Editor’s note:
In the era of personalized medicine, a critical appraisal new developments and controversies are essential in order to derived tailored approaches. In addition to its educative aspect, we expect these discussions to help younger researchers to refine their own research strategies.
Pros: After stereotactic ablative radiotherapy for a peripheral early-stage non-small cell lung cancer, radiological suspicion of a local recurrence can be sufficient indication to proceed to salvage therapy
Introduction
Stereotactic ablative radiotherapy (SABR), also known as “stereotactic body radiation therapy” (SBRT), has revolutionized the treatment of early-stage non-small cell lung cancer (NSCLC), providing an effective treatment option for medically-inoperable patients. Modern advancements in the planning and targeting of radiotherapy have allowed SABR to deliver ablative doses as high as 150 Gy (when converted to 2 Gy per fraction) in a precise and highly conformal manner (1). After SABR, rates of primary tumor control are excellent, in excess of 90% at 5 years (2). These promising results have led to suggestions that SABR may be comparable to the historic gold standard, surgical resection, as first-line treatment in operable patients. Three randomized control trials (RCTs)—the STARS trial, the ROSEL trial and ACOSOG Z4099—attempted to compare SABR and surgical resection, but all closed prematurely due to insufficient enrollment. A pooled analysis of the patients accrued to STARS and ROSEL suggested that, at a minimum, there was equipoise between the two treatments, with significantly better overall survival demonstrated in the patients receiving SABR (3). More robust RCT evidence is still awaited, and at least two RCTs examining this question are ongoing including the STABLE-MATES and SABR-Tooth trials (4).
Although SABR has been widely adopted over the past decade (5), there is ongoing uncertainty in assessing treatment response and detecting local recurrence (LR). Following SABR, radiation-induced lung injury (RILI) is common, which manifests as local changes to the lung parenchyma on CT imaging that are usually asymptomatic. Both acute (within 6 months) and late (after 6 months) changes have been previously described and can obscure the detection of residual and recurrent disease (6). Acute changes have been categorized as one of 4 types: diffuse consolidation, patchy consolidation, diffuse ground-glass opacities, or patchy ground-glass opacities. Late changes typically manifest as a modified conventional pattern, mass-like fibrosis or scar-like fibrosis (7). The possible mass-like appearance of RILI is likely a product of the highly conformal treatment (8), and this appearance may mimic the growth pattern of locally recurrent disease. Benign CT changes may continue to evolve in morphology and severity up to 2 years following SABR (7), which can further impair the detection of LR during the critical period of time when LRs are most likely to occur (9). Predicting which cases of RILI may be at increased risk of recurrence is also challenging, with initial response to treatment and rate of tumor shrinkage not being associated with ultimate local control (10).
Some patients who develop LR after SABR may be candidates for salvage treatments, including surgical resection or repeat SABR (11). Accurate and early detection of LR following SABR is a critical first step to ensuring that recurrences are managed efficiently. For patients with imaging findings suspicious for LR, we argue that radiologic evidence of recurrence can be sufficient to detect LR, and that patients should not be denied the option of salvage treatment if a biopsy is unsafe or contraindicated.
Biopsy: proceed with caution
Ideally, all salvage treatment decisions would be informed by a definitive pathologic diagnosis. The reality, however, is that lung biopsies are imperfect investigations, they are associated with a risk of complications, and pathologic interpretation can be difficult when sampling an irradiated area. Even in patients who have not undergone radiation in the past, the performance characteristics of CT-guided biopsies may be suboptimal. In a retrospective analysis of 242 patients, CT-guided fine needle aspiration biopsies (CT-FNAB) failed to achieve a definitive diagnosis in 20% of cases (inadequate tissue) compared with only a 3% non-diagnostic rate with CT-guided core biopsies (CT-CB) (12). Although highly specific (99.1%), CT-CB are prone to false negatives, with a reported negative predictive value of only 73.3% (13). Furthermore, the accuracy of CT-CB appears to worsen in lung lesions <1.5, >5 cm (increased extent of necrosis) and those with a benign histology (14). A meta-analysis of 32 studies revealed that CT-CB and CT-FNAB have high overall complication rates of 39% and 24% respectively. The bulk of these events were termed “minor complications”, however, they are not negligible sources of morbidity as they included transient hemoptysis and pneumothorax not requiring intervention. Major complications, including pneumothorax requiring intervention and hemothorax, occurred at a rate of 5.7% with CT-CB and 4.4% with CT-FNAB. The overall pooled risk of any pneumothorax was 25% for CT-CB (15). In radiated lesions, accurate assessment of biopsy specimens may be further obscured by fibrotic and necrotic changes. In one report, a patient required 11 needle passes over 3 different biopsy attempts before a diagnosis of recurrence was made (16).
Recurrence versus fibrosis: a non-invasive approach
The limitations of biopsy, including the performance characteristics, risks of complications, and difficulty with interpretation, suggest that they should only be pursued when there is a high likelihood of the biopsy results changing management.
Recent studies have demonstrated that CT imaging findings, termed high risk features (HRFs) (Table 1), can be useful without biopsy to identify LR. The HRFs were first evaluated by Huang et al., who conducted an analysis to determine the performance characteristics of these features. Their study matched 12 patients with biopsy-proven recurrence to 24 patients without recurrence, and found that several of the HRFs were significantly associated with LR. The top performing HRFs, with both a sensitivity and specificity over 80%, were: growth after 12 months, bulging margins, and craniocaudal growth, which was a newly identified HRF in that study. Although several HRFs had good sensitivity and specificity when considered individually, the presence of multiple features in a single patient (≥3 features) achieved superior results with excellent specificity and sensitivity scores of >90% (17). Most of the HRFs (all except for loss of linear margin) were subsequently validated in a separate, independent study, and similar performance characteristics were demonstrated. With separate validation completed, these HRFs should be considered appropriate for clinical use (18).
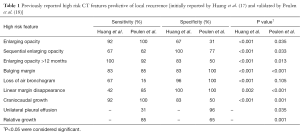
Full table
In patients with a suspected LR, FDG-PET may be useful as an adjunct to the CT-based HRFs, although its role is not as well-defined. Using FDG-PET scans to help distinguish between fibrosis and LR is confounded by the risk of false positives due to the increased metabolic activity related to RILI. Several studies have reported, however, that using a threshold SUVmax of ≥5, or greater than SUVmax prior to treatment, may be a more reliable predictor of LR (6).
Conclusions
Ongoing challenges in accurately distinguishing between LR and RILI on follow-up imaging have complicated decision-making regarding salvage therapy. Biopsies may establish a definitive diagnosis, but at a significant risk of morbidity and inaccurate results, demanding careful consideration regarding their use. HRFs are a validated tool that can indicate a high risk of recurrent disease. Indeed, even in the setting of a newly diagnosed, untreated pulmonary nodule, several guidelines suggest that proceeding to treatment without a biopsy is an appropriate approach when the risk of malignancy is high (19,20), and it is reasonable to extend that paradigm to the post-treatment setting.
For patients who have the option of undergoing biopsy, the available evidence suggests that in the presence of radiologic evidence highly suggestive of LR (e.g., ≥3 HRFs), the pre-test probability of malignancy is sufficiently high that the risk of biopsy likely outweighs any potential benefits. In these situations, a negative biopsy would not be expected to be sufficiently reassuring that there is actually no recurrence present.
For cases where a biopsy is not possible due to significant comorbidities, patient refusal, or an inaccessible lesion, proceeding to salvage therapy based on strong radiologic findings alone is reasonable, as the alternative (i.e., continued observation) puts the patient at risk of progression and metastases.
For all cases, we recommend discussion at a multidisciplinary tumour board to aid with decision-making, and all decisions need to be made in conjunction with the patient after weighing the risks and benefits of the different options.
Acknowledgements
Dr. Palma is supported by a Clinician-Scientist Grant from the Ontario Institute for Cancer Research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fowler JF, Tomé WA, Fenwick JD, et al. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys 2004;60:1241-56. [Crossref] [PubMed]
- Timmerman RD, Hu C, Michalski J, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. International Journal of Radiation Oncology Biology Physics 2014;90:S30. [Crossref]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Chen H, Louie AV. SABR vs. Limited Resection for Non-small Cell Lung Cancer: Are We Closer to an Answer? Curr Treat Options Oncol 2016;17:27. [Crossref] [PubMed]
- Pan H, Simpson DR, Mell LK, et al. A survey of stereotactic body radiotherapy use in the United States. Cancer 2011;117:4566-72. [Crossref] [PubMed]
- Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335-42. [Crossref] [PubMed]
- Dahele M, Palma D, Lagerwaard F, et al. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol 2011;6:1221-8. [Crossref] [PubMed]
- Takeda A, Kunieda E, Takeda T, et al. Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumor recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2008;70:1057-65. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Takenaka R, Shibamoto Y, Miyakawa A, et al. The Fate of Residual Tumor Masses That Persist After Stereotactic Body Radiotherapy for Solitary Lung Nodules: Will They Recur? Clin Lung Cancer 2016;17:406-11. [Crossref] [PubMed]
- Hearn JW, Videtic GM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol 2012;46:19-22. [Crossref] [PubMed]
- Montaudon M, Latrabe V, Pariente A, et al. Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 2004;14:1234-40. [Crossref] [PubMed]
- Yeow KM, Tsay PK, Cheung YC, et al. Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol 2003;14:581-8. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-148. [Crossref] [PubMed]
- Allibhai Z, Cho BC, Taremi M, et al. Surgical salvage following stereotactic body radiotherapy for early-stage NSCLC. Eur Respir J 2012;39:1039-42. [Crossref] [PubMed]
- Huang K, Senthi S, Palma DA, et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol 2013;109:51-7. [Crossref] [PubMed]
- Peulen H, Mantel F, Guckenberger M, et al. Validation of High-Risk Computed Tomography Features for Detection of Local Recurrence After Stereotactic Body Radiation Therapy for Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;96:134-41. [Crossref] [PubMed]
- British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]


