Statistic and dosimetric criteria to assess the shift of the prescribed dose for lung radiotherapy plans when integrating point kernel models in medical physics: are we ready?
Introduction
The dose calculation algorithms integrated in a radiotherapy Treatment Planning System (TPS) compute the medical prescribed dose (PD) into a representation of the delivered dose (DD), of the same expected value in gray, to the patient, itself translated in monitor units (MUs) actually delivered by the radiotherapy machine. This is a fundamental fiducial chain between the treatment desired by the medical oncologist and the physical dose, and clinical effect, truly obtained in the patient. As everyone knows the relation between the PD and the DD is not yet exact in all anatomical situations or with all calculation algorithms. Since the generalization of predictive and personalized dosimetry with 2 and 3D dose distributions and dose volume histograms (DVH) one could easily forget this caveat and imagine to see the truth on treatment plan. This is almost right for density rather homogeneous anatomic regions as brain, pelvis, abdomen, etc. but it is still a search for density very heterogeneous regions as chest because of the very low density of lungs. The progress toward always better calculation algorithms is not linear. Impressive progresses have been made considering heterogeneities but with low consideration regarding the real physical processes of dose deposition, and more recently increasing consideration is given to dose deposition mechanism’s going closer and closer to Monte Carlo simulation results, taken as reference. Most of the Radiation Oncology departments had, in the recent years, to go through successive changes of dose calculation algorithms according to the evolution of those software’s. In this paper, the evolution of the relation between the PD and the DD is examined through the most current situation of change from pencil beam convolution with modified batho (PBC-MB) to anisotropic analytical algorithm (AAA). The point of view is the quantification of the altered PD to consider, when one wishes to keep on with the same physical DD or clinical results, when implementing such changes (1-3). This concern should, of course be extended to organs at risk (OARs), or integrate dose escalation, but this is out of the scope of this report which focus on the methodological issues.
Methods
Dose calculation algorithms
The most commonly dose calculation models for photon beam therapy, as pencil kernel and point kernel, were used in this study. The dose calculations were performed using PBC-MB as pencil kernel model and AAA as point kernel model. Both algorithms were integrated in Eclipse® TPS (Version 8.1; Varian Medical Systems, Palo Alto, CA, USA). The pencil beam convolution (PBC) computes the dose to the patient as the superposition of the total energy released per mass unit within an energy deposition kernel. The kernel represents the spread of energy from the primary photon interaction site throughout the volume (4-7). For inhomogeneity correction, PBC-MB method first calculates a relative dose distribution within a water-equivalent medium, and then adds an inhomogeneity correction factor. In the AAA all energy from a photon interaction is deposited either in the forward beam direction or along one of 16 lateral transport lines, all located in the plane perpendicular to the incident beam direction. AAA presents more accurate algorithm as compared to pencil kernel algorithm (8-10).
Clinical cases and treatment planning
Nine lung cancer cases have been included in this study. Radiation oncologists delineated the anatomic borders of planning target volume (PTV) and OARs. The treatment plans were 3D conformal plans using multi-leaf collimators (MLC). The average target volume was 394.0±194.0 mL, treated with a mean PD of 58.8 Gy (range, 50.8–66 Gy) and n=34 beams. For each patient, three treatments plans were generated using exactly the same beams arrangements (11,12). In plan 1, the dose was calculated using PBC-MB. In plan 2, the dose was calculated using AAA and the same PD as plan 1. In plan 3, the dose was calculated using AAA with MUs obtained from PBC-MB as input. The plan 3, having the MUs from plan 1, shows a display of the dose distributions of the former treatments, taken as references, recalculated with the new algorithm. In all plans, the PD is considered at a single reference point at the isocentre. The reference treatment plan was generated according to the clinical experience of the department and the ICRU recommendations (13,14). The validation of a treatment plan requires that 95% of PTV should be covered by 100% of the PD and the maximum dose within the PTV was under 107% of PD. For OARs, the dose constraints were respected. In our point of view, the choice of plan 1, with pencil kernel model, as the reference was justified by the clinical experience accumulated over several years with the corresponding algorithms.
Dosimetric criteria’s
The MUs characterize the irradiation time from linac and the dose criteria’s to validate a treatment plan are based on DVH parameters. Thus, the three criteria were used:
DD: the MUs from the plan 1 and 2 were compared. Then, the delivered dose at isocentre (Diso) recalculated in plan 3 was compared with the initial PD in plans 1 and 2.
DVH indices: for each PTV the calculated dose to 95% of the target volume (D95) from all plans were compared.
Gamma analysis
The γ-index combines two criteria including the dose difference in percentage (ΔDose) and the distance-to-agreement (DTA) in millimeters. An ellipse is used to determine the acceptable region. The γ-value ≤1 represents fulfillment of the criteria (15). Our goal was to quantify the magnitude of the impact of dose calculation models on “real” DD. The Dicom images including dose distribution from pencil kernel and point kernel models, for each patient were exported to RIT-113® (Dosimetry System Version 5.2, Radiological Imaging Technology, Inc., CO, USA). The pixels with γ≤1 present the pixels having the same dose distribution. The pixels with γ>1 show under/overestimated dose associated with new dose calculation model compared to reference one. Using 3%/3 mm, the 95% of pixels should have γ≤1.
Statistical analysis
The bootstrap simulation method was used to estimate the minimum number of fields “beams” to observe a significant difference between algorithm models. Then, the data resulting from the simulation was used to estimate the 95% of confidence interval (95% CI). This consisted in taking 1,000 random samples of size “n”, with n=5 to n=34. For each sample size, P value was computed using Wilcoxon signed-rank test (16). The dose difference is considered significant, if P<0.05. The statistical correlation between calculated doses was evaluated using Spearman’s correlation coefficient (ρ).
Medical decisions
The objective of the comparison between pencil kernel and point kernel is to check if the PD should be readjusted. We considered that if there is a statistically significant, P<0.05, for dosimetric indices, the PD should be readjusted. The significant differences reflect with 95% of confidence existing differences between algorithms. To make a medical decision, three successive evaluations were carried out using MUs, Diso and D95, as mentioned above. Figure 1 shows which medical decision could be considered regarding the alteration of the PD when moving from reference algorithm to new one.
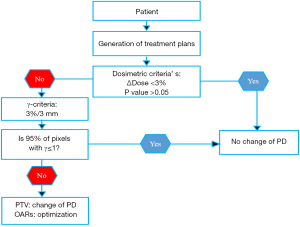
Results
Comparison of DD
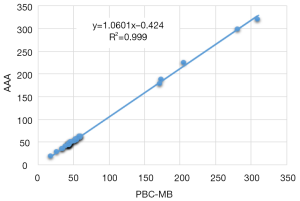
MUs: the AAA in plan 2 calculated significantly more MUs than the PBC-MB in plan 1 using the same PD. The 95% CI for ΔMUs evaluated with bootstrap simulation was (3.9; 5.5). The Wilcoxon test showed a significant difference, with P<0.001 and the data showed a strong correlation, with ρ>0.9. Figure 2 shows the correlation between the MUs from pencil kernel with point kernel. Figure 3 shows the computed average P value for each sample size, going from n=5 to n=34. It can be seen in Figure 3 that eight beams would have been sufficient to observe a significant difference between AAA versus PBC-MB.

Diso: using the same MUs form PBC-MB algorithm, the AAA in plan 3 calculated significantly less dose than initially prescribed in plans 1 and 2, as shown in Figure 4, with P=0.03 and ρ=0.99. The 95% CI for ΔDiso evaluated by bootstrap simulation was (3.3; 5.8).

Comparison of calculated dose to 95% of PTV
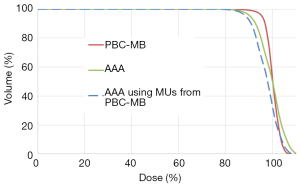
The AAA in plans 2 and 3 calculated significantly less D95% than the PBC-MB in plan 1. The 95% CI for ΔD95 was (10.0; 15.0), with P<0.01 and ρ=0.9. Figure 5 shows the cumulative DVH from all plans. It can be seen that AAA in plans 2 and 3 calculated significantly less D95 for the PTV.
Gamma analysis
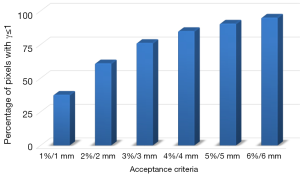
The tolerance limit (TL), 95% of pixels having γ≤1, is not respected at all using routinely γ criteria with 2%/2 mm or 3%/3 mm. However, to satisfy the γ tolerance, at least 6%/6 mm is needed. Figure 6 shows the percentage of pixels having γ≤1, by varying dose-difference and DTA criteria. The significant difference for dose distribution is due to the wide range of electron transport in the lung. The point kernel algorithms are more accurate than pencil kernel algorithms due to their ability to approximately model the electron transport.
Discussion
There are numerous studies recommending integrating carefully, into clinical use, the new dose calculation algorithms (17-19). Recently, Chaikh et al. [2014] reported that the change of dose calculation algorithm might be associated with the adjustment of the dose prescription for clinical purpose (3). In this paper we compared three parameters using two calculation algorithms. Our comparisons were based on 34 fields. The comparison of MUs, as first step, made these changes obvious. For the same PD, the MUs were increased when moving from PBC-MB to AAA. The differences in MUs were influenced by the field size, the anatomical structures surrounding the lung, the beam incidence and orientation. A statistical evaluation based on Wilcoxon’s test showed a significant difference, also confirmed with the 95% CI of the existing differences. Consequently, keeping the same PD, the risk due to the change from PBC-MB to AAA was an increased dose to the target. In addition, the bootstrap simulation indicated that the significant differences between dose calculation algorithms could be ascertained with as little as n=8 beams. The results obtained from MUs were confirmed by the comparison of dosimetric indices and 2D gamma analysis, with P<0.05. When the dose distribution was calculated by AAA, using the same PD, the dose difference was more than 3%/3 mm for all cases. Considering the results from 2D gamma, the major of pixel values don’t meet the criteria 95% of pixels with γ≤1 using routinely recommendation 2%/2 mm or 3%/3 mm (20,21). The results confirm that an optimization of beam weights and arrangement should be performed, for heterogeneity correction with point kernel model, to protect the OARs in thorax region including spinal cord, esophagus, heart and healthy lungs. All these organs were affected by turning-on the heterogeneity correction with AAA, since the secondary electrons go more through the organs due to the lower density of lungs.
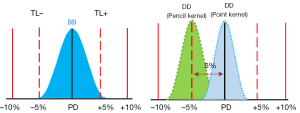
Considering AAA is to be a more accurate algorithm, the comparison between the AAA and PBC-MB provides an indication of the dose-difference for real DD for a decade using PBC-MB. Considering, a satisfaction outcomes with the former algorithm, our results suggest a reduction of 5% for Diso to respect the dose conformity to PTV using point kernel model. Figure 7 shows an illustration for the recommended DD with a TL and the real DD from pencil beam model and point kernel model. The data used to determine the DD were obtained from bootstrap simulation method “in-silico” from treatment plans using both dose calculation models. The TL =±5%, used in the illustration, for dose deviations were suggested in ICRU reports (13,14). However, the real clinical outcome, such as tumor control probability and normal tissue complication probability as endpoint, should be used to determine the DD ± TL. Ideally, more appropriate radiobiological models with clinical parameters, real clinical trials outcomes and clinical experience are needed to better estimate the radiotherapy outcomes.
Conclusions
This paper shows that the alterations of dose estimations are quite important when changing the calculation algorithm in radiotherapy. It is at least of the order of magnitude of dosimetric cumulated uncertainties considered as inacceptable (>5%). Therefore, these alterations need to be known and taken into account in the process of quality assurance in radiation oncology. This alteration could be an increment or a reduction of the PD according to the type of the new algorithm which is substituted to the former one. Actually, in our virtual course to more and more accuracy, hopefully toped some days by the MC simulation, the changes are not all going in the same direction. This could be a source of misunderstanding between the radiation oncologists and their associated medical physicists. Moreover, many parameters are influencing these results and it is difficult to imagine finding the truth all done in the literature. Ideally, each radiation oncology department should be able to assess this question and it is interesting to see that rather simple tools are existing and are powerful enough to allow making a valuable study with a small set of patients, any department could find among its own workflow.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: We declare that the article does not require a Statement of Ethics, since all the clinical material was anonymized CT-scans images used for dosimetric repeated assay’s at a remote time from the real treatment of the patients as mentioned in section (2.2). Absolutely no information concerning the patients, themselves, were used, so no consent were necessary. The study has been carried out in the University Hospital of Grenoble, France.
References
- Knöös T, Wieslander E, Cozzi L, et al. Comparison of dose calculation algorithms for treatment planning in external photon beam therapy for clinical situations. Phys Med Biol 2006;51:5785-807. [Crossref] [PubMed]
- Herman Tde L, Hibbitts K, Herman T, et al. Evaluation of pencil beam convolution and anisotropic analytical algorithms in stereotactic lung irradiation. J Med Phys 2011;36:234-8. [Crossref] [PubMed]
- Chaikh A, Balosso J. Should the dose prescription be readjusted when using tissues density corrections algorithms for radiation oncology? J Case Rep Onc Ther 2014;1:118.
- Task Group No. 65, the Radiation Therapy Committee of the American Association of Physicists in Medicine. Tissue inhomogeneity corrections for MV photon beams. Madison, WI: Medical Physics Publishing, 2004. Available online: https://www.aapm.org/pubs/reports/RPT_85.pdf
- Ahnesjö A, Aspradakis MM. Dose calculations for external photon beams in radiotherapy. Phys Med Biol 1999;44:R99-155. [Crossref] [PubMed]
- Batho HF. Lung corrections in cobalt 60 beam therapy. J Can Assoc Radiol 1964;15:79-83. [PubMed]
- El-Khatib E, Battista JJ. Improved lung dose calculation using tissue-maximum ratios in the Batho correction. Med Phys 1984;11:279-86. [Crossref] [PubMed]
- Van Esch A, Tillikainen L, Pyykkonen J, et al. Testing of the analytical anisotropic algorithm for photon dose calculation. Med Phys 2006;33:4130-48. [Crossref] [PubMed]
- Rana S. Clinical dosimetric impact of Acuros XB and analytical anisotropic algorithm (AAA) on real lung cancer treatment plans Int J Cancer Ther Oncol 2014;2:02019. review. [Crossref]
- Ojala J. The accuracy of the Acuros XB algorithm in external beam radiotherapy – a comprehensive review. Int J Cancer Ther Oncol 2014;2:020417. [Crossref]
- Chaikh A, Giraud JY, Balosso J. A method to quantify and assess the dosimetric and clinical impact resulting from the heterogeneity correction in radiotherapy for lung cancer. Int J Cancer Ther Oncol 2014;2:020110. [Crossref]
- Chaikh A, Balosso J. The use of radiobiological TCP and NTCP models to validate the dose calculation algorithm and readjust the prescribed dose. Radiotherapy and Oncology 2016;118:S24. [Crossref]
- ICRU Report No. 50. Prescribing, Recording and Reporting Photon Beam Therapy. International Commission on Radiation Units and Measurements, Bethesda, Maryland, 1993. Available online: http://www.icru.org/home/reports/prescribing-recording-and-reporting-photon-beam-therapy-report-50
- ICRU Report No. 62. Prescribing, Recording and Reporting Photon Beam Therapy supplement to ICRU Report 50, International Commission on Radi-ation Units and Measurements, Bethesda, Maryland 1999.
- Chaikh A, Giraud JY, Balosso J. A 3D quantitative evaluation for assessing the changes of treatment planning system and irradiation techniques in radiotherapy. Int J Cancer Ther Oncol 2014;2:02033. [Crossref]
- Chaikh A, Giraud JY, Perrin E, et al. The choice of statistical methods for comparisons of dosimetric data in radiotherapy. Radiat Oncol 2014;9:205. [Crossref] [PubMed]
- Morgan AM, Knöös T, McNee SG, et al. Clinical implications of the implementation of advanced treatment planning algorithms for thoracic treatments. Radiother Oncol 2008;86:48-54. [Crossref] [PubMed]
- Chaikh A, Balosso J. NTCP Variability in Radiotherapy of Lung Cancer When Changing the Radiobiologic Models and the Photon Dose Calculation Algorithms. J Cancer Clin Oncol 2016;2:100108.
- Chaikh A, Balosso J. Assessing the shift of radiobiological metrics in lung radiotherapy plans using 2D gamma index. Transl Lung Cancer Res 2016;5:265-71. [Crossref] [PubMed]
- Van Dyk J, Barnett RB, Cygler JE, et al. Commissioning and quality assurance of treatment planning computers. Int J Radiat Oncol Biol Phys 1993;26:261-73. [Crossref] [PubMed]
- Nielsen TB, Wieslander E, Fogliata A, et al. Influence of dose calculation algorithms on the predicted dose distribution and NTCP values for NSCLC patients. Med Phys 2011;38:2412-8. [Crossref] [PubMed]


