
Impact of omitting clinical target volume in radiotherapy for locally advanced non-small cell lung cancer: a propensity score matching analysis
Highlight box
Key findings
• Omitting the clinical target volume (CTV) did not substantially affect the progression-free survival (PFS) and overall survival (OS) rates of patients with locally advanced (LA) non-small cell lung cancer (NSCLC). Moreover, the regional and distant recurrence rates were not increased due to this omission. By omitting the CTV following gross tumor volume (GTV) matching, the radiation dosage to unaffected lung, esophagus, and heart tissues was significantly reduced. This reduction mitigated damage to immune cells, thereby diminishing the frequency of pulmonary inflammatory responses. Thus, fewer instances of radiation pneumonitis (RP) were observed, which in turn enhanced patients’ quality of life and decreased mortality rates related to RP.
What is known, and what is new?
• Defining the range of the planned target volume (PTV) in the radiotherapy target volume of LA-NSCLC is a significant challenge for radiation oncologists.
• By optimizing the delineation of the target volume, the clinical prognosis of NSCLC patients can be improved.
What is the implication, and what should change now?
• Omitting the CTV leads to a substantial reduction in radiation exposure to the lungs, esophagus, and heart. This approach also decreases the damage caused to the peripheral blood lymphocytes, which in turn decreases the incidence of RP and enhances the quality of life of LA-NSCLC patients.
Introduction
Radiotherapy is the primary treatment for locally advanced (LA) unresectable non-small cell lung cancer (NSCLC). The National Comprehensive Cancer Network (NCCN) guidelines recommend the use of three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT) in conjunction with platinum-doublet, chemotherapy as the standard regimen for LA-NSCLC (1). The 5-year overall survival (OS) rate of patients who receive simultaneous chemoradiotherapy is 15–25% (1). However, significant toxic effects related to this anti-tumor treatment limit its overall efficacy. Therefore, mitigating the adverse effects of radiotherapy and chemotherapy to enhance treatment adherence is a crucial strategy for prolonging the survival of patients with unresectable LA-NSCLC (2).
Durvalumab was approved as a consolidation therapy after concurrent chemoradiotherapy for unresectable LA-NSCLC (3). Given the increasing likelihood that more immune checkpoint inhibitors will be used in consolidation treatments, medical practitioners need to be aware of immune-related pneumonitis, including radiation pneumonitis (RP) (4). Lung cancer is moderately sensitive to radiotherapy, which has a clear dose-response relationship. Radiation-induced lung injuries represent the most common adverse reactions to lung cancer radiotherapy (5). Such injuries, particularly RP, are frequently irreversible, and may result in fatal outcomes, thus limiting the permissible radiation dose. Consequently, many studies have sought to predict or prevent ≥ grade 3 RP (4,6). In radiotherapy, precise target delineation is essential to deliver sufficient tumor dose while sparing normal lung tissues, thereby reducing the risk of RP (7). Generally, the local control rate increases as the target dose increases. IMRT exhibits significant dosimetric benefits and target conformity, but patients with LA-NSCLC typically present with sizeable primary tumors accompanied by lymph node metastasis (8). If the radiotherapy target volume increases successively from the gross tumor volume (GTV) to the clinical target volume (CTV), and further still to the planned target volume (PTV), the irradiated volume of the surrounding normal tissue also increases incrementally (9). As a secondary effect, this increases radiation doses to the lungs, resulting in damage to normal tissues. Several methodologies can be used to mitigate radiation damage in clinical practice, including strategies such as respiratory gating, image-guided radiation therapy, and adjusting fractionated doses to decrease radiation-related injuries and subsequent complications (10). Current research aims to reduce the CTV in order to further reduce the irradiation scope of the tumor target volume (11). This approach seeks to decrease the rate of serious complications related to radiation thorough, scientific, standardized, and logical target delineation, while ensuring effective radiotherapy. In clinical practice, a large number of patients with unresectable LA-NSCLC are unable to undergo the requisite treatment due to adverse events. These events can severely affect the patients’ treatment responses and survival rates
We performed a retrospective study using propensity score matching (PSM) to evaluate the effects of various target delineation methods. All patients were treated using IMRT with precise positioning. Omitting the delineation of the CTV did not reduce the local control rate of the tumor, nor did it significantly impact patient survival. However, it significantly decreased the incidence of ≥ grade 3 RP and reduced the dose of irradiation to the lungs, heart, and esophagus. The results of our research are expected to inform and optimize radiotherapy plans for LA-NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-409/rc).
Methods
Patients and simulation
Data were retrospectively collected from all consecutive patients with LA-NSCLC at Shanghai Pulmonary Hospital between January 2019 and December 2020. All enrolled patients were treated with IMRT after consultation with surgeons, pulmonalists, oncologists and radiologists. All the patients received biological effective dose (BED10) >64.8 Gy. The exclusion criteria were as follows: concurrent neoplasms, receipt of neoadjuvant therapy, receipt of adjuvant and/or palliative radiotherapy, and inability to complete the prescribed radiotherapy doses. This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. SK0233-67), and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. As a retrospective study, patient consent was obtained when possible but was not mandatory.
The patients were immobilized using either a body mask or a vacuum pad and positioned supine with hands and elbows elevated. For computed tomography (CT) simulation, conventional 3D CT scans were obtained from the patient’s clavicle, lung and mediastinum, extending to the upper abdomen and adrenal level. The resulting images were subsequently transmitted to a computer workstation via the local area network.
Radiation therapy targets and organs at risk (OAR)
The GTV, which includes the intrapulmonary tumor, hilar and mediastinal involvement, and supraclavicular lymph node metastasis, was delineated by experienced physicians in accordance with the ICRU83 report (12). The GTV was enlarged based on tumor imaging and clinical examination, and was subsequently expanded to form the CTV. For squamous cell carcinoma, the GTV was expanded by 6 mm, while for adenocarcinoma, an 8 mm expansion was used. The resulting CTV was then used for further PTV adjustments. In the PTV-C group, the CTV was further expanded by an additional uniform 5 mm margin to account for setup uncertainties and organ motion, thereby generating the PTV-C. In contrast, the PTV-G group omitted the intermediate CTV and applied a direct 5 mm expansion from the GTV to form the PTV-G, effectively using a slightly larger treatment margin around the gross tumor volume. Concurrently, the range of patient respiratory motion was evaluated to inform individualized target volume design. The OAR in radiation therapy comprises the lungs, esophagus, heart, spinal cord, and brachial plexus. The normal lung volume was defined as the total lung volume minus the GTV.
Treatment Planning
All the IMRT treatment plans were formulated using the Pinnacle 9.10 treatment planning system and were completed by a senior physicist. The IMRT scheme was designed using the design method of coplanar 5–7 field direct subfield optimization with 6 MV X-ray and retrograde dynamic optimization. The program accounted for the irradiation volume and target area of the above-mentioned normal tissues. In this study, all patients were treated according to standardized institutional protocols, and the prescription dose was determined primarily based on tumor stage, size, location, and organ-at-risk constraints. Although previous studies have suggested differential radiosensitivity between squamous cell carcinoma and adenocarcinoma (13,14), our radiotherapy regimen did not stratify the prescription dose by histological subtype. Both histologies received a total prescribed dose of 54–60 Gy delivered in 27–30 fractions, in line with the NCCN guidelines for LA-NSCLC. The irradiation frequency of the two groups was once per day, five times per week. The patient’s radiotherapy plan was jointly determined based on the tumor radiotherapy dose volume histogram and isodose curves, as well as a comprehensive evaluation of the patient’s pulmonary function status and the doses to critical organs.
All the radiotherapy plans required no dose hotspots outside the PTV, and the prescribed dose line had to cover 95% of the PTV volume and 100% of the GTV volume. The total course of radiotherapy was 6 weeks.
Due to the possibility of poor tolerance in the Chinese population, the mean lung dose (MLD) was kept below 15 Gy, and the lung volume receiving 20 Gy (V20) was kept below 28%. Doses for other normal structures met the NCCN recommended criteria (15). Prior to treatment, patient’s position was verified by cone beam computed tomography (CBCT). If the displacement errors in any direction were within 5 mm, the patient’s position was corrected, and treatment was administered directly. This IGRT method allowed for precise patient alignment and ensured minimal positional errors during the radiotherapy process.
Patient follow-up evaluation
Treatment response was evaluated 2 months post-radiation therapy following the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Quarterly follow-ups were conducted for the initial 2 years, which included contrast-enhanced CT of chest and abdomen, ultrasonography of the cervical and clavicle lymph nodes, routine blood testing, and comprehensive metabolic tests. To test for distant metastasis, annual bone scans and brain magnetic resonance imaging were also performed. These measures were supplemented with telephone follow-ups. During this period, data regarding toxicity, the pattern of initial recurrence (local/distant/both), and time of death were collected. During treatment, weekly monitoring for toxicity was conducted and graded as per the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 5.0 (NCI CTCAE 5.0). The highest severity score recorded during treatment was used for analysis. The primary outcomes of the study were the incidence of ≥ grade 3 radiation-induced pulmonary and esophagitis toxicity. The secondary outcomes were the objective response rate (ORR), disease control rate (DCR), median progression-free survival (PFS), median OS, initial failure recurrence patterns, and post-radiotherapy changes in the lymphocyte and neutrophil counts. PFS was assessed from randomization to the first event (locoregional progression, distant metastasis, or death), disease progression during therapy, or relapse post-therapy. OS was calculated from treatment commencement to death. Investigators assessed all recurrences.
The focus of the post-radiotherapy patient recurrence analysis was the pattern of the first instances of recurrence, categorized initially as local, regional, or distant failures. Local failures included primary tumors and local recurrent lymph nodes, while regional failures included regional lymphatic nodes (hilar, mediastinal, and supraclavicular). However, primary metastatic regional lymph nodes are often not included in the regional failure of radiotherapy. Based on the relationship between the recurrence site and the radiation treatment area, the local recurrence patterns are classified as In PTV-G and Out PTV-G, as well as In PTV-C and Out PTV-C. Patients’ recurrence ratio in the irradiation field of both the PTV-G and PTV-C groups was calculated. The PTV in the treatment plan accounted for a set-up error and a 5-mm organ motion external expansion; the PTV-C range was generated from the virtual CTV.
Statistical analysis
A PSM analysis with a 1:2 ratio was performed to match the baseline characteristics of the PTV-G and PTV-C patients. Propensity score were established on the following covariates: age, gender, performance status (PS), tumor diameter, current smoker (yes/no), clinical tumor stage, and radiation dose.
The statistical analysis was performed using SPSS version 26.0 for Windows (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant. The continuous data are expressed as the mean ± standard deviation. The categorical data are presented as the percentage (number). Differences between treatment groups were assessed using the Mann-Whitney U test and the Chi-squared test for continuous data that did not conform to a normal distribution and categorical data, respectively.
A univariate analysis was performed to compare the clinical data between the PTV-G group and PTV-C group, and to find associated variables in the patients enrolled in the study. The univariate analysis used Chi-squared tests for the categorical variables, and t-tests for the continuous variables that conformed to a normal distribution. The significant variables in the univariate analysis and the covariates considered clinically influential were then analyzed by multivariate (backward) stepwise logistic regression to identify significant variables affecting radiotoxic side effects. Survival probability was evaluated using the Kaplan-Meier estimator, while survival differences between groups were studied using the log-rank test. Follow-up duration was calculated using the reverse Kaplan-Meier method.
Results
Study process and patient characteristics
A total of 255 patients were identified from our local database. After matching on propensity score with a 1:2 ratio, 156 patients were included in the final analysis, with 52 in the PTV-G group and 104 in the PTV-C group (flow chart in Figure 1). All these patients had Eastern Cooperative Oncology Group PS scores of 0–1. Patients in good physical condition received a combination treatment of radiotherapy and chemotherapy. Some patients underwent concurrent chemotherapy, while others underwent sequential chemotherapy for up to 6 cycles after radiotherapy.
Table 1 details the clinicopathological characteristics of all the propensity score-matched patients included in the study.
Table 1
| Clinical characteristics | PTV-G arm (N=52) | PTV-C arm (N=104) | P value |
|---|---|---|---|
| Age | 0.36 | ||
| <65 years | 13 (27.7) | 34 (72.3) | |
| ≥65 years | 39 (35.8) | 70 (64.2) | |
| Gender | 0.23 | ||
| Male | 24 (39.3) | 37 (60.7) | |
| Female | 28 (29.5) | 67 (70.5) | |
| ECOG | 0.29 | ||
| 0 | 13 (41.9) | 18 (58.1) | |
| 1 | 39 (31.2) | 86 (68.8) | |
| Smoking status | 0.87 | ||
| ≤30 pack-years | 23 (32.9) | 47 (67.1) | |
| >30 pack-years | 29 (33.7) | 57 (66.3) | |
| History of ILD disease | 0.53 | ||
| No | 46 (33.1) | 93 (66.9) | |
| Yes | 6 (35.3) | 11 (64.7) | |
| Lung function | 0.12 | ||
| Normal-mild lung dysfunction | 45 (31.0) | 100 (69.0) | |
| Moderate lung dysfunction | 7 (63.6) | 4 (36.4) | |
| Location type | |||
| Central type | 23 (28.4) | 58 (71.6) | 0.13 |
| Peripheral type | 29 (38.7) | 46 (61.3) | |
| Histology | 0.17 | ||
| Squamous cell carcinoma | 21 (27.6) | 55 (72.4) | |
| Adenocarcinoma | 28 (36.4) | 49 (63.6) | |
| NSCLC unspecified | 3 (100) | 0 | |
| Clinical stage | 0.13 | ||
| IIIA | 10 (41.7) | 14 (58.3) | |
| IIIB | 33 (36.7) | 57 (63.3) | |
| IIIC | 9 (21.4) | 33 (78.6) | |
| T stage | 0.11 | ||
| T1 | 8 (19.5) | 33 (80.5) | |
| T2 | 5 (13.9) | 31 (86.1) | |
| T3 | 23 (74.2) | 8 (25.8) | |
| T4 | 16 (33.3) | 32 (66.7) | |
| N stage | 0.06 | ||
| N1 | 3 (42.9) | 4 (57.1) | |
| N2 | 40 (37.7) | 66 (62.3) | |
| N3 | 9 (20.9) | 34 (79.1) | |
| Chemotherapy cycles | 0.07 | ||
| ≤4 | 17 (45.9) | 20 (54.1) | |
| >4 | 35 (29.4) | 84 (70.6) | |
| PTV volume (cm3) | 225.50±25.2 | 298.05±14.8 | 0.70 |
The categorical data and ranked data were analyzed using the Chi-squared test, and the continuous data were analyzed using the t-test. Data are presented as n (%) or mean ± standard deviation. ECOG, Eastern Cooperative Oncology Group; ILD, interstitial lung disease; NSCLC, non-small cell lung cancer; PTV, planned target volume; PTV-C, planned target-clinical target volume; PTV-G, planned target volume-gross target.
Radiotherapy-related toxicity
Based on the American Cooperative Organization for Radiation Oncology’s acute radiation injury grading standard, an analysis of the patients with non-hematologic toxicity revealed that the total rate of ≥ grade 3 radiation toxicity was 23.1% (12 patients) in the PTV-G group, and 39.4% (41patients) in the PTV-C group (P=0.02). The incidence of ≥ grade 3 RP was greater in the PTV-C group than the PTV-G group (12.5% vs. 5.7%, respectively; P=0.03). However, no deaths associated with RP were reported. Moreover, the rate of grade 1–2 RP was also significantly lower in the PTV-G group than the PTV-C group (23.1% vs. 39.4%; P=0.041). The contrast observation results revealed no significant difference in the incidence of grade 1–2 radiation-induced esophagitis between the two groups. However, the incidence of grade 3 radiation-induced esophagitis was significantly higher in the PTV-C group than the PTV-G group (15.4% vs. 3.8%; P=0.03) (Figure 2).
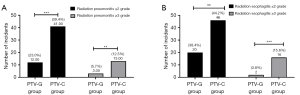
No cases of grade 4 RP or radiation esophagitis were observed in either group. Moreover, the overall incidence of all hematology toxicity related to radiotherapy among the patients with adverse reactions was significantly higher in the PTV-C group than the PTV-G group (38.5% vs. 50.0%; P=0.03). The predominant type of radiation-induced hematological toxicity was lymphocyte damage. The adverse reaction rates for the two groups are detailed in Table 2.
Table 2
| Toxic reactions | Grade 1–2, n (%) | Grade ≥3, n (%) | |||||
|---|---|---|---|---|---|---|---|
| PTV-G (n=52) | PTV-C (n=104) | P | PTV-G (n=52) | PTV-C (n=104) | P | ||
| Non-hematological | |||||||
| Radiation pneumonitis | 12 (23.1) | 41 (39.4) | 0.04 | 3 (5.8) | 13 (12.5) | 0.03 | |
| Death | – | – | – | – | – | – | |
| Radiation esophagitis | 20 (38.5) | 46 (44.2) | 0.15 | 2 (3.8) | 16 (15.6) | 0.03 | |
| Nausea | 25 (48.1) | 48 (46.2) | 0.99 | 2 (8.5) | 6 (4.9) | 0.10 | |
| Vomiting | 15 (28.8) | 36 (34.6) | 0.55 | 5 (9.6) | 10 (2.5) | 0.85 | |
| Anorexia | 16 (30.8) | 22 (21.2) | 0.48 | 1 (1.9) | 4 (2.5) | 0.96 | |
| Fatigue | 17 (32.7) | 43 (41.3) | 0.18 | 2 (3.8) | 8 (3.7) | 0.38 | |
| Total | 105 (33.6) | 236 (27.8) | 0.32 | 12 (27.6) | 57 (41.7) | 0.02 | |
| Hematological | |||||||
| Leukopenia | 31 (59.6) | 67 (64.4) | 0.28 | 8 (15.4) | 25 (24.0) | 0.23 | |
| Neutropenia | 28 (53.8) | 48 (46.2) | 0.96 | 6 (11.5) | 16 (15.4) | 0.93 | |
| Thrombocytopenia | 19 (36.5) | 49 (47.1) | 0.25 | 4 (7.7) | 6 (5.8) | 0.42 | |
| Elevated ALT | 20 (38.5) | 47 (45.2) | 0.79 | 2 (3.8) | 5 (4.8) | 0.75 | |
| Total | 98 (47.1) | 211 (50.7) | 0.22 | 20 (38.5) | 36 (50.0) | 0.03 | |
ALT, alanine aminotransferase; PTV-C, planned target-clinical target volume; PTV-G, planned target volume-gross target.
Univariate and multivariate logistic regression analyses of clinical and dosimetric parameters for radiation injury in the lung cancer cohort indicated that RP was correlated with moderate lung dysfunction [hazard ratio (HR) =1.37; P=0.03], more than 4 chemotherapy cycles (HR =1.47, P=0.025), the MLD (Gy) (HR =1.75, P=0.029), and lung V20 (%) (HR =18.3%, P=0.058). Similarly, the multivariate logistic regression analysis of patients suffering from radiation esophagitis showed that comorbidities (HR =1.59, P=0.019) and the mean esophagus dose (cGy) (HR =1.06, P=0.01) were relevant factors (Table 3). The dosimetric parameters and OAR dosimetric characteristics are set out in Table S1.
Table 3
| Factors | Radiation pneumonitis | Radiation esophagitis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Age | |||||||||||
| ≤65 years | 1.00 | – | – | 1.00 | – | – | |||||
| >65 years | 1.21 (0.44–2.29) | 0.10 | – | – | 1.04 (0.62–1.46) | 0.865 | – | – | |||
| Gender | |||||||||||
| Male | 1.00 | – | – | 1.00 | – | – | |||||
| Female | 1.41 (0.74–1.88) | 0.31 | – | – | 1.19 (0.89–1.48) | 0.51 | – | – | |||
| Smoking history | |||||||||||
| ≤30 pack-years | 1.00 | – | – | 1.00 | – | – | |||||
| >30 pack-years | 2.05 (0.91–3.90) | 0.27 | – | – | 1.06 (0.75–3.19) | 0.420 | – | – | |||
| Comorbidities | |||||||||||
| No | 1.00 | – | – | 1.00 | 1.00 | ||||||
| Yes | 1.46 (0.49–1.51) | 0.61 | – | – | 1.76 (1.02–1.99) | 0.016 | 1.59 (1.06–2.10) | 0.019 | |||
| Lung function | |||||||||||
| Normal-mild lung dysfunction | 1.00 | 1.00 | 1.00 | – | – | ||||||
| Moderate lung dysfunction | 1.88 (1.49–2.21) | 0.028 | 1.37 (1.19–2.13) | 0.03 | 1.64 (0.41–1.91) | 0.054 | – | – | |||
| ECOG PS | |||||||||||
| 0 | 1.00 | – | – | 1.00 | – | – | |||||
| 1 | 2.08 (0.81–5.10) | 0.086 | – | – | 1.86 (0.61–2.82) | 0.09 | – | – | |||
| 2 | 1.46 (0.68–3.12) | 0.30 | 0.92 (0.81–2.65) | 0.228 | |||||||
| Location type | |||||||||||
| Peripheral | 1.00 | – | – | 1.00 | – | – | |||||
| Central | 0.84 (0.42–1.20) | 0.298 | – | – | 0.92 (0.52–1.58) | 0.850 | – | – | |||
| Stage | |||||||||||
| IIIA | 1.00 | – | – | 1.00 | – | – | |||||
| IIIB | 1.45 (0.84–2.49) | 0.118 | – | – | 1.49 (1.12–2.21) | 0.09 | – | – | |||
| IIIC | 1.09 (0.72–2.25) | 0.215 | – | – | 1.39 (1.21–2.79) | 0.099 | – | – | |||
| Pathology | |||||||||||
| Squamous | 1.00 | 0.115 | – | – | 1.00 | 0.296 | – | – | |||
| Adenocarcinoma | 0.80 (0.46–1.88) | 0.60 | – | – | 1.02 (0.48–2.19) | 0.965 | – | – | |||
| NSCLC unspecified | 1.92 (0.98–1.99) | 0.058 | – | – | 1.69 (0.89–4.40) | 0.125 | – | – | |||
| Chemotherapy cycle | |||||||||||
| ≤4 cycles | 1.00 | 1.00 | 1.00 | – | – | ||||||
| >4 cycles | 1.66 (1.52–2.92) | 0.042 | 1.47 (1.17–2.77) | 0.025 | 0.97 (0.32–1.95) | 0.125 | – | – | |||
| Treatment approach | |||||||||||
| Concurrent chemotherapy | 1.00 | – | – | 1.00 | – | – | |||||
| Sequential chemotherapy | 1.50 (0.59–1.82) | 0.06 | – | – | 1.58 (1.05–1.97) | 0.047 | – | – | |||
| Mean heart dose (Gy) | 1.47 (0.77–2.17) | 0.21 | – | – | 0.93 (0.43–1.38) | 0.039 | – | – | |||
| Mean lung dose (Gy) | 1.25 (1.08–1.79) | 0.018 | 1.75 (1.48–3.79) | 0.029 | 1.97 (0.67–2.17) | 0.127 | – | – | |||
| Mean esophagus dose (cGy) | 2.12 (1.68–3.20) | 0.437 | – | – | 1.32 (1.04–2.44) | 0.02 | 1.06 (1.00–1.84) | 0.01 | |||
| Lung V20 (%) | 19.5(18.11–21.76) | 0.051 | 18.3 (17.31–20.19) | 0.058 | 23.5 (20.13–24.22) | 0.068 | – | – | |||
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NSCLC, non-small cell lung cancer; V20, percentage volume receiving 20 Gy or higher.
A weekly analysis during and following radiotherapy (at 1 month) was conducted to track changes in the neutrophil count, lymphocyte count, and neutrophil-to-lymphocyte ratio (NLR) in the RP patients. The lymphocyte count [(0.4±0.12) ×109] 1-month post-radiotherapy was significantly lower in the patients with ≥ grade 3 RP than those with mild RP [(0.8±0.22) ×109] (P=0.01) (Figure 3A). Conversely, the neutrophil count was significantly higher in the severe RP group than the mild RP group [(4.6±0.17) ×109 vs. (4.6±0.17) ×109, P<0.001] (Figure 3B). The NLR was greater in the severe RP group than in the mild RP group, which suggests that the NLR may be useful in predicting RP severity post-lung cancer radiotherapy (P<0.001) (Figure 3C).

Clinical responses to treatment
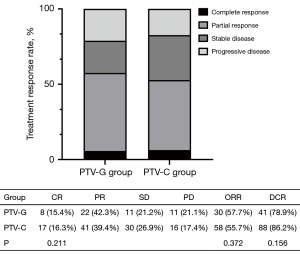
In the PTV-G group, 30 patients (57.7%) achieved an objective response, of whom 8 had complete responses (15.4%) and 22 had partial responses (42.3%). In the PTV-C group, 58 patients (55.8%) achieved an objective response, of whom 17 had complete responses (16.3%), and 41 had partial responses (39.4%). ORRs were 57.7% in the PTV-G group versus 55.8% in the PTV-C group (P=0.37), while DCRs were 78.8% versus 84.6%, respectively (P=0.16), with no significant differences observed between groups. Similarly, the complete response rate did not differ significantly between the two groups (15.4% vs. 16.3%, P=0.21) (Figure 4).
OS
Of the 156 assessable patients, 121 (77.6%) received a definitive radiotherapy dose (≥60 Gy), and 117 (75.0%) received protocol-specified chemotherapy. Definitive radiotherapy doses were completed by 78.8% (41 patients) and 76.9% (80 patients) of the patients in the PTV-G and PTV-C groups, respectively (P=0.81). Correspondingly, the combination of radiotherapy and chemotherapy was completed by 86.5% (45 patients) and 69.2% (72 patients) of the patients in the PTV-G and PTV-C groups, respectively (P=0.23). The completion rate for the radical radiotherapy and chemotherapy combination was higher in the PTV-G group than the PTV-C group, but this difference was not statistically significant.
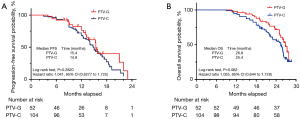
The follow-up of the treated patients continued until December 2022. In relation to the surviving patients, the PTV-G group had a median follow-up period of 24.1 months (range, 17.3–29.7 months), while that of the PTV-C group was 25.2 months (range, 16.1–29.1 months). The PTV-G group had a median PFS time of 15.4 months, while that of the PTV-C group was 14.8 months; the difference was not statistically significant [P=0.28; hazard ratio (HR): 1.041, 95% confidence interval (CI): 0.6277–1.725] (Figure 5A). The PTV-G group had a median OS time of 26.8 months, while that of the PTV-C group was 25.4 months. No significant difference was observed between the two groups in terms of OS (P=0.06; HR: 1.055, 95% CI: 0.644–1.728) (Figure 5B). The 1-year and 2-year OS rates were 94.2% and 71.1% in the PTV-G group, and 90.4% and 55.8% in the PTV-C group, respectively. Kaplan-Meier curves for PFS for the PTV-G and PTV-C groups are shown in Figure 5A.
Radiation recurrence patterns
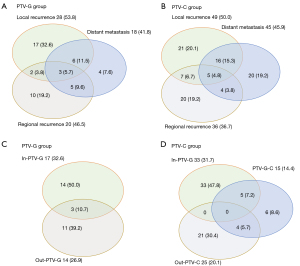
In the PTV-G group, local recurrence was observed in 28 patients (53.8%), regional recurrence in 20 patients (46.5%), and distant recurrence in 18 patients (41.8%) (Figure 6A). In the PTV-C group, local recurrence was observed in 49 patients (50.0%), regional recurrence in 36 patients (36.7%), and distant recurrence in 45 patients (45.9%) (Figure 6B). In relation to the relationship between tumor recurrence location and the radiation field, our analysis showed that in the PTV-G group, 17 patients (32.7%) had recurrences in the PTV-G radiation field, 14 (26.9%) had recurrences outside the radiation field, and 3 (10.7%) had recurrences both inside and outside the field (Figure 6C). Conversely, in the PTV-C group, 33 patients (31.7%) had recurrences in the PTV-C field, 25 (20.1%) had recurrences outside the PTV-C field, and none had recurrences both inside and outside the field (Figure 6D).
Discussion
In the field of lung cancer radiotherapy, radiation lung injury, which is categorized as early radiation pneumonia or late radiation pulmonary fibrosis, is a significant cause of concern. Radiation pneumonia is a major side effect and the most frequent complication of thoracic tumor radiotherapy, and has an incidence ranging from 5% to 30%. In more severe cases (≥ grade III), the incidence ranges from 10% to 20%, and the fatality rate, which represents the highest potential harm, can reach 15% (16). Shi et al. noted that there are various risk factors for RP, including patient age, lung cancer type, chemotherapy history, lung function, and radiotherapy parameters. A thorough evaluation and examination pre-radiotherapy can significantly diminish RP (17). However, an increase in the lung irradiation dose and volume leads to a rise in its incidence.
In this study, there was no significant variation in the mean dose to lung tumors between the CTV-inclusive and CTV-omitted groups. The short-term total response rates, 1- and 2-year survival rates, and distant metastasis rates were similar between the two groups. In relation to the toxicity and side effects, there was a significant decrease in the incidence of ≥3 grade radiation pneumonia in the clinical target group. These results could be due to the omission of the CTV target area in the PTV-G group, which reduced the lung tissue and heart irradiation volume. Consequently, V5, V20, and V30, and the MLD values in the lung tissue showed a decrease, enhancing the safety of the radiotherapy. The incidence of radiation pneumonia was found to be significantly reduced in the patients in the PTV-G group.
Delays in treatment can arise due to acute inflammation during the later stages of radiotherapy, and insufficient doses may affect the local tumor control rate. Upon the formation of radioactive fibrosis, there is a significant reduction in the patient’s lung function. Currently, due to the absence of specific drugs in the treatment, the treatment period is extended, which adversely affects patients’ quality of life, necessitates follow-up treatments, and increases the distant tumor metastasis rate.
Presently, the principles of radiation therapy for lung tumors dictate that the radiation dose to the tumor should be guaranteed, and the radiation dose to normal tissue should be minimized. Emphasis is placed on reducing the dose to the lung tissue as much as possible during lung cancer radiotherapy. Notable predictors of RP comprise volume of lung receiving ≥20 Gy (V20), Volume of lung receiving≥30 Gy (V30), and the MLD in both lungs. According to a dosimetry study, a close relationship exists between the volume and dose of radiation and the incidence of radiation injury (18). Recently, precision radiotherapy has been recommended as a means to improve radiotherapy efficacy. It aims to offer precise diagnosis, positioning, planning, and treatment (19). Through optimizing the body position fixation methods, controlling physiological organ movement, the appropriate choice of imaging examination technology, and the accurate delineation of the target area, it is possible to control the ideal dose volume and provide better protection to normal tissues (20). Precision planning represents the most intricate stage; however, target delineation is the cornerstone of accurate planning. The results of this clinical retrospective study revealed a significant reduction in the occurrence of ≥3 grade RP and radiation esophagitis in the PTV-G group. Notably, patients in the PTV-G group had a considerably higher completion rate of concurrent chemoradiotherapy than those in the PTV-C group, reflecting improved patient compliance and tolerability.
The potential of radiotherapy dose escalation has been a point of interest for some time. However, the pivotal RTOG 0617 study (21) did not find any survival advantage related to dose escalation (74 Gy in 37 fractions), as higher radiation doses to the immune system were correlated with poorer survival outcomes (22). Similarly, a notable factor in reduced patient survival is increased mortality from escalated cardiopulmonary toxicity. Therefore, a promising approach lies in selectively intensifying the radiation dose to the most aggressive tumor regions, while mitigating the radiation dose to the neighboring normal tissues and OAR. This could improve the efficacy of radiotherapy’ in LA-NSCLC patients (23). In this study, after adjusting for the margin of PTV among the patients, we observed a significant dose reduction in the normal tissue of the PTV-G group (e.g., heart V40: 5.48% vs. 9.32%, P=0.03; MLD and lung V20/V30 also showed favorable trends). Thus, excluding the CTV could elevate the radiation dose to the tumor target volume without escalating the toxicity to the normal tissues, which in turn, could enhance local tumor control.
Currently, prophylactic radiotherapy is not broadly endorsed in the treatment of lung cancer. However, consideration should be given to the viability of further reducing the target volume, ensuring only the dose to the GTV. Cai et al. showed that IMRT that omitted the CTV and added 0.8 cm to the GTV to form the PTV improved patient survival rates and local control, and notably decreased the occurrence of RP (24).
The benefit associated with omitting the CTV is not limited to fewer normal tissues (e.g., the lung, esophagus, and heart) being exposed to high-dose radiation. It also involves a reduction in the irradiation field, which minimizes the effect of radiation on peripheral blood lymphocytes. From a mechanistic perspective, inflammation is a primary cause of radiation-induced lung injuries. The abundance of lymphocytes signals the immune system’s regulatory potential, the neutrophil count reflects the degree of unspecific inflammatory responses, and the NLR reflects both lymphocytic and neutrophil-associated immune pathways (25). It is known that the NLR is correlated within infectious pneumonia and the prognosis of chronic obstructive pulmonary disease patients. Our findings showed that compared to the RP ≤ grade 2 group, the NLR of the RP ≥ grade 3 group was significantly higher at 1, 2, and 3 months post-radiotherapy. This shows the elevated inflammation levels in lung cancer patients with RP post-radiotherapy and associated reduced lymphatic immune system regulation. Consequently, the NLR might serve as an early indicator of RP.
In the current landscape, the continuous evolution of medical equipment and technology is leading to shrinking radiation fields, and has resulted in a shift from the prophylactic irradiation of lymphatic drainage areas to involved field irradiation. Nonetheless, there are numerous challenges in clinical practice. The most significant issue lies in the external range of the CTV and the PTV, as the CTV is a subclinical tumor region encompassing the GTV and is associated with treatment outcomes. The demarcation area of the CTV primarily depends on the scope of the tumor invasion and dissemination, as well as an understanding of the probability of tumor occurrence. The determination of the CTV must also account for tumor type, location, histology, and the individual differences of the patients (26). In defining the CTV, considerations are limited to static images and the biological behavior of the tumor, and they fail to factor in organ motion, set-up errors, and the chosen radiotherapy technique.
Currently, most facilities follow the conventional target delineation methodology outlined in the ICRU 83 report, which emphasizes CTV expansion based on anatomical and histopathological features. Clinical findings indicate a high prevalence of RP among patients, resulting in poor treatment tolerance. Thus, due to treatment discontinuation or incomplete irradiation resulting from intolerable toxicities, the prescribed dose often cannot be adequately delivered via conventional CTV-inclusive approaches, potentially compromising tumor control. A reduction in the GTV radiation dose targeted at potential microinvasive lesions inevitably compromises the therapeutic effect. However, the GTV is typically the primary source of local recurrence after local radiotherapy for the tumor, and by omitting the CTV delineation, normal tissue irradiation can be minimized (24,27). As normal organ function persists, subclinical lesions around the GTV will receive some level of irradiation, and a combined treatment with intravenous chemotherapy can control the progression of these subclinical lesions to a certain extent in clinical practice. Therefore, this study examined whether omitting clinical targets significantly affects the therapeutic and adverse effects of lung cancer radiotherapy.
In terms of the clinical effectiveness results, there was no evidence of reduced short-term treatment efficacy in the PTV-G group. Several factors could contribute to this clinical benefit. First, contemporary lung cancer radiotherapy normally applies non-prophylactic irradiation, ensuring the GTV’s dose for the tumor. The exclusion of subclinical targets aligns with the modern trend toward smaller radiation fields and involved-field irradiation in precision radiotherapy. During radiation therapy, even if specific areas, such as the ipsilateral hilum of the lung, mediastinum, and paratracheal lymph nodes, fall outside the radiotherapy target area, incidental radiation doses reaching 40–50 Gy may still be applied, which can have therapeutic advantages for subclinical lesions. Second, the omission of the clinical target area reduces radiation doses to normal tissues, such as the lung, esophagus, and heart, which subsequently reduces the toxic side effects and increases patient tolerance.
The limitations of this retrospective study should be acknowledged. First, the non-prospective, randomized controlled design and small sample size of the study constrained the analysis, shortening the follow-up period, and resulting in insufficient long-term patient survival and side-effect observations. Second, only a limited number of patients in this study received consolidation immunotherapy, which is now a standard treatment for advanced lung cancer and known to enhance tumor treatment efficacy. The interaction between immunotherapy and pneumonia remains an area for further clinical research, and the clinical significance of omitting the CTV in radiotherapy for lung cancer patients requires further investigation. While our retrospective findings provide valuable insights into the feasibility of omitting the CTV, prospective, randomized studies are necessary to validate our approach. In the future, we intend to increase the sample size and extend the follow-up period to gather more comprehensive data on long-term survival and treatment-related side effects. These studies will be crucial in confirming the clinical benefits and safety of reduced treatment target volume techniques.
Conclusions
Omitting the CTV did not significantly decrease the PFS and OS of the patients with LA-NSCLC, nor did it increase the rates of regional and distant recurrence. Omitting the CTV after GTV matching substantially reduced irradiation exposure to normal lung, esophagus, and heart tissues, thereby decreasing damage to immune cells and the onset of pulmonary inflammatory responses. This decrease in the occurrence of RP improved patients’ quality of life and minimized RP-related mortality. Thus, omitting the clinical target region appears feasible, albeit further prospective studies are necessary.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-409/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-409/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-409/prf
Funding: This work was partially supported by multiple grants, including grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-409/coif). P.C. reports research grants from Roche, Amgen, Boehringer Ingelheim, Takeda, Merck, AstraZeneca, and Novartis; speaker’s honoraria from Roche, Takeda, Gilead, AstraZeneca, Merck, Thermo Fisher, Janssen, Pfizer, and Novartis; support for attending meetings from AstraZeneca, Pfizer, Janssen, Merck, Gilead, Daiichi Sankyo, Takeda, Novartis, and Eli Lilly; and participation in advisory/data safety monitoring boards from Pfizer, Chugai, Boehringer Ingelheim, Takeda, Janssen, Novartis, AstraZeneca, MSD, and Roche, outside the submitted work. B.A. reports research grant from MSD avenir; consulting fees from Novartis, Astellas, and Sanofior; honoraria from Sanofi, Astra Zeneca, BMS, MSD and Astellas; and support for attending meetings from Janssen, MSD, Pfizer, IPSEN Pharma, and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. SK0233-67), and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. As a retrospective study, patient consent was obtained when possible but was not mandatory.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wiesweg M, Eberhardt WE, Schuler M, et al. Treatment of early and locally advanced stages of non-small cell lung cancer. Inn Med (Heidelb) 2022;63:717-23. [Crossref] [PubMed]
- Thureau S, Mallet R, Gouel P, et al. What dose escalation in the treatment of locally advanced non-small cell lung cancer? Cancer Radiother 2022;26:890-3. [Crossref] [PubMed]
- Borghetti P, Imbrescia J, Volpi G, et al. Chemo-radiotherapy plus durvalumab for loco-regional relapse of resected NSCLC. Radiat Oncol 2022;17:124. [Crossref] [PubMed]
- Takeda Y, Kusaba Y, Tsukita Y, et al. The efficacy profiles of concurrent chemoradiotherapy with intensity-modulated radiotherapy followed by durvalumab in patients with unresectable stage III non-small cell lung cancer: A multicenter retrospective cohort study. Clin Transl Radiat Oncol 2022;37:57-63. [Crossref] [PubMed]
- Zhao N, Yang R, Wang J, et al. An IMRT/VMAT Technique for Nonsmall Cell Lung Cancer. Biomed Res Int 2015;2015:613060. [Crossref] [PubMed]
- Ghafoori P, Marks LB, Vujaskovic Z, et al. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22:37-47; discussion 52-3.
- Xia F, Zhou L, Yang X, et al. Is a clinical target volume (CTV) necessary for locally advanced non-small cell lung cancer treated with intensity-modulated radiotherapy? -a dosimetric evaluation of three different treatment plans. J Thorac Dis 2017;9:5194-202. [Crossref] [PubMed]
- Ergen SA, Dincbas FO, Yücel B, et al. Risk factors of radiation pneumonitis in patients with NSCLC treated with concomitant chemoradiotherapy--Are we underestimating diabetes?--Turkish oncology group (TOG)/Lung cancer study group. Clin Respir J 2020;14:871-9. [Crossref] [PubMed]
- Wang H, Chen H, Gu H, et al. A novel IMRT planning study by using the fixed-jaw method in the treatment of peripheral lung cancer with mediastinal lymph node metastasis. Med Dosim 2018;43:46-54. [Crossref] [PubMed]
- Giuranno L, Ient J, De Ruysscher D, et al. Radiation-Induced Lung Injury (RILI). Front Oncol 2019;9:877. [Crossref] [PubMed]
- Kawahara D, Imano N, Nishioka R, et al. Prediction of radiation pneumonitis after definitive radiotherapy for locally advanced non-small cell lung cancer using multi-region radiomics analysis. Sci Rep 2021;11:16232. [Crossref] [PubMed]
- Hodapp N. The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther Onkol 2012;188:97-9. [Crossref] [PubMed]
- Kepka L, Socha J. Dose and fractionation schedules in radiotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2021;10:1969-82. [Crossref] [PubMed]
- Zou L, Chu L, Xia F, et al. Is clinical target volume necessary?-a failure pattern analysis in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy using intensity-modulated radiotherapy technique. Transl Lung Cancer Res 2020;9:1986-95. [Crossref] [PubMed]
- Riely GJ, Wood DE, Ettinger DS, et al. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2024;22:249-74. [Crossref] [PubMed]
- Agrawal S, Kumar S, Lawrence A, et al. Ipsilateral lung dose volume parameters predict radiation pneumonitis in addition to classical dose volume parameters in locally advanced NSCLC treated with combined modality therapy. South Asian J Cancer 2014;3:13-5. [Crossref] [PubMed]
- Shi LL, Yang JH, Yao HF. Multiple regression analysis of risk factors related to radiation pneumonitis. World J Clin Cases 2023;11:1040-8. [Crossref] [PubMed]
- Zhao J, Lei T, Zhang T, et al. The efficacy and safety of simultaneous integrated dose reduction in clinical target volume with intensity-modulated radiotherapy for patients with locally advanced esophageal squamous cell carcinoma. Ann Transl Med 2020;8:1160. [Crossref] [PubMed]
- Yan Y, Fu J, Kowalchuk RO, et al. Exploration of radiation-induced lung injury, from mechanism to treatment: a narrative review. Transl Lung Cancer Res 2022;11:307-22. [Crossref] [PubMed]
- Hanania AN, Mainwaring W, Ghebre YT, et al. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019;156:150-62. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Huang Y, Zhang W, Yu F, et al. The Cellular and Molecular Mechanism of Radiation-Induced Lung Injury. Med Sci Monit 2017;23:3446-50. [Crossref] [PubMed]
- Liu X, Shao C, Fu J. Promising Biomarkers of Radiation-Induced Lung Injury: A Review. Biomedicines 2021;9:1181. [Crossref] [PubMed]
- Cai S, Shi A, Yu R, et al. Feasibility of omitting clinical target volume for limited-disease small cell lung cancer treated with chemotherapy and intensity-modulated radiotherapy. Radiat Oncol 2014;9:17. [Crossref] [PubMed]
- De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol 2010;28:5301-10. [Crossref] [PubMed]
- Ottolenghi A, Smyth V, Trott KR. The risks to healthy tissues from the use of existing and emerging techniques for radiation therapy. Radiat Prot Dosimetry 2011;143:533-5. [Crossref] [PubMed]
- Bradley JD, Hu C, Komaki RR, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:706-14. [Crossref] [PubMed]


