
Impact of coronavirus disease 2019 on surgery in patients with early-stage lung cancer: the COVIDLungSurg prospective cohort study
Highlight box
Key findings
• In our prospective cohort study, we included 1,734 early-stage lung cancer patients who received surgery between January 2023 and April 2024. Our results showed no significant difference in the rate of postoperative 30-day complications in these patients with and without preoperative coronavirus disease 2019 (COVID-19). Preoperative COVID-19 was not associated with increased risk of postoperative complications. Moreover, the time since COVID-19 to surgery did not show any association with the risk of postoperative complications.
What is known and what is new?
• Previous studies reported an increased risk of postoperative complications (sometimes pulmonary complications or mortality) in patients with a history of COVID-19. All these studies were conducted before the emergence of the Omicron variant and the use of COVID-19 vaccination. However, there is a paucity of evidence on whether COVID-19 has a negative impact on the perioperative outcomes of these patients. Our study prospectively enrolled early-stage lung cancer patients to investigate the association between COVID-19 and postoperative complications during the Omicron epidemic wave.
What is the implication, and what should change now?
• Our findings suggest an update to the current guidelines on the timing of surgery for patients with preoperative COVID-19, at least for early-stage lung cancer patients with a history of mild COVID-19.
Introduction
There have been over 700 million cases of coronavirus disease 2019 (COVID-19) reported globally (1). As the number of people who had COVID-19 rises, it will be increasingly common for patients with a history of COVID-19 to undergo surgery. Previous studies have found that a prior history of COVID-19 is associated with worse postoperative outcomes and complications (2-7). A seven-eight weeks delay of surgery after the diagnosis of COVID-19 was recommended, which could lower the postoperative mortality and morbidity (2,5).
While postponing surgery could lower the risk of postoperative complications, it also resulted in delayed medical care for many patients, potentially allowing their conditions to worsen while they waited. Moreover, these studies were mainly conducted in the early phase of the COVID-19 pandemic. As the pandemic develops, its characteristics continue to evolve. On the one hand, a greater number of people have been fully vaccinated against the virus, providing protection against COVID-19 (8). As of June 4, 2022, about 87% of people in China had been fully vaccinated against the coronavirus (9). On the other hand, the Omicron variant emerged and has become the dominant variant since the end of January 2022 (10,11). Although the Omicron variant is more transmissible than other variants, it is less pathogenic compared to earlier ones, resulting in less severe disease (8,12-15). More recent studies using data from the Omicron epidemic wave have shown that the risk of developing postoperative complications associated with preoperative COVID-19 is lower than that in previous phases (16,17).
Surgery is the standard treatment for early-stage lung cancer (18). With the use of minimally invasive surgery, an early-stage lung cancer patient can recover quickly and be discharged within one week after surgery (19,20). There is a paucity of evidence that whether COVID-19 has a negative impact on the perioperative outcomes of these patients. Therefore, we started this multicenter, prospective, observational cohort study to investigate the association between COVID-19 and postoperative complications in early-stage lung cancer patients during the Omicron epidemic wave. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1276/rc).
Methods
Study design and participants
This was a multicenter, prospective cohort study conducted in five hospitals in China. Ethics approval has been obtained from Ethics Committees of Ruijin Hospital (Approval No. 2023-03), Anhui Chest Hospital (Approval No. K2023-001), Shanghai Ninth People’s Hospital (Approval No. SH9H-2023-T104-1), Shanghai Tongji Hospital (Approval No. K-2023-002), and Guangdong Provincial People’s Hospital (Approval No. KY2023-589-01). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. All participating patients provided written informed consent. Adults aged 18 years or older were eligible for the study if they had been clinically diagnosed with stage I/II lung cancer and were scheduled to undergo surgery. The staging of the patients was in accordance with the 8th edition of the tumor node metastasis (TNM) classification for lung cancer (21). The surgery types included wedge resection, segmental resection, sleeve resection, lobectomy, or pneumonectomy under general anesthesia. The Charlson comorbidity index (CCI) was calculated to reflect the burden of comorbid conditions. Patients were classified as having preoperative COVID-19 based on any one of the following criteria: (I) positive reverse transcription polymerase chain reaction (RT-PCR) nasopharyngeal swab taken at any time before surgery; (II) positive rapid antigen test performed at any time before surgery. All the included patients were tested negative on RT-PCR at the time of surgery. The eligible patients should sign the informed consent and be able to complete the observation and follow-up. The American Society of Anesthesiologist (ASA) score of the included patients should be I-III. Patients with suspected diagnosis of COVID-19 without the positive results of RT-PCR or antigen test were excluded. Patients who were diagnosed with COVID-19 between postoperative day 0–30 were excluded. Other exclusion criteria included patients who were unable to cooperate or describe the treatment response, clinically diagnosed stage III/IV lung cancer patients, or patients who received neoadjuvant treatment before surgery. The severity of the patients’ COVID-19 was determined using the World Health Organization clinical progression scale (22). This study was registered at ClinicalTrials.gov (NCT05684549).
Outcomes
The primary outcome was the rate of postoperative 30-day complication. Postoperative complication was defined as any complication occurring within 30 days from surgery. The severity of complications was evaluated based on the Clavien-Dindo classification of surgical complications (23). The secondary outcomes included total length of hospital stay, postoperative stay, and postoperative 30-day mortality.
Statistical analysis
Continuous variables that followed a normal distribution were reported as mean [standard deviation (SD)], and the Student’s t-test was used for comparison. For variables that did not follow a normal distribution, they were presented as median [interquartile range (IQR)], and compared using the Wilcoxon rank-sum test between the two groups. Unadjusted baseline characteristics and comparisons between patients with and without prior COVID-19 were analyzed using standard descriptive statistics. Multivariable logistic regression models adjusting for potential confounders were utilized to estimate the odds ratios (ORs) of postoperative complication.
Propensity score matched (PSM) analysis was performed to control the baseline characteristics between patients with and without history of COVID-19. Each patient’s propensity score was calculated from a multivariable logistic regression model with covariates including sex, age, clinical TNM staging, smoking history, vaccination status, and surgery type. Patients in the two groups were matched 1:1 using the nearest-neighbor method with a caliper of 0.05. After PSM analysis, we compared the rate of postoperative complication and other perioperative outcomes between the two groups.
The associations between the time since COVID-19 to surgery and postoperative complications were evaluated on a continuous scale with restricted cubic spline curves based on logistic regression model. To balance best fit and overfitting in the main splines for postoperative complications, the number of knots, between three and seven, was chosen as the lowest value for the Akaike information criterion. We also performed post-hoc subgroup analysis to see the associations between the time since COVID-19 to surgery and postoperative complications using logistic regression and restricted cubic spline models.
Statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA) and R (version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were 2-sided and considered significant at P<0.05.
Results
Between January 2023 and April 2024, a total of 2,005 patients were screened and 1,734 patients were included in the final analysis (Figure S1). There were 238 patients (13.7%) without a history of COVID-19 [95 men (39.9%); median age: 60 (IQR, 52–68) years] and 1,496 patients (86.3%) with a history of COVID-19 [527 men (35.2%); median age: 56 (IQR, 45–66) years]. Only one patient received open thoracotomy. Among patients who received minimally invasive surgery, 1,707 patients received video-assisted thoracoscopic surgery, and 26 patients received robotic-assisted thoracoscopic surgery. Baseline characteristics of the total sample, unweighted sample, and PSM sample were shown in Table 1. The comorbidities of the included patients were shown in Table S1. All patients completed the postoperative 30-day follow-up.
Table 1
| Characteristic | Total sample (N=1,734) | Unweighted sample | PSM sample | ||||||
|---|---|---|---|---|---|---|---|---|---|
| History of COVID-19 (N=1,496) |
No history of COVID-19 (N=238) | SMD | History of COVID-19 (N=232) |
No history of COVID-19 (N=232) | SMD | ||||
| Sex | 0.097 | 0.053 | |||||||
| Female | 1,112 (64.1) | 969 (64.8) | 143 (60.1) | 147 (63.4) | 141 (60.8) | ||||
| Male | 622 (35.9) | 527 (35.2) | 95 (39.9) | 85 (36.6) | 91 (39.2) | ||||
| Age (years) | 57 [46–66] | 56 [45–66] | 60 [52–68] | 0.378 | 60 [53–68] | 60 [53–68] | 0.002 | ||
| Smoking history | 0.135 | 0.049 | |||||||
| Never smoked | 1,520 (87.7) | 1,321 (88.3) | 199 (83.6) | 201 (86.6) | 197 (84.9) | ||||
| Smoker | 214 (12.3) | 175 (11.7) | 39 (16.4) | 31 (13.4) | 35 (15.1) | ||||
| Vaccination status | 0.104 | 0.065 | |||||||
| Fully vaccinated | 1,538 (88.7) | 1,334 (89.2) | 204 (85.7) | 205 (88.4) | 200 (86.2) | ||||
| Non-fully vaccinated | 196 (11.3) | 162 (10.8) | 34 (14.3) | 27 (11.6) | 32 (13.8) | ||||
| CCI | 0.385 | 0.125 | |||||||
| 0 | 526 (30.3) | 482 (32.2) | 44 (18.5) | 39 (16.8) | 44 (19.0) | ||||
| 1 | 442 (25.5) | 382 (25.5) | 60 (25.2) | 70 (30.2) | 60 (25.9) | ||||
| 2 | 392 (22.6) | 337 (22.5) | 55 (23.1) | 59 (25.4) | 55 (23.7) | ||||
| 3+ | 374 (21.6) | 295 (19.7) | 79 (33.2) | 64 (27.6) | 73 (31.5) | ||||
| Clinical TNM staging | 0.248 | 0.082 | |||||||
| IA1 | 713 (41.1) | 633 (42.3) | 80 (33.6) | 74 (31.9) | 79 (34.1) | ||||
| IA2 | 723 (41.7) | 623 (41.6) | 100 (42.0) | 103 (44.4) | 100 (43.1) | ||||
| IA3 | 220 (12.7) | 176 (11.8) | 44 (18.5) | 45 (19.4) | 42 (18.1) | ||||
| IB | 44 (2.5) | 37 (2.5) | 7 (2.9) | 5 (2.2) | 7 (3.0) | ||||
| IIA | 24 (1.4) | 18 (1.2) | 6 (2.5) | 4 (1.7) | 3 (1.3) | ||||
| IIB | 10 (0.6) | 9 (0.6) | 1 (0.4) | 1 (0.4) | 1 (0.4) | ||||
| Surgery type | 0.258 | 0.073 | |||||||
| Wedge resection | 679 (39.2) | 586 (39.2) | 93 (39.1) | 80 (34.5) | 83 (35.8) | ||||
| Segmentectomy | 564 (32.5) | 507 (33.9) | 57 (23.9) | 52 (22.4) | 57 (24.6) | ||||
| Lobectomy | 491 (28.3) | 403 (26.9) | 88 (37.0) | 100 (43.1) | 92 (39.7) | ||||
Data are presented as n (%) or median [interquartile range]. CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; PSM, propensity score matched; SMD, standardized mean difference; TNM, tumor node metastasis.
In the unadjusted analysis, there was no difference in the rates of 30-day postoperative complications in patients with and without a history of COVID-19 (9.2% vs. 11.8%, P=0.20) (Table 2). One patient with a history of COVID-19 died of gastrointestinal bleeding within the first postoperative 30 days. After adjusting for sex, age, smoking history, vaccination status, CCI, clinical TNM staging, and surgery type, patients with a history of COVID-19 had a similar rate of postoperative 30-day complications compared with patients without a history of COVID-19 [OR, 0.99; 95% confidence interval (CI): 0.63 to 1.60; P=0.96] (Table S2). After PSM analysis, there were 232 patients in each group (Table 1), and there was no difference in the rate of 30-day postoperative complications in the two groups (11.2% vs. 11.6%, P=0.89) (Table S3).
Table 2
| Characteristic | History of COVID-19 (N=1,496) | No history of COVID-19 (N=238) | P value |
|---|---|---|---|
| Total hospital stay (days) | 7 [6–8] | 7 [6–9] | 0.04 |
| Postoperative hospital stay (days) | 3 [3–5] | 3 [3–5] | 0.70 |
| Chest tube duration (days) | 3 [2–4] | 3 [2–4] | 0.71 |
| Chest tube drainage (mL) | 258 [120–530] | 260 [130–496] | 0.54 |
| 30-day mortality | 1 (0.07) | 0 (0) | >0.99 |
| Postoperative complications | 137 (9.2) | 28 (11.8) | 0.20 |
| Clavien Dindo I–II | 110 (7.4) | 18 (7.6) | 0.91 |
| Pleural effusion | 24 (1.6) | 6 (2.5) | 0.31 |
| Atrial fibrillation | 8 (0.5) | 2 (0.8) | 0.91 |
| Hypertension | 8 (0.5) | 2 (0.8) | 0.91 |
| Pneumothorax | 7 (0.5) | 2 (0.8) | 0.80 |
| Air leak | 44 (2.9) | 8 (3.4) | 0.72 |
| Pneumonia | 6 (0.4) | 1 (0.4) | >0.99 |
| Atelectasis | 1 (0.07) | 0 (0) | >0.99 |
| Hypoproteinemia | 1 (0.07) | 0 (0) | >0.99 |
| Pulmonary embolus | 1 (0.07) | 0 (0) | >0.99 |
| Chylothorax | 1 (0.07) | 0 (0) | >0.99 |
| Peptic ulcer | 1 (0.07) | 0 (0) | >0.99 |
| Subcutaneous emphysema | 8 (0.5) | 2 (0.8) | 0.91 |
| Thrombocytopenia | 1 (0.07) | 0 (0) | >0.99 |
| Delirium | 1 (0.07) | 0 (0) | >0.99 |
| Clavien Dindo grade III–IV | 27 (1.8) | 9 (3.8) | 0.05 |
| Subcutaneous emphysema | 8 (0.5) | 2 (0.8) | 0.91 |
| Pneumothorax | 15 (1.0) | 4 (1.7) | 0.55 |
| Air leak | 7 (0.5) | 0 (0) | 0.60 |
| Pleural effusion | 10 (0.7) | 4 (1.7) | 0.22 |
| Bronchopleural fistula | 1 (0.07) | 0 (0) | >0.99 |
| Clavien Dindo grade V | 0 (0) | 1 (0.4) | 0.14 |
| Gastrointestinal bleeding | 0 (0) | 1 (0.4) | 0.14 |
Data are presented as median [interquartile range] or n (%). COVID-19, coronavirus disease 2019.
Of the 1,496 patients with a history of COVID-19, 1,458 patients had mild diseases, 37 patients had moderate diseases, and one patient had a severe disease. Nearly 90% of the COVID-19 occurred after November 2022 (Table S4). The median time from COVID-19 to surgery was 26 weeks (IQR, 14–48 weeks). The postoperative complication rates by time since COVID-19 to surgery are shown in Table S5. The distribution of estimated risk for the continuous variable of time since COVID-19 to surgery was analyzed graphically using splines (Figure 1). The results showed that the 95% CI included the OR of 1.0 for any time since COVID-19 to surgery, which indicated that time since COVID-19 to surgery was not associated with postoperative complication (Figure 1B). The results of subgroup analysis also confirmed that time since COVID-19 to surgery was not associated with postoperative complication (Figure S2). In the multivariable analysis, the following factors were associated with higher risk of postoperative complications: smoker (OR, 2.13; 95% CI: 1.22–3.69; P=0.01), higher CCI, e.g., 2 vs. 0 (OR, 4.72; 95% CI: 1.56–14.7; P=0.01), higher clinical TNM staging, e.g., IB vs. IA1 (OR, 4.69; 95% CI: 1.89–11.5; P<0.001) and larger surgical resection, e.g., segmentectomy vs. wedge resection (OR, 2.26; 95% CI: 1.28–4.12; P=0.01) (Table 3). However, time since COVID-19 to surgery was not associated with postoperative complication (OR, 1.00; 95% CI: 0.99–1.01; P=0.41). Fully vaccinated patients did not show a decreased risk of developing postoperative complications (OR, 0.86; 95% CI: 0.48–1.61; P=0.61). Patients with moderate or severe COVID-19 did not have increased risk of developing postoperative complications compared with patients with mild COVID-19 (OR, 2.04; 95% CI: 0.62–5.66; P=0.20).
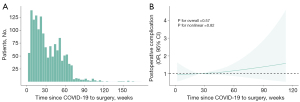
Table 3
| Characteristic | OR | 95% confidence interval | P value |
|---|---|---|---|
| Time since COVID-19 to surgery | 1.00 | 0.99–1.01 | 0.41 |
| Sex | |||
| Female | 1 (reference) | ||
| Male | 1.14 | 0.72–1.79 | 0.56 |
| Age (years) | 0.97 | 0.94–1.01 | 0.21 |
| Smoking history | |||
| Never smoked | 1 (reference) | ||
| Smoker | 2.13 | 1.22–3.69 | 0.01 |
| Vaccination status | |||
| Non-fully vaccinated | 1 (reference) | ||
| Fully vaccinated | 0.86 | 0.48–1.61 | 0.61 |
| CCI | |||
| 0 | 1 (reference) | ||
| 1 | 1.77 | 0.74–4.32 | 0.21 |
| 2 | 4.72 | 1.56–14.7 | 0.01 |
| 3+ | 4.33 | 1.10–17.3 | 0.04 |
| Clinical TNM staging | |||
| IA1 | 1 (reference) | ||
| IA2 | 1.40 | 0.83–2.39 | 0.22 |
| IA3 | 1.50 | 0.74–3.04 | 0.26 |
| IB | 4.69 | 1.89–11.5 | <0.001 |
| IIA | 3.33 | 0.99–10.3 | 0.04 |
| IIB | 1.43 | 0.07–9.67 | 0.75 |
| Surgery type | |||
| Wedge resection | 1 (reference) | ||
| Segmentectomy | 2.26 | 1.28–4.12 | 0.01 |
| Lobectomy | 3.95 | 2.20–7.34 | <0.001 |
| Severity of COVID-19 | |||
| Mild | 1 (reference) | ||
| Moderate or severe | 2.04 | 0.62–5.66 | 0.20 |
CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; OR, odds ratio; TNM, tumor node metastasis.
Discussion
In this multicenter, prospective cohort study, we aimed to investigate the impact of preoperative COVID-19 on postoperative complications and other perioperative outcomes in patients with early-stage lung cancer. Our findings showed that preoperative COVID-19 was not associated with increased risk of postoperative complications. Patients with a short time between COVID-19 and surgery had a similar risk of postoperative complication compared to those with a longer time.
The results of this study are different from previous studies that examined the relationship between the history of COVID-19 and surgical outcomes using data from the earlier phase of the COVID-19 pandemic. These studies found that the history of COVID-19 was associated with higher rate of postoperative complications (2-7,24-29). In the early outbreak of the COVID-19 pandemic, the COVIDSurg Collaborative found that perioperative COVID-19 was associated with higher mortality in patients at 235 hospitals in 24 countries (3). The higher risk of postoperative complications associated with COVID-19 was also confirmed in a US population (4,5). According to their results, surgery performed eight weeks after COVID-19 diagnosis was not associated with increased postoperative complications (5). Another international prospective cohort study found that surgery performed more than seven weeks after COVID-19 diagnosis was associated with a similar mortality risk to baseline (2). Based on these findings, a seven-week delay of surgery following COVID-19 was recommended (2). In our study, both the results of the PSM analysis and logistic regression showed that the history of COVID-19 was not associated with higher risk of postoperative complication in early-stage lung cancer patients receiving surgery. We also found that time since COVID-19 to pulmonary surgery was not associated with postoperative complications. The difference in results from our study may be explained by the high proportion of fully vaccinated population, high proportion of patients with mild COVID-19 (97.5%, 1,458/1,496), and the prevalence of Omicron variant. In our study, 88.7% (1,538/1,734) patients were fully vaccinated, which is close to the reported COVID-19 vaccination rate of 87% in China (9). Our results are consistent with another study conducted during the period of Omicron variant in a population where the majority has been fully vaccinated, which showed that preoperative COVID-19 was not associated with increased postoperative respiratory morbidity (16).
Previous studies found that compared with unvaccinated patients, vaccinated patients had lower risk of developing postoperative complications (7,30,31). However, in our study, fully vaccinated patients did not show a decreased risk of postoperative complication compared with non-fully vaccinated patients. Previous studies also found that the association between the time since COVID-19 to surgery and the risk of postoperative complication varied greatly based on the severity of COVID-19 (7,30). In their studies, patients with mild COVID-19 did not demonstrate an increased risk of postoperative complication, regardless of the time between COVID-19 and surgery. However, patients with moderate or severe COVID-19 had a significantly higher risk of postoperative complications (7,30). In our study, we did not find an increased risk of postoperative complications in patients with moderate or severe COVID-19 compared with patients with mild COVID-19, which might be partly explained by the low proportion of patients with moderate or severe COVID-19 included in our study (2.5%, 38/1,496). We also find factors that were associated with higher risks of postoperative complications: smoking, higher CCI, higher clinical TNM staging, and more extensive surgery methods.
The rate of postoperative complication in our study was 9.5% (165/1,734). In patients with and without history of COVID-19, the rates of postoperative complication were 9.2% (137/1,496) and 11.8% (28/238), respectively. Since the patients in our study underwent different types of surgery, a direct comparison of the rate of postoperative complication is not feasible. Previous studies reported that the rate of postoperative complication in minimally invasive lobectomy ranged from 10.0% to 18.4% (19,32). For minimally invasive segmentectomy, the rate varied between 13.2% to 17.9% (20,33,34). The rate of postoperative complication in wedge resections is much lower than that of lobectomy and segmentectomy, ranging from 3.1% to 3.3% (35,36). Most complications in this study were mild and not clinically important. Only 2.1% (37/1,734) patients had grade III or higher complications (1.8% in patients with a history of COVID-19 and 4.2% in patients without a history of COVID-19).
There are several limitations in our study. First, the information about COVID-19 in each patient was provided by the patients themselves, and the accuracy largely depended on the reliability of the patients’ statements. Meanwhile, there might have been asymptomatic patients who were unaware of their COVID-19 infection. Second, despite using valid statistical methods, there might still be residual confounding factors in this observational study. Third, as this study focused on the impact of COVID-19 on the early-stage lung cancer patients undergoing surgery, the results might not be generalizable to other surgical populations. Therefore, our results should be interpreted cautiously when extrapolating to patients undergoing other kinds of surgeries. It should also be noted that the study results were collected during the Omicron era, a phase characterized by high vaccination rates and less severe disease. These factors are critical to understanding how the findings might apply today, where the overall burden of COVID-19 is likely lower in many parts of the world.
Conclusions
In conclusion, the results of our study showed that in early-stage lung cancer patients who underwent surgery, preoperative COVID-19 was not associated with higher risk of postoperative complications during the Omicron variant era and in a population where the majority has been fully vaccinated. The time from COVID-19 to surgery was also not associated with postoperative complications. These findings suggest an update to the current guidelines on the timing of surgery for patients with preoperative COVID-19, at least for early-stage lung cancer patients with a history of mild COVID-19.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1276/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1276/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1276/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2024-1276/coif). H.L. reports that this study was supported by National Natural Science Foundation of China (Nos. 82372855 and 82072557), Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2023ZD04), Novel Interdisciplinary Research Project from Shanghai Municipal Health Commission (No. 2022JC023), Shanghai Municipal Education Commission - Gaofeng Clinical Medicine Grant (No. 20172005, the 2nd round of disbursement), National Key Research and Development Program of China (No. 2021YFC2500900). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval has been obtained from Ethics Committees of Ruijin Hospital (Approval No. 2023-03), Anhui Chest Hospital (Approval No. K2023-001), Shanghai Ninth People’s Hospital (Approval No. SH9H-2023-T104-1), Shanghai Tongji Hospital (Approval No. K-2023-002), and Guangdong Provincial People’s Hospital (Approval No. KY2023-589-01). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. All participating patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. WHO COVID-19 dashboard 2024. Available online: https://data.who.int/dashboards/covid19/cases?n=c
- COVIDSurg Collaborative. GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia 2021;76:748-58. [Crossref] [PubMed]
- Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020;396:27-38. [Crossref] [PubMed]
- Outcomes and Their State-level Variation in Patients Undergoing Surgery With Perioperative SARS-CoV-2 Infection in the USA: A Prospective Multicenter Study. Ann Surg 2022;275:247-51. [Crossref] [PubMed]
- Deng JZ, Chan JS, Potter AL, et al. The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. Ann Surg 2022;275:242-6. [Crossref] [PubMed]
- Bryant JM, Boncyk CS, Rengel KF, et al. Association of Time to Surgery After COVID-19 Infection With Risk of Postoperative Cardiovascular Morbidity. JAMA Netw Open 2022;5:e2246922. [Crossref] [PubMed]
- Verhagen NB. Severity of Prior Coronavirus Disease 2019 is Associated With Postoperative Outcomes After Major Inpatient Surgery. Ann Surg 2023;278:e949-56. [Crossref] [PubMed]
- Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022;376:e069761. [Crossref] [PubMed]
- Statista. Share of people fully or partly vaccinated against coronavirus COVID-19 in China from August 2021 to June 2022. 2022. Available online: https://www.statista.com/statistics/1279024/china-coronavirus-covid-19-vaccination-rate/
- Data OWi. Share of SARS-CoV-2 sequences that are the omicron variant. 2024. Available online: https://ourworldindata.org/grapher/covid-cases-omicron?country=GBR%7EFRA%7EBEL%7EDEU%7EITA%7EESP%7EUSA%7EZAF%7EBWA%7EAUS
- CoVariants. Overview of Variants/Mutations 2024. Available online: https://covariants.org/per-variant
- Uraki R, Kiso M, Iida S, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature 2022;607:119-27. [Crossref] [PubMed]
- Araf Y, Akter F, Tang YD, et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol 2022;94:1825-32. [Crossref] [PubMed]
- Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022;399:1303-12. [Crossref] [PubMed]
- Suzuki R, Yamasoba D, Kimura I, et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 2022;603:700-5. [Crossref] [PubMed]
- Garnier M, Constantin JM, Cinotti R, et al. Association of preoperative COVID-19 and postoperative respiratory morbidity during the Omicron epidemic wave: the DROMIS-22 multicentre prospective observational cohort study. EClinicalMedicine 2023;58:101881. [Crossref] [PubMed]
- McInerney CD, Kotzé A, Bacon S, et al. Postoperative mortality and complications in patients with and without pre-operative SARS-CoV-2 infection: a service evaluation of 24 million linked records using OpenSAFELY. Anaesthesia 2023;78:692-700. [Crossref] [PubMed]
- Expert Consensus Panel. The American Association for Thoracic Surgery (AATS) 2023 Expert Consensus Document: Staging and multidisciplinary management of patients with early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2023;166:637-54. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]
- Zhang Y, Chen C, Hu J, et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg 2020;160:1363-72. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20:e192-7. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Knisely A, Zhou ZN, Wu J, et al. Perioperative Morbidity and Mortality of Patients With COVID-19 Who Undergo Urgent and Emergent Surgical Procedures. Ann Surg 2021;273:34-40. [Crossref] [PubMed]
- Doglietto F, Vezzoli M, Gheza F, et al. Factors Associated With Surgical Mortality and Complications Among Patients With and Without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surg 2020;155:691-702. [Crossref] [PubMed]
- COVIDSurg Collaborative. GlobalSurg Collaborative. Effects of pre-operative isolation on postoperative pulmonary complications after elective surgery: an international prospective cohort study. Anaesthesia 2021;76:1454-64. [Crossref] [PubMed]
- Benson RA, Nandhra S. Outcomes of Vascular and Endovascular Interventions Performed During the Coronavirus Disease 2019 (COVID-19) Pandemic. Ann Surg 2021;273:630-5. [Crossref] [PubMed]
- Jonker PKC, van der Plas WY, Steinkamp PJ, et al. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: A Dutch, multicenter, matched-cohort clinical study. Surgery 2021;169:264-74. [Crossref] [PubMed]
- Odozor CU, Kannampallil T, Ben Abdallah A, et al. Post-acute sensory neurological sequelae in patients with severe acute respiratory syndrome coronavirus 2 infection: the COVID-PN observational cohort study. Pain 2022;163:2398-410. [Crossref] [PubMed]
- SenthilKumar G. Preoperative SARS-CoV-2 infection increases risk of early postoperative cardiovascular complications following noncardiac surgery. Am J Physiol Heart Circ Physiol 2023;324:H721-31. [Crossref] [PubMed]
- Le ST, Kipnis P, Cohn B, et al. COVID-19 Vaccination and the Timing of Surgery Following COVID-19 Infection. Ann Surg 2022;276:e265-72. [Crossref] [PubMed]
- Miyajima M, Maki R, Arai W, et al. Robot-assisted vs. video-assisted thoracoscopic surgery in lung cancer. J Thorac Dis 2022;14:1890-9. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- Lee MO, Jin SY, Lee SK, et al. Video-assisted thoracoscopic surgical wedge resection using multiplanar computed tomography reconstruction-fluoroscopy after CT guided microcoil localization. Thorac Cancer 2021;12:1721-5. [Crossref] [PubMed]
- Tsutani Y, Kagimoto A, Handa Y, et al. Wedge resection versus segmentectomy in patients with stage I non-small-cell lung cancer unfit for lobectomy. Jpn J Clin Oncol 2019;49:1134-42. [Crossref] [PubMed]


