Response to editorial titled ‘Intrapulmonary lymph node retrieval: unclear benefit for aggressive pathologic dissection’
To the editor,
The TNM staging system is currently our best prognostic tool in lung cancer, but poor application of this tool is an increasingly recognized worldwide problem in thoracic oncology (1-3). The main deficiency appears to be suboptimal pathologic lymph node staging, an important problem because lymph node metastasis is the gravest prognostic feature in patients without distant metastasis, who are candidates for curative surgical intervention. The statistics are startling: 17% of lung cancer resections in the US have no lymph nodes examined (pNX) (4), 40-50% of all resections (67% of resections with ‘pN0/pN1’ disease) have no mediastinal lymph nodes examined (5,6), 12% of patients have no hilar/intrapulmonary (N1) lymph nodes examined (7), the median total lymph node count is only 4-5 and less than 15% of patients have more than 10 lymph nodes examined (8-10).
Patients with pNX resections are usually managed post-operatively as though known to be pN0, but have a 5-year survival rate closer to patients with pN1 (4). Patients with ‘pN0/pN1 disease’ and no mediastinal lymph nodes examined have an 11% excess lung cancer-specific mortality risk compared to identical stage patients with one or more examined mediastinal lymph node (6). Multiple studies reveal a sequential improvement in survival of patients with ‘pN0’ disease with examination of more lymph nodes. All these studies suggest the minimum required number of lymph nodes is greater than 10 (8-10). Our recent analysis of the pN0 population in the US Surveillance, Epidemiology, and End Results database suggests that the lowest mortality risk occurs in those with 18-20 lymph nodes (Osarogiagbon and Yu, unpublished data).
Even in patients with lymph node metastasis, several groups, analyzing different databases from around the world, have consistently reported the direct association between the number of lymph node metastasis and survival (11-15). Indeed, there is an ongoing debate about whether or not the number of lymph nodes with metastasis or the ratio of lymph nodes with and without metastasis may be a more powerful prognostic factor than the anatomic location of lymph node metastasis, which is the sole basis of the current AJCC/UICC lymph node staging system (13,16).
What is going on here? We believe the problem is the risk of sampling error and stage mis-attribution (‘the Will Rogers phenomenon’) with incomplete nodal examination. It seems logical that when we do not examine lymph nodes, we will not detect lymph node metastasis. In recognition of this, the Association of Directors of Anatomic and Surgical Pathology made the recommendation to ‘submit every node for microscopic examination’ (17).
The problem of poor lymph node examination can be conceptually localized to three sites: events in the operating room, the communication between the operating room and pathology laboratory, and events in the pathology laboratory. Examination of hilar (station 10) and mediastinal (stations 2-9) lymph nodes requires surgical harvest of these nodes, without which pathology examination is impossible; correct identification of lymph node specimens and secure transfer from the operating room to the pathology laboratory are mandatory, loss of specimens in transit or poor identification of the provenance of specimens will impair the pathology examination; finally, proper pathology department processes to ensure thorough examination of submitted specimens is vital to achievement of optimal pathologic staging. A breakdown in any of these links in the chain of events will severely impair proper examination.
Successful correction of the problem of poor nodal staging requires understanding of the extent to which problems arise at each of these sites, in order to logically design and implement corrective interventions. For example, it can be argued that no matter what transpires in the operating room and in transit from there to the pathology laboratory, proper pathology examination protocols should assure that pNX resections are rare in patients who undergo lobectomy (or more extensive) resections in the absence of neo-adjuvant therapy, because lymph nodes from stations 11 to 14 are present in the resected lung specimen.
Against this background, we evaluated current routine pathology examination processes. We tested our hypothesis that low N1 lymph node counts indicate non-examination of a significant proportion of lymph nodes present within the lung resection specimen by fastidiously re-examining discarded lung resection specimens after completion of the official pathology examination. In calculating our sample size for this project, we estimated that a 20% increase in number of lymph nodes examined, a 10% increase in number of lymph node metastasis detected, and a change in pathologic stage in 5% of patients would all be clinically meaningful. What did we find? Lymph node retrieval increased by 137%, detection of N1 lymph nodes with metastasis increased by 165%, pathologic up-staging in 11% of patients, and missed lymph node metastasis in 12% of patients with ‘pN0’. Clearly, the recommendation that all lymph nodes in the resection specimen should be examined is not being followed. Therefore, we suggested that current pathology examination protocols need to be improved.
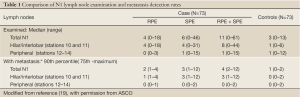
The editorial by Brzezniak and Giaccone ‘Intrapulmonary lymph node retrieval: unclear benefit for aggressive pathologic dissection’ summarized our results succinctly and accurately, except for a couple of points (18). As shown in Table 1, which is taken from the reference publication (19), majority of the discarded lymph nodes retrieved by our re-dissection protocol, and majority of the retrieved lymph nodes with metastasis, were from the hilar/interlobar zone (mostly station 11). We found relatively few lymph nodes, with or without metastasis, in the peripheral zones (stations 12-14). Therefore, the excursus about the different survival implications of peripheral lymph node metastasis and hilar/interlobar (stations 10-11) metastasis, entirely supports our concern that the missed lymph node metastasis we demonstrated will probably have a significant survival impact. Secondly, although we compared the dissection time of sequential batches of 10 re-dissections in our analysis of the evolution of efficiency of the special dissection protocol, the specimens were not examined in batches of 10, but rather as they became eligible for examination after completion of the routine pathology examination over the study duration from July 2010 to August 2011. Furthermore, the dissections were all performed by a pathology technician working in a community hospital. Therefore, we believe the improvements are feasible in any institution engaged in the business of providing surgical and pathology services to patients with lung cancer.
Full Table
We agree that our study was not designed to directly examine the impact on survival. Clearly, it will take a prospective comparative effectiveness study, with cost-effectiveness components, to quantify the impact and cost of this corrective intervention. However, the impact of our findings on the quality and outcomes of care for lung cancer patients is potentially great. We are developing a prospective institutional randomization study of a simplified modification of this pathology dissection protocol, in combination with an intra-operative quality improvement intervention in which a specially designed surgical specimen collection kit would be used to help surgeons perform a standardized systematic lymph node dissection (20). This study, titled Strategies to Improve Lymph node Examination in Non-small cell lung Tumors (SILENT) is currently in development through the US clinical research cooperative group SWOG. It will address the pathologic upstaging rate as its primary endpoint and relapse-free survival as one of a number of secondary endpoints. It should provide definitive information on the opportunity to improve patient survival with these two relatively simple corrective interventions. However, until then, it is important to recognize that absence of proof is not proof of absence of a survival benefit from these simple, commonsense interventions.
Acknowledgements
Disclosure: Dr. Osarogiagbon has a patent application under consideration for a specimen collection kit, the other authors declare no conflicts of interest.
References
- Gephardt GN, Baker PB. Lung carcinoma surgical pathology report adequacy: a College of American Pathologists Q-Probes study of over 8300 cases from 464 institutions. Arch Pathol Lab Med 1996;120:922-7.
- Farooq A, Osarogiagbon RU, Allen JW, et al. Accuracy and comprehensiveness of pathology reportage after lung cancer resection. J Clin Oncol 2009;27:15s (abstr 6523).
- Verhagen AF, Schoenmakers MC, Barendregt W, et al. Completeness of lung cancer surgery: is mediastinal dissection common practice? Eur J Cardiothorac Surg 2012;41:834-8.
- Osarogiagbon RU, Yu X. Comparative survival of resected node-negative non-small cell lung cancer with and without lymph node examination in the SEER database. J Thorac Oncol 2012;7:abstr 131.
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056.
- Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol 2012;7:1798-806.
- Allen JW, Farooq A, O’Brien TF, et al. Quality of surgical resection for nonsmall cell lung cancer in a US metropolitan area. Cancer 2011;117:134-42.
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50.
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6.
- Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer 2009;115:851-8.
- Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol 2006;1:120-5.
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg 2008;85:211-5.
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8.
- Jonnalagadda S, Smith C, Mhango G, et al. The number of lymph node metastases as a prognostic factor in patients with N1 non-small cell lung cancer. Chest 2011;140:433-40.
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9; discussion 1619-20.
- Rusch VW, Giroux DJ. Nodal staging in lung cancer: lymph node location or number? J Thorac Oncol 2011;6:237-8.
- Association of Directors of Anatomic and Surgical Pathology. Recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Clin Pathol 2001;115:799-801.
- Brzezniak C, Giaccone G. Intrapulmonary lymph node retrieval: unclear benefit for aggressive pathologic dissection. Transl Lung Cancer Res 2012;1:230-3.
- Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30:2823-8.
- Osarogiagbon RU, Miller LE, Ramirez RA, et al. Use of a surgical specimen-collection kit to improve mediastinal lymph-node examination of resectable lung cancer. J Thorac Oncol 2012;7:1276-82.


