements. Intra-observer agreement was evaluated by comparing the two measurements from the same observer, while inter-observer agreement was assessed by comparing measurements between the two observers. HU on unenhanced chest CT at the base of the first lumbar vertebrae within the mid-nucleus pulposus circle was used to compute BMD (Figure 1).
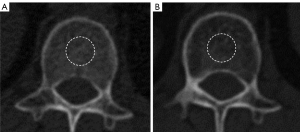
Time normalization for BMD
The relative rates of change of CT parameters must be normalized by the criteria of previous studies due to the variations in the time interval between the two CT scans before and after treatment in different patients (18). Since the study’s median period between two CT scans was 246 days, we used that number as the standard duration to determine each parameter’s relative rate of change, which we expressed as ΔBMD: ΔBMD = (post-treatment BMD − pre-treatment BMD) × median time interval/(time interval × pre-treatment BMD). After determining the ideal cutoff value with the X-tile program (version 3.6.1), all patients were categorized into two groups: those with severe BMD loss (lower ΔBMD, ΔBMD ≤−0.12) and those with non-severe BMD loss (higher ΔBMD, ΔBMD >−0.12).
Definition and evaluation of data
Each patient was followed up until May 2024. Follow-up chest CT scans were compared with baseline imaging in accordance with the Response Evaluation Criteria for Solid Tumors version 1.1 (RECIST 1.1) (19) to determine the length of progression-free survival (PFS) and the ratios of overall response rate (ORR) and disease control rate (DCR) between the two groups. ORR and DCR were calculated based on the number of complete responses (CRs), partial responses (PRs), and cases of stable disease (SD). PFS was defined as the time from the initiation of ICI therapy to the onset of tumor progression or patient death. OS was defined as the time from the start of ICI therapy to the final follow-up or patient death.
Statistical analysis
With the use of X-tile software (Yale University School of Medicine, New Haven, CT, USA), critical values (BMD-related indices) were calculated. Based on survival curves, this software offers a thorough way to divide cohorts into those with low and high levels of marker expression. The differences in PFS and OS between the two groups were compared using the Kaplan-Meier method and the log-rank test. The Cox proportional risk model was used for both univariate and multivariate analysis. Hazard ratio (HR) and confidence intervals (CIs) were computed, and variables with univariate P values of less than 0.1 were added to the multivariate Cox regression model. Using univariate Cox models, PFS and OS risk ratios were determined for each subgroup and displayed as forest plots. Independent factors were used to construct a nomogram model, and the consistency index (C-index) and time-dependent area under the subjects’ work receiver operating characteristic (ROC) curve values were used to assess the nomogram model’s predictive power. To compare observed and anticipated survival measured at the 12- and 18-month milestones of patient survival following ICIs, we constructed a calibration plot. Intragroup correlation coefficients (ICCs) were used to measure the intra-group consistency of body composition characteristics; ICC values greater than 0.75 indicated full repeatability. R version 4.3.0 (R Foundation) and SPSS version 26.0 (IBM, Armonk, NY, USA) were used for all analyses. All tests were two-tailed, and statistical significance was defined as a P value less than 0.05.
Results
Patient characteristics
A total of 300 SCLC patients (109 in the severe BMD loss group and 191 in the non-severe BMD loss group) were involved in this study, all of whom were treated with ICIs. The baseline clinical and demographic details of the patients treated with ICIs are presented in Table S2. Among all patients, a higher percentage of patients were male and aged <65 years old, which may be related to epidemiology. In contrast, laboratory test parameters were not significantly different in the two groups, which reduces the bias of confounders in subsequent analyses.
In addition, to explore the predictive value of ΔBMD in non-immunotherapy patients, we selected a total of 258 SCLC patients who received concurrent standard chemotherapy at three centers to investigate the prognostic effectiveness of ΔBMD in non-immunotherapy patients. ΔBMD was time-standardized, and the optimal threshold for ΔBMD classification was determined by using X-tile software to classify the patients into the low group (n=114) and the high group (n=144). Baseline characteristics of patients not treated with ICI are shown in Table S3. The non-severe BMD loss group had a higher squamous cell carcinoma antigen, A/G ratio, and a greater number of patients under 65 years than the severe BMD loss group. In addition, the severe BMD loss group had a higher Ki67 and a higher prevalence of hyperlipidemia.
Optimal cutoff values for ΔBMD
The most optimal cut values for ΔBMD classification were found using the X-tile program (Figure S1A,S1B). Specifically, we utilized the OS outcome as a reference; based on making sure the OS of the two groups followed the same trend at all important moments, we chose the point where the variation in outcome between the two groups was the highest. After deciding that the last essential value is −0.12, 109 patients fell into the severe BMD loss group and 191 patients fell into the non-severe BMD loss group.
Tumor response
Table S4 shows tumor response. Short-term treatment results for the two groups were somewhat similar overall. With no significant distinction (ORR, P=0.43; DCR, P=0.12), ORR and DCR were 27.6% and 72.4% in the severe BMD loss group and 32.1% and 80.5% in the non-severe BMD loss group respectively. Especially in the severe BMD loss group, more patients reached PD than in the non-severe BMD loss group (27.6% vs. 19.6%).
Intra- and inter-observer comparison
The assessment was repeated 2 weeks later using the same method with intra- and inter-observer agreement of 0.91 (95% CI: 0.88 to 0.94) and 0.90 (95% CI: 0.84 to 0.93), respectively.
Survival analysis
Ninety of the 109 patients (82.6%) in the group with severe BMD loss and 106 of the 191 patients (55.5%) in the group with non-severe BMD loss died during the median follow-up of 13.0 months [interquartile range (IQR), 17.0–29.0 months]. Patients with severe BMD loss group and those with non-severe BMD loss group were compared using Kaplan-Meier survival curves for PFS and OS. Log-rank test showed that the PFS in the group with severe BMD loss (5.8 vs. 7.7 months, P=0.04) and OS (11.6 vs. 19.9 months, P<0.001) were shorter than those of the non-severe BMD loss group (Figure 2A,2B).
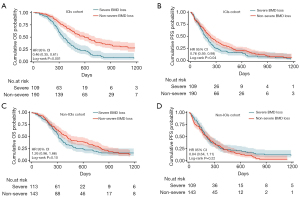
In non-ICI patients (chemotherapy cohort) (258 cases) we found that: OS was longer in the non-severe BMD loss group than in the severe BMD loss group, although there was no statistical difference (P=0.10, Figure 2C), there was no significant difference in PFS between the two groups (P=0.22, Figure 2D).
Cox regression analysis and subgroup analysis
Carcinoembryonic antigen, clinical stages, A/G ratio, prior radiation therapy, type of ICIs and ΔBMD group were identified as potential predictors of PFS, univariate regression study revealed several possible predictors of OS: clinical stages, alkaline phosphatase, carcinoembryonic antigen, neuron specific enolase, prior radiation therapy, and ΔBMD group. The multivariate regression analysis used these factors in addition. In multivariate analysis, the extensive disease (ED) (HR =1.527; 95% CI: 1.001–2.331; P=0.049), higher alkaline phosphatase (HR =1.004; 95% CI: 1.001–1.006; P=0.001), and higher carcinoembryonic antigen (HR =1.000; 95% CI: 1.000–1.001; P=0.01), without prior radiation therapy (HR =1.592; 95% CI: 1.131–2.242; P=0.008), and in the severe BMD loss group (HR =2.169; 95% CI: 1.543–3.049; P<0.001) were significantly associated with shorter OS (Table 1), ED (HR =1.828; 95% CI: 1.289–2.591; P<0.001), without prior radiation therapy (HR =1.449; 95% CI: 1.086–1.931; P=0.01), higher carcinoembryonic antigen (HR =1.000; 95% CI: 1.000–1.000; P=0.005) and higher A/G ratio (HR =1.023; 95% CI: 1.004–1.042; P=0.02) were significantly associated with shorter PFS (Table 2).
Table 1
| Parameters | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender | |||||
| Male | Reference | – | – | ||
| Female | 0.805 (0.501–1.293) | 0.37 | – | – | |
| Age | |||||
| <65 years | Reference | – | – | ||
| ≥65 years | 1.096 (0.824–1.458) | 0.53 | – | – | |
| ECOG status | |||||
| 0 | Reference | – | – | ||
| ≥1 | 1.071 (0.677–1.693) | 0.77 | – | – | |
| Clinical stages | |||||
| Limited disease | Reference | Reference | |||
| Extensive disease | 1.835 (1.285–2.618) | <0.001 | 1.527 (1.001–2.331) | 0.049 | |
| Smoking | |||||
| No | Reference | – | – | ||
| Yes | 0.890 (0.671–1.181) | 0.42 | – | – | |
| Alkaline phosphatase | 1.004 (1.002–1.005) | <0.001 | 1.004 (1.001–1.006) | 0.001 | |
| BMI | 1.015 (0.988–1.044) | 0.28 | – | – | |
| Hypertension | |||||
| No | Reference | – | – | ||
| Yes | 0.907 (0.644–1.277) | 0.58 | – | – | |
| Hyperlipidemia | |||||
| No | Reference | – | – | ||
| Yes | 1.154 (0.861–1.548) | 0.34 | – | – | |
| Ki67 | 0.569 (0.196–1.653) | 0.30 | – | – | |
| Ca | 1.674 (0.615–4.561) | 0.31 | – | – | |
| Drinking | |||||
| No | Reference | – | – | ||
| Yes | 0.818 (0.594–1.126) | 0.22 | – | – | |
| Carcinoembryonic antigen | 1.000 (1.000–1.001) | 0.01 | 1.000 (1.000–1.001) | 0.01 | |
| A/G ratio | 0.984 (0.934–1.037) | 0.55 | – | – | |
| Neuron specific enolase | 1.003 (1.001–1.005) | 0.003 | 1.001 (0.999–1.003) | 0.45 | |
| Prior radiation therapy | |||||
| No | Reference | Reference | |||
| Yes | 0.575 (0.429–0.771) | <0.001 | 0.628 (0.446–0.884) | 0.008 | |
| Type of ICIs | |||||
| PD-1 | Reference | – | – | ||
| PD-L1 | 1.050 (0.508–2.168) | 0.90 | – | – | |
| Group | |||||
| Severe BMD loss | Reference | Reference | |||
| Non-severe BMD loss | 0.459 (0.346–0.610) | <0.001 | 0.461 (0.328–0.648) | <0.001 | |
A/G ratio, albumin to globulin ratio; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; OS, overall survival.
Table 2
| Parameters | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender | |||||
| Male | Reference | – | – | ||
| Female | 1.036 (0.689–1.560) | 0.86 | – | – | |
| Age | |||||
| <65 years | Reference | – | – | ||
| ≥65 years | 0.896 (0.695–1.154) | 0.39 | – | – | |
| ECOG status | |||||
| 0 | Reference | – | – | ||
| ≥1 | 1.027 (0.693–1.522) | 0.89 | – | – | |
| Clinical stages | |||||
| Limited disease | Reference | Reference | |||
| Extensive disease | 1.927(1.412–2.625) | <0.001 | 1.828 (1.289–2.591) | <0.001 | |
| Smoking | |||||
| No | Reference | – | – | ||
| Yes | 0.899 (0.699–1.155) | 0.41 | – | – | |
| Alkaline phosphatase | 1.001 (0.999–1.003) | 0.29 | – | – | |
| BMI | 1.000 (0.974–1.027) | >0.99 | – | – | |
| Hypertension | |||||
| No | Reference | – | – | ||
| Yes | 1.099 (0.814–1.484) | 0.54 | – | – | |
| Hyperlipidemia | |||||
| No | Reference | – | – | ||
| Yes | 0.921 (0.705–1.203) | 0.54 | – | – | |
| Ki67 | 0.940 (0.355–2.493) | 0.90 | – | – | |
| Ca | 1.227 (0.559–2.692) | 0.61 | – | – | |
| Drinking | |||||
| No | Reference | – | – | ||
| Yes | 0.818 (0.594–1.126) | 0.22 | – | – | |
| Carcinoembryonic antigen | 1.000 (1.000–1.001) | 0.01 | 1.000 (1.000–1.000) | 0.005 | |
| A/G ratio | 1.023 (1.004–1.042) | 0.02 | 1.023 (1.004–1.042) | 0.02 | |
| Neuron specific enolase | 1.000 (0.999–1.002) | 0.59 | – | – | |
| Prior radiation therapy | |||||
| No | Reference | Reference | |||
| Yes | 0.719 (0.556–0.931) | 0.01 | 0.690 (0.518–0.920) | 0.01 | |
| Type of ICIs | |||||
| PD-1 | Reference | Reference | |||
| PD-L1 | 1.305 (1.013–1.681) | 0.04 | 1.261 (0.952–1.672) | 0.11 | |
| Group | |||||
| Severe BMD loss | Reference | Reference | |||
| Non-severe BMD loss | 0.761 (0.589–0.984) | 0.04 | 0.829 (0.621–1.107) | 0.20 | |
A/G ratio, albumin to globulin ratio; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PFS, progression-free survival.
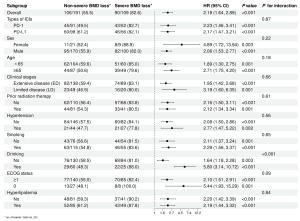
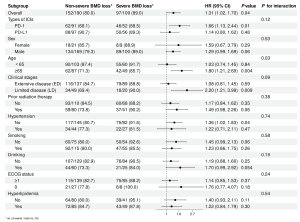
We performed subgroup analyses based on baseline characteristics of patients and obtained similar results for each subgroup OS, the risk of short OS was high in the severe BMD loss group compared with the non-severe BMD loss group (Figure 3). In addition, we found an interaction between alcohol consumption and bone loss status, specifically, a higher risk of short OS was observed for severe bone loss in patients who consumed alcohol compared to patients who did not consume alcohol. Severe BMD loss was associated with a higher risk of short PFS than non-severe BMD loss in four subgroups: PD-1, aged ≥65 years, limited disease (LD), without hypertension, whereas similar results were not found in other subgroups (Figure 4). In addition, there was an interaction between age and bone loss.
Construction of the nomogram model
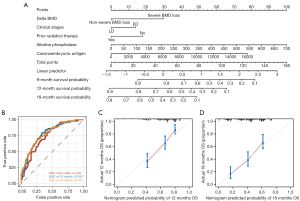
The five independent prognostic factors identified to be associated with OS were integrated to construct the nomogram model (Figure 5A). The projection range of the total score on the corresponding scale was 0–160, and the 9-, 12-, and 18-month survival rates of SCLC patients treated with ICIs were predicted within this projection range. The nomogram model predicted 9-, 12-, and 18-month OS probabilities between 0.9–0.1, 0.9–0.1, and 0.8–0.1, respectively. The ROC curves over time showed that the area under the curve (AUC) of the 9-, 12-, and 18-month OS curves were 0.743, 0.782, and 0.781, respectively (Figure 5B), with a C-index of 0.701 (95% CI: 0.684–0.801). Furthermore, the OS calibration curves at 12 and 18 months after treatment demonstrated a good agreement between the actual data and the nomogram predictions (Figure 5C,5D).
Discussion
ICIs can be used in clinical decision-making for extensive-stage SCLC patients; however, due to the metastatic nature of SCLC and multiple mechanisms, it is difficult for clinicians to differentiate between patients who are more amenable to ICIs. Our study is the first to predict the prognosis of SCLC based on the indicator of bone mass change. And our results showed that more severe bone loss was associated with shorter OS and also with shorter PFS. In addition, we explored the prognostic value of ΔBMD in SCLC patients with non-ICIs. The results were that there was no significant difference in the prognosis of ΔBMD for SCLC patients with non-ICIs.
Several factors may explain the association between changes in BMD and poor prognosis in cancer patients undergoing ICIs. Immune system-derived cytokines, including macrophage colony-stimulating factor (M-CSF), interleukin (IL), transforming growth factor β (TGF-β), prostaglandins, and interferon γ, are responsible for the differentiation of osteoblast precursors into osteoclasts. The balance between osteoblasts and osteoclasts is upset by the cytokines released by tumor cells themselves, leading to bone resorption and the creation of an immune-suppressive milieu known as the “vicious circle” (20,21). This idea was also supported by a clinical study that showed a significant reduction in BMD in lung cancer patients compared to those who did not have cancer (22).
Research has demonstrated that during treatment, changes in body composition frequently accompany malignancies, such as muscle, bone mass, and adiposity, which are strongly associated with patient prognosis (23-25). It has been demonstrated that BMD may have predictive value for a number of cancer types, including hepatocellular carcinoma and breast cancer (26,27). Analyzing 11 studies (2,330 individuals) on the association between gastrointestinal cancers and BMD, Watanabe et al. (28) revealed that bone loss was independently associated with a poor prognosis in these patients. Similar findings have been documented in the case of gastrointestinal tumors. This is consistent with the findings of Tao et al. on 106 patients with gastric cancer, where changes in bone mass before and after treatment were independent prognostic factors for gastric cancer (29). In this study, changes in BMD before and after treatment were independent factors in the prognosis of patients with SCLC.
In our study, we found an interaction between alcohol consumption and bone loss profile, specifically, patients who consumed alcohol [odds ratio (OR) =1.715; 95% CI: 1.191–2.470] experienced a higher risk of short OS for severe bone loss compared to patients who did not consume alcohol in a subgroup analysis.
Some studies have shown that alcohol use is a risk factor for bone loss in cancer patients (22), this may be relevant.
In addition to this, we developed a nomogram containing body composition parameters to predict the prognosis of SCLC patients treated with ICI. The ROC curves over time showed AUCs of 0.743, 0.782, and 0.781 for the 9-, 12-, and 18-month OS curves, respectively, with a C-index of 0.701 (95% CI: 0.663–0.739). To the best of our knowledge, this is the first column-line plot containing body composition parameters for predicting the prognosis of SCLC treated with ICI. Based on our nomogram, a more personalized chemotherapy regimen plan and a shorter follow-up period for the high-risk subgroup should be implemented.
There are limitations in this study. First, this study is retrospective, and larger prospective clinical trials are needed to validate our results in the future. Second, the lack of control and monitoring of diet and exercise during patient follow-up may have affected the accuracy and reliability of the findings. Furthermore, we did not verify the body composition values derived from CT scanning with outcomes obtained from alternative modalities such as DXA. Finally, we would like to recognize that the L1 vertebral lamina BMD assessment used in the study is not fully representative of an individual’s overall body composition. Nonetheless, this study provides strong findings suggesting that pre- and post-treatment BMD loss has potential prognostic utility in patients receiving immunotherapy. Through the use of targeted nutritional therapies and the assessment of this body composition parameter in risk stratification, individualized management strategies can be developed for these patients.
Conclusions
Our study found that for SCLC patients, the relative rate of change in BMD at baseline and after ICI treatment was strongly associated with long-term prognostic prognosis, although there was no difference in their short-term outcomes. Future research should focus on elucidating the mechanisms underlying the observed association between BMD changes and prognosis, as well as exploring interventions to mitigate bone loss and improve clinical outcomes. Additionally, integrating BMD assessments into routine follow-up may enhance the stratification of patients for tailored therapeutic strategies.
Acknowledgments
We would like to thank all the investigators and patients who participated in this project.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1125/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1125/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1125/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1125/coif). P.S. is an employee of Philips Healthcare. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This retrospective cohort study has been authorized by the institutional review board of the following three hospitals and the local ethics committees: Wuhan Union Hospital (Institutional Review Board No. 0464), Xiangyang No.1 People’s Hospital (Institutional Review Board No. XYYYE20240076), The 900th Hospital of the Joint Logistics Team, Fujian Medical University (Institutional Review Board No. 2023-097). And they waived the obligation for informed consent. Clinical data were evaluated retrospectively, and anonymously.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. [Crossref] [PubMed]
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Arriola E, Wheater M, Galea I, et al. Outcome and Biomarker Analysis from a Multicenter Phase 2 Study of Ipilimumab in Combination with Carboplatin and Etoposide as First-Line Therapy for Extensive-Stage SCLC. J Thorac Oncol 2016;11:1511-21. [Crossref] [PubMed]
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Decazes P, Ammari S, Belkouchi Y, et al. Synergic prognostic value of 3D CT scan subcutaneous fat and muscle masses for immunotherapy-treated cancer. J Immunother Cancer 2023;11:e007315. [Crossref] [PubMed]
- Tamura S, Ashida R, Sugiura T, et al. The prognostic impact of skeletal muscle status and bone mineral density for resected distal cholangiocarcinoma. Clin Nutr 2021;40:3552-8. [Crossref] [PubMed]
- Müller L, Mähringer-Kunz A, Auer TA, et al. Low bone mineral density is a prognostic factor for elderly patients with HCC undergoing TACE: results from a multicenter study. Eur Radiol 2023;33:1031-9. [Crossref] [PubMed]
- Engblom C, Pfirschke C, Zilionis R, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science 2017;358:eaal5081. [Crossref] [PubMed]
- Brom VC, Strauss AC, Sieberath A, et al. Agonistic and antagonistic targeting of immune checkpoint molecules differentially regulate osteoclastogenesis. Front Immunol 2023;14:988365. [Crossref] [PubMed]
- Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002;359:1929-36. [Crossref] [PubMed]
- Lee H, Park S, Kwack KS, et al. CT and MR for bone mineral density and trabecular bone score assessment in osteoporosis evaluation. Sci Rep 2023;13:16574. [Crossref] [PubMed]
- Chassagnon G, Revel MP. Lung cancer screening: Current status and perspective. Diagn Interv Imaging 2016;97:949-53. [Crossref] [PubMed]
- Lou J, Gong B, Li Y, et al. Bone mineral density as an individual prognostic biomarker in NSCLC patients treated with immune checkpoint inhibitors. Front Immunol 2024;15:1332303. [Crossref] [PubMed]
- Wang X, Zhang C, Cao F, et al. Nomogram of Combining CT-Based Body Composition Analyses and Prognostic Inflammation Score: Prediction of Survival in Advanced Epithelial Ovarian Cancer Patients. Acad Radiol 2022;29:1394-403. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Liu C, Wang M, Xu C, et al. Immune Checkpoint Inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J Immunol Res 2021;2021:8970173. [Crossref] [PubMed]
- Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res 2017;36:108. [Crossref] [PubMed]
- Huang JF, Tan QC, Bai H, et al. Bone mineral density, osteopenia and osteoporosis among US adults with cancer. QJM 2022;115:653-60. [Crossref] [PubMed]
- Hacker UT, Hasenclever D, Linder N, et al. Prognostic role of body composition parameters in gastric/gastroesophageal junction cancer patients from the EXPAND trial. J Cachexia Sarcopenia Muscle 2020;11:135-44. [Crossref] [PubMed]
- Aleixo GFP, Shachar SS, Nyrop KA, et al. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol 2020;145:102839. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Sharma P, Parikh ND, Yu J, et al. Bone mineral density predicts posttransplant survival among hepatocellular carcinoma liver transplant recipients. Liver Transpl 2016;22:1092-8. [Crossref] [PubMed]
- Tseng OL, Dawes MG, Spinelli JJ, et al. Utilization of bone mineral density testing among breast cancer survivors in British Columbia, Canada. Osteoporos Int 2017;28:3439-49. [Crossref] [PubMed]
- Watanabe J, Saitsu A, Miki A, et al. Prognostic value of preoperative low bone mineral density in patients with digestive cancers: a systematic review and meta-analysis. Arch Osteoporos 2022;17:33. [Crossref] [PubMed]
- Tao C, Hong W, Yin P, et al. Nomogram Based on Body Composition and Prognostic Nutritional Index Predicts Survival After Curative Resection of Gastric Cancer. Acad Radiol 2024;31:1940-9. [Crossref] [PubMed]


