Transthoracic needle aspiration in solitary pulmonary nodule
Introduction
Lung cancer remains the leading cause of cancer deaths worldwide. With the established role of computed tomography (CT) screening for lung cancer, and the broad application of high-resolution CT, the solitary pulmonary nodule (SPN) are increasingly detected. Accurate assessment, proper treatment and timely surgical resection of malignant pulmonary nodules will be highly beneficial to the survival of patients with lung cancer (1). Therefore, the discovery rate of SPN is evidently elevated: most of them are benign, but some of them are lung cancer. The diagnosis of this kind of nodules is difficult and obtaining tissue samples to conduct pathology examination is the key point. The main ways to obtain a specimen for pathology diagnosis include exfoliative cell examination of sputum, bronchoscopy, transthoracic needle aspiration (TTNA), video-assisted thoracic surgery (VATS) and open-lung biopsy. The exfoliative cell examination of sputum is easy and non-invasive, but its positive rate is low. VATS and open-lung biopsy must be conducted under general anaesthesia, with risk, surgical trauma and high cost; nevertheless, some patients cannot undergo general anaesthesia. Bronchoscopy has a great diagnosis value in central type lung nodules, but the determination value in peripheral SPN is limited. TTNA, as a minimally invasive diagnostic method, has been widely used in the diagnosis of small nodules. In 1976, Haaga and Alfidi (2) reported the first case of CT-guided pulmonary puncture biopsy, and after that, this technology has been continuously developing and updating. By reviewing the latest literature, we summarized the relevant notes and strategies about TTNA in SPN diagnosis.
TTNA as diagnostic tool
Currently, an accepted definition of SPN is a single, well circumscribed, radiographic opacity <30 mm in diameter surrounded by aerated lung and not associated with atelectasis, hilar enlargement, or pleural effusion (3). SPN can be caused by a variety of factors, including malignant diseases, or a range of benign lesions. In recent years, an important type of pulmonary nodules has gradually increased, namely the subcentimeter nodules, which refer to those with a diameter <8 mm. Studies have shown that sub-centimeter lung nodules have an overall low degree of malignancy (4). Improved imaging techniques such as high-resolution chest CT scan, result in the most common identification of small and often sub-centimetric SPN (5). With high-resolution CT, lung nodules can be categorized in a more accurate and detailed way. Ground-glass opacity (GGO) is a particular type of pulmonary nodules: is a sign of slightly increased density on the CT where the bronchial and vascular textures are still visible (1). Although most SPN is benign, the pathology of the nodule is crucial to a patient with a history of cancer even if the SPN is small and peripheral. Also, even in small pulmonary lesions <1 cm, the overall malignancy rate is as high as, or slightly lower than that in nodules >1 cm (5). TTNA is a minimally invasive diagnostic method, with a high positive diagnostic rate, less injury and low cost; so, it has been widely used in the routine diagnosis of SPN. Diagnosis of TTNA on small nodules has the following features:
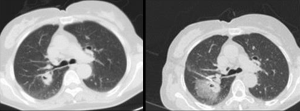
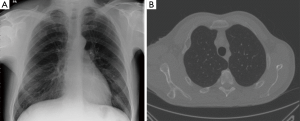
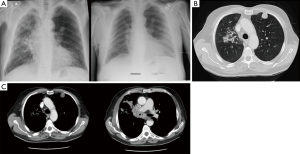
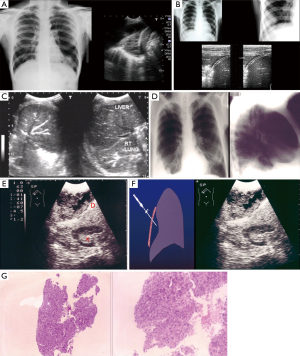
- Wide adaptation range. Except for central type lesions, the diagnostic rate of bronchoscopy on the peripheral type and diffuse lesions is little while TTNA can be applied both in central type lesions or peripheral type and diffuse lesions, as long as there is no apparent adhesion in blood vessels (Figure 1). It is vital that other SPN mimics are excluded on imaging alone, and invasive procedures avoided (Figure 2A,B);
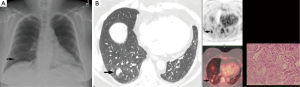
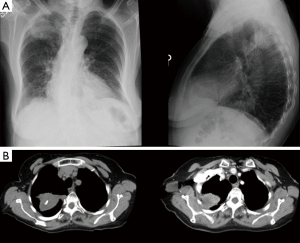
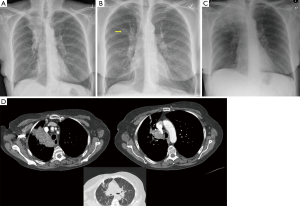
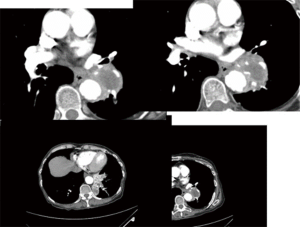
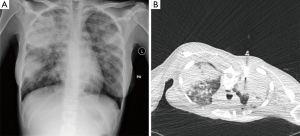
- TTNA has a high accuracy. CT scan can clearly show the location, density of pulmonary nodules and anatomic relationship between lesions and the surround tissues, and it can also locate the puncture site, the angle and the depth accurately. For lesions about 0.5–1 cm, it can also successfully conduct biopsy under CT guidance. It is important to emphases: (I) extrinsic/thoracic wall lesions can mimic an SPN; (II) malignant SPN can increase as well as reduce in size; (III) benign lesions may co-exist; (IV) some lesions with a wider differential such as BAC almost always need tissue sampling; (V) not all calcified lesions are benign (Figures 2-8);
- High diagnostic accuracy. TTNA is a well-established, useful procedure. However, the diagnostic accuracy of TTNA depends on the size and location of the lesion, as well as the guidance technique, and decreases from over 90% to 25% when the malignant nodule is small (<1 cm), and to 70% when the lesion is benign. As many as 29% of patients whose conditions were not diagnosed as malignant on trans-thoracic needle biopsy were ultimately found to have carcinoma (5). According to literature reports, the sensitivity and specificity of lung biopsy are separately 86% and 98.8% while its sensitivity and specificity can reach 91%, 94% by combining with perspective and CT guidance (7,8). The accuracy of puncture is evidently related to the location, depth and size of lesions; for nodules <2 cm, the total diagnosis accuracy of CT-guided puncture is about 77.2% (9); while for nodules with the diameter about 0.5–0.7 cm, its sensitivity is only 50%. At the same time, a number of aspirates also seriously influence the diagnosis of lesion nature; for nodules with the diameter <1 cm, only 77% lesion sampling can satisfy the pathology diagnosis (10); according to reports by Tsukada et al., the diagnosis accuracy of diameter 6–10, 11–20, 21–30 mm are separately 66.7%, 78.9% and 86.7% (11);
- High safety. Although TTNA is a safe and reliable examination method, it is still a traumatic investigation, so there are still some complications. The main complications of TTNA mainly include pneumothorax and haemorrhage. According to literature reports, the incidence of pneumothorax is about 10–40% while the incidence of pulmonary injury is about 26–33% (12). The occurrences of these complications are not only related to nodule size, depth to the chest wall and patients’ basal lung function, but also related to the preoperative preparation, operation technology and patients’ cooperation.
Tips and pitfalls
The direct purpose of TTNA is to improve the diagnosis accuracy of nodules. Before the procedure, patients’ clinical and imaging materials should be fully understood. If it is an enhanced scanning, sites with evident enhancement should be chosen for puncture. If it is a benign tumour, a puncture needle with larger diameter should be selected to obtain enough pathology tissue. If it is tuberculosis, polymerase chain reaction-tuberculosis DNA (PCR-TB DNA) and acid-fast bacillus examination should be conducted on aspirates. CT characteristics of the focal localization scanning should be analyzed seriously. If there is any larger lesion, sites with evident enlargement should be chosen as the puncture targets and liquefactive necrosis tissue should be avoided. If a Franseen needle is selected, movement at the time of negative suction and cutting should be softly and each puncture time should be shortened as shorter as possible to reduce the influence of focal haemorrhage on obtaining the final samples. To maintain a certain amount of negative pressure, the selection of syringes is also critical. If the syringe volume is too small, it is not sufficient to produce a proper negative pressure effect, but if it is too large, it will be difficult to operate, so syringes of 10 or 20 mL will be a right choice. After pulling out the needle, samples should be carefully searched and selected, especially when there is plenty of sludge blood in the aspirates. If the operator is a lack of experience, he/she should conduct the selection, film preparation, submission and deciding whether there should be another puncture with the assistance of a pathology physician or a clinical physician. Therefore, the operator’s experience is paramount. The extension-type automated biopsy gun should be selected because one puncture can keep several materials, which will make treatment available for several times. This set of puncture equipment is expensive, while Franseen needle is cheaper and its repeat utilization rate is high. Besides, for patients with blood in the sputum after operation or patients with a highly suspected malignant tumour or those who are not satisfying with the puncture results, Sputum cytology examination should be conducted after the process. Since biopsy may quickly make cancer cells fall off and flow out along the bronchi, so the positive rate of post-operative cytological examination of sputum is often higher. The skills of core biopsy, combination use, and aspiration in our present cohort were higher than those reported by Yamagami et al. (13). Differences in lesion characteristics (the length of aerated lung or the proportion of experienced operators) may affect diagnostic accuracy. The number of specimens and biopsy methods are significantly different between success or failure groups. Hiraki et al. reported that the acquisition of a larger number of samples significantly increases diagnostic accuracy because the sampling error decreases (14). However, the rate of pneumothorax in patients with a single puncture is significantly less than in patients with three tentatives. With the coaxial technique, core biopsy could be performed without other pleural punctures and with a reduction of the risk of pneumothorax. A higher rate of complications was reported using the more approaches compared with a single technique in a study by Klein et al. (15). While simultaneously considering the risks and benefits, it is important to decide first the number of specimens and to choose the right method. Regarding the methods, the diagnostic accuracies of aspiration, core biopsy, and the combination of techniques were respectively 93.4%, 95.2%, and 100.0%. Yamagami et al. investigated the efficacy of the combination use of core biopsy and aspiration compared with each method alone (13). Lung biopsy is needed to determine the particular cell type of lung cancer. Moreover, the current trend of using receptor antagonists as chemotherapeutic agents requires more tissue to determine the presence of specific receptors and perform various kinds of immunohistochemical staining. Also, when a lesion is shown to be benign, clarification of the particular cell type may be necessary. Core biopsy or a combination of core biopsy and TTNA is required for higher diagnostic accuracy and more pathologic information. It is not obvious whether the consistency of the nodule is a significant factor associated with diagnostic accuracy. Hur et al. reported that the diagnostic accuracy of aspiration is significantly lower for evaluating pure GGO nodules than mixed GGO nodules (16). On the other hand, the sensitivity, the specificity, and the accuracy of TTNA were not significantly different between pure GGO nodules and mixed GGO nodules according to Yamauchi et al. (17). Considering that a diagnosis of adenocarcinoma made up most of the false-negative biopsy results, adenocarcinomas presenting as pure GGO nodules may also show low diagnostic yield on TTNA (18). Within the past decade, new techniques have emerged that offer guidance through the tracheobronchial tree during bronchoscopy to help reach and biopsy the nodule, such as electromagnetic navigation bronchoscopy (ENB) and endobronchial ultrasonography (EBUS) (Figure 9) (19,20). The diagnostic accuracy of peripheral pulmonary nodule by EBUS and ENB is 46–86.2% (21-23), and 62.5–76.9% respectively (24,25). Steinfort et al. (26) comprehensively analyzed 1,420 EBUS biopsies of the peripheral pulmonary tumour from 16 studies, with a sensitivity of 0.73 (P<0.05, 95% CI: 0.70–0.76). Also, a meta-analysis of 15 studies involving 1,033 patients with SPN by Gex et al. (27) showed that diagnostic accuracy of pulmonary nodules with ENB was 73.9% (P<0.05, 95% CI: 68.0–79.2). Based on the above data, we suggest that the diagnostic accuracy of SPN with TTNA is significantly higher than that with EBUS or ENB. Therefore, the positive predictive factors of TTNA of pulmonary nodules are correlated to nodule size (the larger the diameter, the better the accuracy), non-calcific density (the higher the density, the better the accuracy), and distance between the nodule and the pleural plane (the shorter the distance, the better the accuracy). The most common negative predictive factor of TTNA is the wrong placement of the needle tip, not appreciated in the native axial images but retrospectively observed in the sagittal and axial oblique CT images. The diagnostic accuracy of cytologically assisted TTNA can, therefore, be improved by the use of imaging, which is useful to plan the needle path while performing needle aspiration (28).
Conclusions
Selecting the appropriate diagnosis method according to the clinical features of SPN patients can maximally improve the diagnostic accuracy and avoid adverse reactions. For TTNA, the risk of pneumothorax increases if the nodule is near the hilar or away from the surface, or the puncture path passes pulmonary bulla, or when the patient’s lung function is compromised. The risk of haemorrhage is high in a biopsy if the nodule is located near large blood vessels, such that other diagnostic methods should be considered in this case. Overall, TTNA has several advantages such as high diagnostic rate, low cost, and manageable adverse reactions. With proficient operating skills and precise positioning of puncture, the diagnostic accuracy of TTNA can be significantly improved, and its complications can be minimized. Thus, this conventional method is still useful if it is the appropriate approach based on the SPN features. The development of new technology will add more complementary values to the traditional ones.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhan P, Xie H, Xu C, et al. Management strategy of solitary pulmonary nodules. J Thorac Dis 2013;5:824-9. [PubMed]
- Haaga JR, Alfidi RJ. Precise biopsy localization by computer tomography. Radiology 1976;118:603-7. [Crossref] [PubMed]
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [Crossref] [PubMed]
- Slattery MM, Foley C, Kenny D, et al. Long-term follow-up of non-calcified pulmonary nodules (<10 mm) identified during low-dose CT screening for lung cancer. Eur Radiol 2012;22:1923-8. [Crossref] [PubMed]
- Sa YJ, Kim JJ, Du Kim Y, et al. A new protocol for concomitant needle aspiration biopsy and localization of solitary pulmonary nodules. J Cardiothorac Surg 2015;10:104. [Crossref] [PubMed]
- Khan AN, Al-Jahdali HH, Allen CM, et al. The calcified lung nodule: What does it mean? Ann Thorac Med 2010;5:67-79. [Crossref] [PubMed]
- Lacasse Y, Wong E, Guyatt GH, et al. Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax 1999;54:884-93. [Crossref] [PubMed]
- Cheung JY, Kim Y, Shim SS, et al. Combined fluoroscopy- and CT-guided transthoracic needle biopsy using a C-arm cone-beam CT system: comparison with fluoroscopy-guided biopsy. Korean J Radiol 2011;12:89-96. [Crossref] [PubMed]
- Ohno Y, Hatabu H, Takenaka D, et al. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003;180:1665-9. [Crossref] [PubMed]
- Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002;225:823-8. [Crossref] [PubMed]
- Tsukada H, Satou T, Iwashima A, et al. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 2000;175:239-43. [Crossref] [PubMed]
- Yeow KM, See LC, Lui KW, et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol 2001;12:1305-12. [Crossref] [PubMed]
- Yamagami T, Iida S, Kato T, et al. Combining fine-needle aspiration and core biopsy under CT fluoroscopy guidance: a better way to treat patients with lung nodules? AJR Am J Roentgenol 2003;180:811-5. [Crossref] [PubMed]
- Hiraki T, Mimura H, Gobara H, et al. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest 2009;136:1612-7. [Crossref] [PubMed]
- Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology 1996;198:715-20. [Crossref] [PubMed]
- Hur J, Lee HJ, Nam JE, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2009;192:629-34. [Crossref] [PubMed]
- Yamauchi Y, Izumi Y, Nakatsuka S, et al. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT fluoroscopic guidance for ground-glass opacity pulmonary lesions. Eur J Radiol 2011;79:e85-9. [Crossref] [PubMed]
- Choi SH, Chae EJ, Kim JE, et al. Percutaneous CT-guided aspiration and core biopsy of pulmonary nodules smaller than 1 cm: analysis of outcomes of 305 procedures from a tertiary referral center. AJR Am J Roentgenol 2013;201:964-70. [Crossref] [PubMed]
- McNulty W, Cox G, Au-Yong I. Investigating the solitary pulmonary nodule. BMJ 2012;344:e2759. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [Crossref] [PubMed]
- Yoshikawa M, Sukoh N, Yamazaki K, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest 2007;131:1788-93. [Crossref] [PubMed]
- Eberhardt R, Ernst A, Herth FJ. Ultrasound-guided transbronchial biopsy of solitary pulmonary nodules less than 20 mm. Eur Respir J 2009;34:1284-7. [Crossref] [PubMed]
- Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesiEur Respir J 2007;29:1187-92.
- Lamprecht B, Porsch P, Pirich C, et al. Electromagnetic navigation bronchoscopy in combination with PET-CT and rapid on-site cytopathologic examination for diagnosis of peripheral lung lesions. Lung 2009;187:55-9. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- De Filippo M, Saba L, Concari G, et al. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol Med 2013;118:1071-81. [Crossref] [PubMed]


