磁共振成像在肺癌精准放疗中的应用
背景
肺癌是全球死亡率最高的癌症。2012年,全球预计新增病例数达到了1 825万例[1]。大部分(85%~90%)肺癌主要的组织学类型为非小细胞肺癌(NSCLC)。约30%的NSCLC患者处于疾病晚期。手术治疗在该类患者中作用不大,放疗联合化疗是大多数患者的首选治疗方法[2-3]。该病预后很差(5年生存率为15%~30%)[4-5],并且在过去几十年中变化不大,因此迫切需要研究来提高疾病的治疗效果。近年来,临床试验通过改善准确性和改变分次、剂量递增及同步全身治疗的强化治疗,研究提高放疗比例的作用[3]。将新技术纳入放疗中可通过促进治疗的个性化来进一步给患者带来益处,从而使得个体化的治疗强化成为可能。示例之一就是将胸部MRI与直线加速器结合(MR-Linac)[6]。
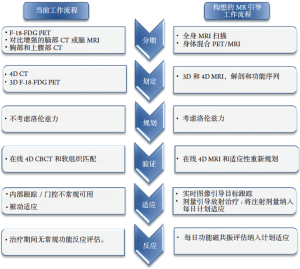
大多数肺癌患者由于肺实质组织密度低,信噪比差,呼吸运动和心脏运动的存在等因素,导致胸部MRI的价值有限[7]。然而,代表7个国际研究中心合作的大西洋MR-Linac协会正在努力克服这些问题,并将这些技术引入到适应性放疗工作流程中(图1)。MRI可应用于放疗的各个阶段:从疾病分期和患者选择、目标和风险器官(organs at risk,OAR)描绘,图像引导的适应性治疗到评估治疗反应。在放疗的这些阶段中,每个阶段都有可能获得增量收益,MR引导和适应性放疗可能为肺癌患者个体化治疗提供一个平台。
本篇综述讨论了当前根治性肺癌放射治疗途径的最新进展和局限性,并概述了可用的MRI技术,将MRI引入到肺癌放疗工作流程中面临的挑战,以及研究将其转化为潜在临床益处的机会。
搜索策略和选择标准
在PubMed通过关键词“肺癌放射治疗”“肺癌MRI”和“MR-Linac”,搜索从1986年1月—2017年4月期间的相关文献。为了防止遗漏文献,辅助进行手工搜索。只对英文文献进行审查。根据与本篇综述的相关性,所有作者都在最终参考文献的选择上达成了一致意见。
疾病分期和患者选择
肺癌准确的疾病分期有助于治疗决策和指导预后。现代治愈—目标的放疗试验要求患者具有最新的全身F-18氟代脱氧葡萄糖定位发射断层扫描(F-18-FDG PET)计算机断层扫描(CT),且在原发肿瘤分期中显示其优于单独进行CT或者F-18-FDG PET[8-9]。F-18-FDG PET对孤立性肺结节、胸腔内病理性淋巴结和远处转移性疾病的评估具有较高的敏感性[10]。
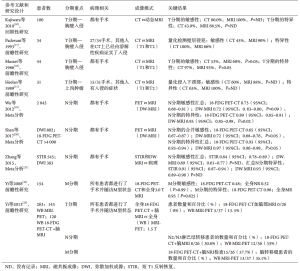
传统观点认为胸部MRI在常规肺癌疾病分期中使用有限。但与CT及18-FDG PET-CT相比,MRI具有更好的软组织对比度,更有助于评估纵隔或胸壁浸润[11-12]。美国临床卓越研究所(national institute of clinical excellence,NICE)、美国国家综合癌症网络(national comprehensive cancer network,NCCN)和美国胸科医师学会(the American College of Chest Physicians,ACCP)指南强调了使用MRI评估肺上沟瘤的可切除性[13-15](表1)。MRI在其他肺部肿瘤的原发肿瘤(T)分期方面的证据有限,但研究正在进行中(图2)。目前NICE指南明确指出,“不应该常规进行MRI检查”用于在肺上沟瘤或疑似胸壁侵犯的情况下辅助进行疾病分期[13]。在其他研究中对使用MRI评估孤立性肺结节进行了回顾[9]。
Full table
当考虑用MRI对胸部恶性肿瘤的淋巴结(N)分期时,发表的数据并不一致。3个Meta分析的解释(表1)受到个体试验诊断标准和MRI脉冲序列不同的变化的限制,导致汇集的敏感性和特异性发生变化。一些单独的试验也包含在多个Meta分析中。尽管有这些局限性的存在,数据表明弥散加权(DW)MRI对NSCLC的N分期具有高特异性(高达0.72;95%CI:0.63~0.80)(表1)[19-21]。所有的研究都强调了在DW-MRI的常规临床实践中诊断病理性淋巴结的一般建议可以作出之前诊断标准的标准化的需求[21,24]。在诊断标准化达成共识之后,需要进一步的方法学检测,优先在大型多学科试验中进行,以便更接近将这些有前景的技术应用于常规临床工作流程(表1)[24]。
关于转移性(M)疾病的分期,MRI的主要作用是检测脑转移。但越来越多的证据提倡使用全身MRI,采用快速采集,以用于评估转移性疾病[25-26]。一项比较165例3.0特斯拉(T)全身MRI和18-FDG PET-CT的NSCLC患者的研究显示,成像方式在分期准确性方面没有统计学差异。全身MRI可以更有效地检测脑部(5例全体核磁共振成像,1例为PET-CT)和肝转移(4例MRI和0例PET/CT的真阳性病例,但有3例MRI为假阳性)。相反来自同一患者队列的数据提示,18-FDG PET-CT可能更有助于检测远处淋巴结和软组织疾病[22],但是这项工作需要在更大的患者队列研究中进一步验证。与18-FDG PET-CT相比,MRI在脑和肝转移灶检测中的优越性归因于这些器官中的生理FDG更新,这可能阻碍PET中的转移性疾病可视化以及改善与MRI的软组织对比度[9]。进一步发展成为共引进注册FDG PET-MRI影像。与18-FDG PET-CT相比,18-FDG PET-MRI混合系统可提供较高的软组织对比度,较少的辐射暴露[27],早期的数据结果令人满意(表1)[23],但是需要进一步研究新的示踪剂并结合运动[9]。
MRI避免了PET成像分期的许多缺点,包括放射性示踪剂合成和运输;可以受血糖水平影响的标准化摄取变量(SUV)测量的准确性;部分体积平均效应;恢复系数和辐射暴露[28]。由于患者数目较少,支撑胸部MRI用于分期的大部分证据尚处于初始阶段。进一步的研究应集中在调查潜在的解决方案,以精心设计多中心研究克服胸部MRI挑战(表2)。预计未来的影像学发展可能会扩大MRI在分期肺癌患者中的作用。
Full table
靶标和OAR划定
靶组织和正常组织的准确成像对于放疗至关重要。基线计划CT扫描构成了目标和OAR划定的基础,并且形成剂量指标。这个阶段的不准确之处会贯穿到所有后续阶段。
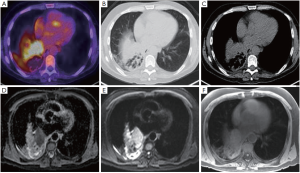
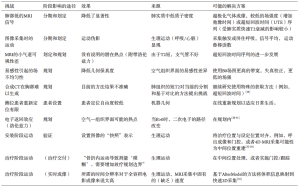
对于胸部放疗,要描绘的OAR包括肺、食道、心脏和脊髓,以及某些患者的臂丛、气管、主要支气管、主要血管和胸壁。OAR描绘的内部和内部观察者差异(表3)已有报道,虽然通过使用胸部CT,OAR图谱可以改善食管和心脏的描绘重现性[38],但仍待改进。关于臂丛神经轮廓,Kong等指出“在CT扫描上勾画臂丛神经是具有挑战性的”[39],而在需要确定臂丛神经位置的情况下推荐进行CT-MRI融合。即使使用图谱的食管和心脏等OAR受CT扫描变异的影响(表3),也可以通过增加MRI来更加一致地描述(图3)。鉴于心脏剂量与肺癌根治性放疗后总生存率的相关性[41,43],人们对于量化心脏亚结构的辐射暴露越来越感兴趣[40-42]。
Full table
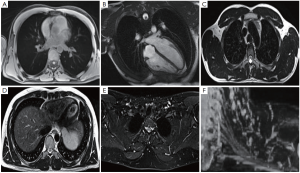
将F-18-FDG PET-CT成像纳入放疗中,可以改善肺靶标的再现性[8,10]。一项重要的研究比较了11位临床医生的描绘变异性,结果显示通过增加自由呼吸的F-18-FDG PET,目标描述的观察者间变化从单独CT的标准偏差1.0 cm降低到添加PET的0.4 cm[44]。为了描绘肿瘤靶点,MRI为PET成像提供了更好的空间分辨率[45]。已经建立了将MRI整合到放射治疗规划路径中以便描绘头颈部,中枢神经系统和骨盆靶点[46-50]。由于合适的胸部MRI序列的发展相当困难,来自肺癌患者的可比较研究的公开数据十分有限。尽管如此,临床仍存在需求,特别是对于侵入纵隔或邻近实质肺部改变(例如,远端崩溃/巩固)的肿瘤,其中准确的疾病程度评估仍然十分困难。在评估放疗过程中存在严重急性出血风险时,还需要改进评估大的纵隔血管侵袭风险(例如主动脉和肺动脉)。MR-Linac协会目前正在努力优化放疗计划的胸部图像(图3)。胸部OAR和靶标描绘的另一个考虑因素是呼吸运动。多年来,已经开发了各种技术来评估和解释靶标运动[51],其中应用最广泛的运动评估技术是呼吸相关的或4D CT扫描。关于目标运动的信息可以用于创建个性化的包含运动的目标体积[51]。近来有关呼吸相关18-FDG PET-CT扫描的研究取得了进展,正在研究关于4D 18-FDG PET-CT在放疗计划中的临床应用[8],然而这并不常用于临床实践。用于放疗的4D MRI图像的发展(表2)仍然具有挑战性[32,52-53]。4D MRI可提供高空间分辨率信息来创建运动管理治疗计划[53-54](例如,使用内部靶体积或中间位置方法)[55]。大西洋MR-Linac联盟的研究重点是开发几何精确的胸部MRI序列,以获得最佳的OAR和靶标可视化,以改善呼吸运动存在的描绘重复。
治疗计划
治疗计划的目标是达到计划剂量与靶体积的一致性,同时最小化周围正常组织的剂量。在对靶标进行识别和勾画之后,为了解决微观疾病的扩展问题(临床靶体积(clinical target volume,CTV)),建立和放射不确定性(计划靶体积(planning target volume,PTV)),在此过程中存在固有的不确定性和不准确性,需要增加边界的设置分量。历来,肿瘤总体积(gross tumour volume,GTV)与CTV边界是根据病理标本分析的人口数据生成的[56]。基于标准人群的CTV-PTV利润率因机构不同而不同,这反映了设置技术,成像频率和验证策略的差异[57-58]。
首先,MRI有可能改变放疗计划的方法。需要研究相关的MRI发现与病理标本的相关性,以研究是否可以调整原发肿瘤和淋巴结的GTV-CTV边缘。与此同时,胸椎MRI具有降低CTV-PTV边缘的潜力,因为改进的目标轮廓再现性可以减少对CTV-PTV边缘的系统性错误判断[59]。最近的一项研究表明,将MR序列添加到CT和PET并不会导致观察者变异性减少,但是评论说这可能是由于MR序列轮廓的观察者经验有限所致[60]。尽管其他放射治疗平台可能允许通过治疗适应的方法来减少间隙内运动,MR引导的治疗单位为基于目标和OAR的分数内变化的额外适应提供可能性[61-63]。根据预先设定的OAR限制(同种毒性方法),治疗边缘的减少将保留更大的正常组织或提供个体化剂量递增的范围[64]。
其次,功能性MRI序列可能被用来提供临床相关癌症标志和正常组织生理学的空间图谱(图4)。有关肿瘤异质性的信息可以整合到放射治疗计划中,以促进非均质剂量的绘制,使用类似的方法进行基于FDG-PET成像的研究(NCT01507428和NCT01024829)[65-66]。在成像上鉴定的肿瘤异质性可以用作预测生物标志物来选择纳入治疗强化试验的患者。最近的发展是利用MRI对肿瘤(缺氧)内的氧剥夺进行成像的能力。缺氧是耐受放疗的重要因素,与肺癌患者生存率低有关[67-69]。人们据此研究了血氧水平依赖性(BOLD)-MRI,并取得了一定的成功,这是由于灌注与缺氧的不完美联系以及对神经的显著敏感性[70-72]。氧增强(OE)-MRI是一种有前景的技术,它依赖于量化血浆和组织间液中的氧[73]。根据肿瘤氧合程度,呼吸道激发诱发R1(参见R2*粗体)(R1的弛豫速率为1/T1,R2为1/T2)即刻和可测量的变化[74-75]。最近研究证实肿瘤难治性部分是临床前模型中缺氧的重要生物标志物[76],而这项技术目前正在肺癌患者中进行早期临床验证(图4)。这种方法具有临床可转化性,并避免了与低氧特异性PET成像相关的几个缺点(如放射性示踪剂的制造和质量保证的复杂性,图像对比度差,以及在成像[77]之前,患者需要等待更长时间的放射性示踪剂注射)。迄今为止,这些因素已经阻碍了低氧成像在放射剂量照射的临床试验或低氧靶向治疗中的整合。
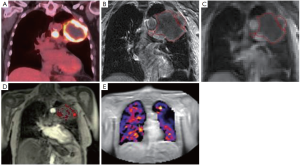
再者,实施这些MR引导的治疗单元可影响治疗计划,因为MR引导的治疗单元的辐射几何结构偏离了常规直线加速器的辐射几何结构。治疗是在一个静态磁场内传递的,由于洛仑兹力[6,49]可以改变二次电子的路径,每个光束的来源,方向和路径以及磁体的强度都会导致成像能力和剂量的变化(表4)。ViewRay MRIdian系统(美国俄亥俄州奥克伍德村的ViewRay公司)将1个0.35特斯拉(T)磁铁与3个钴-60(60Co)磁源相结合,在一个旋转台架上以120°间隔排列,目的是提高处理效率,通过以不同的光束角度同时发射辐射,以最小化光束干涉。使用ViewRay对肺癌患者进行规划研究表明,可以规划临床可行性治疗[85-87]。与传统直线加速器相比,当考虑立体定向放射治疗(stereotactic body radiotherapy,SBRT)治疗中央位置早期疾病时,临床医生认为与100%的直线加速器计划相比,90%的60Co计划具有临床可行性。此外,60Co计划中的所有计划均导致OAR剂量高于直线加速器计划,但是这只是对正常肺低剂量有统计学意义[87]。对于局部晚期疾病患者,只有有限的数据是可用的,但在60Co方案中报道的平均肺部剂量较高[85]。
其他MR引导的治疗单元已经设计成将直线加速器与MRI扫描仪相结合,再次具有磁体定位,强度和定向的变化(表4)。MR-Linac联盟的七名成员购买了由Elekta和Philips开发的临床原型,其结合了1.5 T宽孔径MRI扫描仪和7 MV直线加速器。这种混合机器有目的地被设计成具有更高的磁性强度,以优化信噪比,从而提供诊断质量的图像[88]。许多研究调查了肺癌患者在不同强度磁场下治疗的剂量学结果[30-31,89]。对于早期的小肿瘤,计划采用内嵌式磁体定位,磁场强度的增加与GTV的平均剂量增加有关[89]。在局部先进的疾病计划中,采用垂直磁体方向,当比较1.5 T磁场与零磁场(未发表)的计划时,可以看到增加的一致性。关于OAR剂量,与零磁场和1.5 T磁场相比,计划研究表明,在早期和局部晚期疾病,皮肤剂量有一个少量但具备统计学意义的上升趋势[30],同时,在未发表的1.5 T MR-Linac计划中,远端肺部组织(定义为离ITV超过5 cm的任何健康肺组织)的剂量也具有少量(+0.3 Gy)但具备统计学意义(P<0.01)的增加。然而,所有的研究都表明,在1.5 T的MR-Linac可以为早期和局部晚期肺癌患者生成临床可接受的计划。人们预期,一旦MRI引导的肺癌治疗的自适应元素纳入患者的工作流程中,这将超过先前所观察到的在磁场中计划的剂量效应。在临床前和临床研究中,我们需要研究适应性工作流程对MR导向治疗单元的全部潜在益处。
治疗验证
治疗中的目标是计划的和放疗的剂量分布与目标和周围正常组织之间的一致性。在过去的10年中,锥束CT(cone-beam computed tomography,CBCT)的广泛应用为3D和4D图像提供了靶体积的软组织定义,可以与之前或甚至在每日治疗期间的计划扫描进行比较,由于CBCT采集的体积特性,与诊断性CT相比,图像受到更高程度的散射,因此图像质量更差,但是与旧的二维(2D)兆伏电压相比,仍然提供了用于验证的优良软组织信息(MV)电子门户图像(EPI)[90]。此外,成像软件的进步已经允许通过计划CT扫描快速采集、重建和记录CBCT图像,从而可以评估验证和参考计划图像之间的差异,并通过纠正床面的方式对计划等角点进行每日在线修正。在线肿瘤匹配的每日CBCT成像目前被认为是用于立体定向放射治疗的早期肿瘤[91-94]和用于常规分割治疗的局部晚期肿瘤的肺癌匹配的最佳成像[58]。
目前的CBCT工作流程有局限性,主要的肿瘤和纵隔淋巴结在CBCT上很难识别,而CT扫描(图5)相比通常可复制性较差[57]。在软组织成像得到改善之前,心脏或脊柱匹配认为是重复性最好的[57]。因此,如果患者在CBCT采集和束流治疗交付之间的位置发生变化将不被接受。

相比之下,具有优越的软组织可视化,MR引导的治疗单元将有可能促进直接原发性肿瘤和纵隔淋巴结匹配预处理(图5)[95],从而可能允许减小CTV-PTV边界的设置分量。此外,随着4D MR技术的迅速发展,能够在5 min[96]内获得和重建4D图像时,在治疗前和治疗期间,每天呼吸模式的验证可助于进一步个性化放射治疗。
每日治疗计划的调整
在预处理CBCT的基础上,每日标准工作流程依赖于3个平移平面的转换来最优地调整计划。这种方法只能纠正由于一致的目标形状和体积的位移造成的误差,并且不能解释分数之间目标的形状和体积的变化。另一个问题是原发肿瘤和淋巴结靶点相对于彼此和OAR的独立置换[97-98]。对于原发肿瘤和淋巴结目标的差异性边界[98]或单独计划和等中心可能有帮助,然而在与中央部疾病和纵隔淋巴结匹配具有挑战性且有可能计划重叠的情况下,这些策略并非没有问题。一项主要是局部晚期肺癌患者的研究评估了1 793例CBCT扫描,显示72%患者胸内解剖发生变化,最常见的变化是35%的肿瘤衰退[61]。观察到的正常解剖变化包括19%的病例表现出肺不张,6%的病例表现出胸腔积液的波动[61]。其他研究报道肿瘤大小的可减小15%~71%[62,99]。治疗期间肿瘤和正常组织解剖结构的变化对靶标和周围的OAR具有剂量测定的重要性,当靶标邻接剂量限制性OAR时,这是特别重要的。在目前的CBCT成像中,对观察到的胸腔内解剖变化的一种方法是使用一种“交通灯协议”,它用于放射医生在治疗时根据匹配触发临床或物理评论[61]。这种方法可能有助于突出考虑重新计划的变化,但不提供每日重新计划解决方案。
为了说明目标和周围正常组织的形状、体积和位置的变化,在传递前立即调整治疗计划的能力是很有吸引力的。当考虑适应性治疗时,需要注意的是,虽然在局部晚期肺癌的根治性治疗过程中可以观察到显著的解剖变化,但是如何将这些变化纳入适应性放射治疗计划的全面理解尚不清楚。最近已经发表的一项Ⅱ期临床试验,研究了在局部晚期NSCLC治疗期间基于CT减少靶体积的概念[100]。在这项研究中,每周对治疗过程进行CT计划扫描,在肿瘤缩小的情况下,划定新的肿瘤体积并制订新的治疗计划。结果表明这种适应性方法降低了毒性和边缘失败率,但是这项工作尚待大规模随机试验的验证。MR-Linac的设想适应性工作流程提供了进一步的研究,可以每天进行重复成像,而不需要额外的CT扫描和相关的伴随辐射照射。
另外,该工作流程具有在治疗之前进行快速在线计划调整的能力[101]。自动在线计划调整的发展和采用是为了大幅简化每日重新规划的过程。在大部分治疗过程中的迭代测序将确保达到OAR最小剂量目标的最佳剂量覆盖率[102]。MR-Linac日常的适应能力可能因此延长治疗的时间窗,使进一步的安全同种毒素的强化治疗成为可能。
实时目标跟踪
如果没有在标准直线加速器上进行“束流”成像,则不能实时考虑实际的分数内部运动。例如,在患者呼吸不规律的情况下,或者如果基线呼吸移位或漂移的形式存在变化,则“束流”成像可能是有意义的。在外周早期肿瘤患者中,有72%的治疗组患者观察到至少3 mm的基线漂移[103]。为了适应这些变化,需要有足够的安全边界,这是在CTV-PTV边缘的产生中考虑的。对内部比例运动适应的选择仍然有限。用于射波刀治疗(机器人放射外科系统)[104-105]的内部基准标记,现在也被应用于Vero gimblinac系统[106]。利用这两个系统,对基准点的实时跟踪允许对目标进行实时跟踪,以提供被充分定位在目标周围或目标中的基准点。在整个治疗过程中跟踪基准点所需的正交kV成像与患者额外的辐射照射有关。虽然短程低分割立体定向放射治疗在临床上可以被接受,但对于局部晚期疾病的常规分割放疗延长疗程的患者,额外的放射线照射会更大。鉴于原发性肿瘤靶区和纵隔淋巴结靶区之间差异运动的可能性[51],局部晚期病变的患者可能需要纵隔和周围肺组织中的多个基准标记,这两者都不切实际,代价昂贵并且会使患者暴露于额外的与插入相关的风险[107]。
“实时成像”有两个重要的先决条件:首先成像必须具有高质量和时间分辨率才能准确反映底层的解剖结构;其次必须以足够的速度获得成像,以便真实反映潜在的肿瘤位置[108]。在“射线开始照射”期间通过肿瘤和OAR可视化,MR-Linac将实现实时的分数阶内MRI引导放射治疗基于动态多叶准直器(MLC)的呼吸运动跟踪已经在计划研究中显示出对肺癌治疗具有剂量学上的有益效果[109],其有利于减少治疗边界,并且使得治疗光束雕刻可以适应内部分数目标改变。基于MRI的实时跟踪已经在许多不同的情况下被模拟[30,110-113],临床研究也正在开发之中。在新型放射剂量增强试验的背景下,MR-Linac的追踪潜力呈现了这种混合型机器的另一个应用。在当前基于最初计划扫描的毒性剂量递增策略[114]的情况下,所设想的MR-Linac工作流程(具有实时的计划内与实际剂量的分数间监测以及所观察到的剂量测定差异的补偿)可以促进从“图像引导”到“剂量引导”治疗,进一步细化个体化毒性剂量方案的优化。
早期评估治疗反应
动态对比增强(DCE)-MRI是一种有前景的功能性MRI技术,其具有成为肿瘤应答和早期正常组织毒性的非侵入性成像生物标志物的潜力。毛细血管通透性的动力学参数(例如Ktrans)一直与直肠癌[115]、头颈癌[116]和宫颈癌[117]对放疗的反应相关,但是肺癌的数据是混合的[118-121],正在进行的研究继续调查这种技术的潜力[122]。为了评估潜在的OAR功能,术后1秒内用力呼气容积可以通过使用DCE-MRI对肺癌患者进行术前肺灌注成像[123]。此外DCE-MRI的动力学变化已被证实可用于区分早期放射性肺炎和晚期放射性纤维化[124]。
这些应用可以提高诊断鉴别能力,并因此改善管理,不仅在完成放疗过程之后,而且贯穿于每天利用MR-Linac获取图像的整个治疗过程中。在肺癌根治性放射治疗中对PET-CT的功能肿瘤变化的研究表明,在治疗过程中,PET的代谢肿瘤体积比CT上可见的肿瘤体积减小了更多[125]。最近发表的一项单臂试验结果证实,在经过大约三分之二的总剂量治疗后,一旦发生代谢变化,就可以对靶点进行剂量调整,从而提供有利的局部疾病控制[126]。目前正在进行基于PET的RTOG 1106随机试验(NCT01507428)。在患者治疗之后离开床前(治疗后的图像)在治疗位置拍摄的规律性(每日)功能性成像序列可以促进基于治疗过程期间的功能改变的治疗适应,类似于RTOG 1106试验的方式。此外,后束功能成像也可以根据正常组织毒性的概率进行适应,例如早期的毒性标记可以作为治疗强化潜在耐受性的选择标准。新的功能成像(如F-18-FDG PET)的新型功能性MRI序列的最优整合仍有待确定,并需要进一步研究包括与病理结果的相关性[45]。
结论
尽管还处于起步阶段,但将MRI纳入放疗治疗中对肺癌患者来说具有不可否认的前景,它可提供个性化增量益处,加强治疗工作流程中的各个环节。更准确的疾病分期和患者选择用于根治性治疗之后将会有更加可重复的肿瘤靶标和OAR描绘。这允许在较小的治疗界线范围下制订治疗计划。在治疗前,根据每日计划适应的可能性,考虑到分数变化和实时图像引导,甚至是剂量指导的治疗,以考虑到分数的变化可以在治疗指标上取得进一步的进展。在治疗过程中定期获得的额外功能成像可以提供关于生物肿瘤特征和正常组织毒性的关键信息,从而有可能指导适应性放疗的进一步临床应用。
在每个步骤的临床意义之前需要克服的技术复杂性(表2),这仍然是大西洋MR-Linac联盟和其他研究组成员积极研究的领域。精心设计的多中心前临床试验与临床研究证明,这些患者在局部疾病和总生存方面获益。
Acknowledgements
Prof. Faivre-Finn and Dr. McDonald gratefully acknowledge the support of the NIHR Biomedical Research Centre and the CRUK ARTNET Network. Prof. Faivre-Finn gratefully acknowledges the CRUK Major Centre. All authors gratefully acknowledge the support of Elekta and Philips to the Altantic MR-Linac consortium.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Website Cancer Research UK. Lung Cancer Incidence Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#heading-Ten
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Christodoulou M, Bayman N, McCloskey P, et al. New radiotherapy approaches in locally advanced non-small cell lung cancer. Eur J Cancer 2014;50:525-34. [Crossref] [PubMed]
- Senan S, Brade A, Wang LH, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:953-62. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial p. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Raaymakers BW, Raaijmakers AJ, Kotte AN, et al. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose deposition in a transverse magnetic field. Phys Med Biol 2004;49:4109-18. [Crossref] [PubMed]
- Wild JM, Marshall H, Bock M, et al. MRI of the lung (1/3): methods. Insights Imaging 2012;3:345-53. [Crossref] [PubMed]
- Konert T, Vogel W, MacManus MP, et al. PET/CT imaging for target volume delineation in curative intent radiotherapy of non-small cell lung cancer: IAEA consensus report 2014. Radiother Oncol 2015;116:27-34. [Crossref] [PubMed]
- Kim HS, Lee KS, Ohno Y, et al. PET/CT versus MRI for diagnosis, staging, and follow-up of lung cancer. J Magn Reson Imaging 2015;42:247-60. [Crossref] [PubMed]
- Grootjans W, de Geus-Oei LF, Troost EG, et al. PET in the management of locally advanced and metastatic NSCLC. Nat Rev Clin Oncol 2015;12:395-407. [Crossref] [PubMed]
- Heelan RT, Demas BE, Caravelli JF, et al. Superior sulcus tumours: CT and MR imaging. Radiology 1989;170:637-41. [Crossref] [PubMed]
- Foroulis CN, Zarogoulidis P, Darwiche K, et al. Superior sulcus (Pancoast) tumors: current evidence on diagnosis and radical treatment. J Thorac Dis 2013;5 Suppl 4:S342-58. [PubMed]
- National Institute for Health and Clinical Excellence. Lung Cancer: the Diagnosis and Treatment of Lung Cancer (CG121). London: NICE; April 2011.
- NCCN clinical practice guidelines in oncology. Non-Small Cell Lung Cancer 2016, Version 3. 2016.
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:369S-99S.
- Kajiwara N, Akata S, Uchida O, et al. Cine MRI enables better therapeutic planning than CT in cases of possible lung cancer chest wall invasion. Lung Cancer 2010;69:203-8. [Crossref] [PubMed]
- Padovani B, Mouroux J, Seksik L, et al. Chest wall invasion by bronchogenic carcinoma: evaluation with MR imaging. Radiology 1993;187:33-8. [Crossref] [PubMed]
- Musset D, Grenier P, Carette MF, et al. Primary lung cancer staging: prospective comparative study of MR imaging with CT. Radiology 1986;160:607-11. [Crossref] [PubMed]
- Wu LM, Xu JR, Gu HY, et al. Preoperative mediastinal and hilar nodal staging with diffusion-weighted magnetic resonance imaging and fluorodeoxyglucose positron emission tomography/computed tomography in patients with non-small-cell lung cancer: which is better? J Surg Res 2012;178:304-14. [Crossref] [PubMed]
- Shen G, Lan Y, Zhang K, et al. Comparison of 18F-FDG PET/CT and DWI for detection of mediastinal nodal metastasis in non-small cell lung cancer: A meta-analysis. PLoS One 2017;12:e0173104. [Crossref] [PubMed]
- Zhang Y, Qin Q, Li B, et al. Magnetic resonance imaging for N staging in non-small cell lung cancer: A systematic review and meta-analysis. Thorac Cancer 2015;6:123-32. [Crossref] [PubMed]
- Yi CA, Shin KM, Lee KS, et al. Non-small cell lung cancer staging: efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imaging. Radiology 2008;248:632-42. [Crossref] [PubMed]
- Yi CA, Lee KS, Lee HY, et al. Coregistered whole body magnetic resonance imaging-positron emission tomography (MRI-PET) versus PET-computed tomography plus brain MRI in staging resectable lung cancer: Comparisons of clinical effectiveness in a randomized trial. Cancer 2013;119:1784-91. [Crossref] [PubMed]
- Sommer G, Stieltjes B. Magnetic resonance imaging for staging of non-small-cell lung cancer-technical advances and unmet needs. J Thorac Dis 2015;7:1098-102. [PubMed]
- Lauenstein TC, Goehde SC, Herborn CU, et al. Whole-body MR imaging: evaluation of patients for metastases. Radiology 2004;233:139-48. [Crossref] [PubMed]
- Schlemmer HP, Schäfer J, Pfannenberg C, et al. Fast whole-body assessment of metastatic disease using a novel magnetic resonance imaging system: initial experiences. Invest Radiol 2005;40:64-71. [Crossref] [PubMed]
- Pichler BJ, Kolb A, Nägele T, et al. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med 2010;51:333-6. [Crossref] [PubMed]
- Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology 2005;236:1011-9. [Crossref] [PubMed]
- Edmund JM, Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol 2017;12:28. [Crossref] [PubMed]
- Menten MJ, Fast MF, Nill S, et al. Lung stereotactic body radiotherapy with an MR-linac - Quantifying the impact of the magnetic field and real-time tumor tracking. Radiother Oncol 2016;119:461-6. [Crossref] [PubMed]
- Bainbridge H, Menten MJ, Fast MF, et al. Dosimetric Implications for Radical Radiation Therapy on the 1.5 Tesla Magnetic Resonance Linear Accelerator (MR-Linac) in Locally-Advanced Non-Small Cell Lung Cancer. Lung Cancer 2017;103:S55. [Crossref]
- Rank CM, Heußer T, Buzan MTA, et al. 4D Respiratory Motion-Compen sated ImageReconstruction of Free-Breathing RadialMR Data With Very High Undersampling. Magn Reson Med 2017;77:1170-83. [Crossref] [PubMed]
- Stemkens B, Tijssen RH, de Senneville BD, et al. Optimizing 4-Dimensional Magnetic Resonance Imaging Data Sampling for Respiratory Motion Analysis of Pancreatic Tumors. Int J Radiat Oncol Biol Phys 2015;91:571-8. [Crossref] [PubMed]
- Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014;72:707-17. [Crossref] [PubMed]
- Stemkens B, Tijssen RH, de Senneville BD, et al. Image-driven, model-based 3D abdominal motion estimation for MR-guided radiotherapy. Phys Med Biol 2016;61:5335-55. [Crossref] [PubMed]
- Collier DC, Burnett SS, Amin M, et al. SG. Assessment of consistency in contouring of normal-tissue anatomic structures. J Appl Clin Med Phys 2003;4:17-24. [Crossref] [PubMed]
- McCall R, MacLennan G, Taylor M, et al. Medical Dosimetry Anatomical contouring variability in thoracic organs at risk. Med Dosim 2016;41:344-50. [Crossref] [PubMed]
- Cui Y, Chen W, Kong FM, et al. Contouring variations and the role of atlas in non-small cell lung cancer radiation therapy: Analysis of a multi-institutional preclinical trial planning study. Pract Radiat Oncol 2015;5:e67-75. [Crossref] [PubMed]
- Kong FM, Ritter T, Quint DJ DJ, et al. Consideration of Dose Limits for Organs At Risk of. Int J Radiat Oncol Biol Phys 2011;81:1442-57. [Crossref] [PubMed]
- Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:1395-402. [Crossref] [PubMed]
- Wollschläger D, Karle H, Stockinger M, et al. Radiation dose distribution in functional heart regions from tangential breast cancer radiotherapy. Radiother Oncol 2016;119:65-70. [Crossref] [PubMed]
- Duane F, Aznar MC, Bartlett F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol 2017;122:416-22. [Crossref] [PubMed]
- McWilliam A, Faivre-Finn C, Kennedy J, et al. Data mining identifies the base of the heart as a dose-sensitive region affecting survival in lung cancer patients. Int J Radiat Oncol Biol Phys 2016;96:S48. [Crossref]
- Steenbakkers RJ, Duppen JC, Fitton I, et al. Reduction of observer variation using matched CT-PET for lung cancer delineation: A three-dimensional analysis. Int J Radiat Oncol Biol Phys 2006;64:435-48. [Crossref] [PubMed]
- Kumar S, Liney G, Rai R, et al. Magnetic resonance imaging in lung: a review of its potential for radiotherapy. Br J Radiol 2016;89:20150431. [Crossref] [PubMed]
- Chuter R, Prestwich R, Bird D, et al. The use of deformable image registration to integrate diagnostic MRI into the radiotherapy planning pathway for head and neck cancer. Radiother Oncol 2017;122:229-35. [Crossref] [PubMed]
- Rasch C, Barillot I, Remeijer P, et al. Definition of the prostate in CT and MRI: A multi-observer study. Int J Radiat Oncol Biol Phys 1999;43:57-66. [Crossref] [PubMed]
- Aoyama H, Shirato H, Nishioka T, et al. Magnetic resonance imaging system for three-dimensional conformal radiotherapy and its impact on gross tumor volume delineation of central nervous system tumors. Int J Radiat Oncol Biol Phys 2001;50:821-7. [Crossref] [PubMed]
- Yeung AR, Vargas CE, Falchook A, et al. Dose-Volume Differences for Computed Tomography and Magnetic Resonance Imaging Segmentation and Planning for Proton Prostate Cancer Therapy. Int J Radiat Oncol Biol Phys 2008;72:1426-33. [Crossref] [PubMed]
- O’Neill BD, Salerno G, Thomas K, et al. MR vs CT imaging: low rectal cancer tumour delineation for three-dimensional conformal radiotherapy. Br J Radiol 2009;82:509-13. [Crossref] [PubMed]
- Cole AJ, Hanna GG, Jain S, et al. Motion Management for Radical Radiotherapy in Non-small Cell Lung Cancer. Clin Oncol (R Coll Radiol) 2014;26:67-80. [Crossref] [PubMed]
- Du D, Caruthers SD, Glide-hurst C, et al. High-Quality T2-Weighted 4-Dimensional Magnetic Resonance Imaging for Radiation Therapy Applications. Int J Radiat Oncol Biol Phys 2015;92:430-7. [Crossref] [PubMed]
- Freedman JN, Collins DJ, Bainbridge H, et al. T2-Weighted 4D Magnetic Resonance Imaging for Application in Magnetic Resonance-Guided Radiotherapy Treatment Planning. Invest Radiol 2017;52:563-73. [Crossref] [PubMed]
- Freedman JN, Collins DJ, Rank CM, et al. Evaluation of 4D-T2w MRI methods for lung radiotherapy treatment planning with application to an MR-linac. ISMRM 25th Annu Meet Exhib 2017:Abstract #2906.
- Wolthaus JW, Sonke JJ, van Herk M, et al. Comparison of Different Strategies to Use Four-Dimensional Computed Tomography in Treatment Planning for Lung Cancer Patients. Int J Radiat Oncol Biol Phys 2008;70:1229-38. [Crossref] [PubMed]
- Grills IS, Fitch DL, Goldstein NS, et al. Clinicopathologic Analysis of Microscopic Extension in Lung Adenocarcinoma: Defining Clinical Target Volume for Radiotherapy. Int J Radiat Oncol Biol Phys 2007;69:334-41. [Crossref] [PubMed]
- Higgins J, Bezjak A, Franks K, et al. Comparison of Spine, Carina, and Tumor as Registration Landmarks for Volumetric Image-Guided Lung Radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1404-13. [Crossref] [PubMed]
- Higgins J, Bezjak A, Hope A, et al. Effect of image-guidance frequency on geometric accuracy and setup margins in radiotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1330-7. [Crossref] [PubMed]
- van Herk M. Errors and Margins in Radiotherapy. Semin Radiat Oncol 2004;14:52-64. [Crossref] [PubMed]
- Karki K, Saraiya S, Hugo GD, et al. Variabilities of Magnetic Resonance Imaging-, Computed Tomography-, and Positron Emission Tomography-Computed Tomography-Based Tumor and Lymph Node Delineations for Lung Cancer Radiation Therapy Planning. Int J Radiat Oncol Biol Phys 2017;99:80-9. [Crossref] [PubMed]
- Kwint M, Conijn S, Schaake E, et al. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol 2014;113:392-7. [Crossref] [PubMed]
- Kataria T, Gupta D, Bisht SS, et al. Adaptive radiotherapy in lung cancer: dosimetric benefits and clinical outcome. Br J Radiol 2014;87:20130643. [Crossref] [PubMed]
- McPartlin AJ, Li XA, Kershaw LE, et al. MRI-guided prostate adaptive radiotherapy - A systematic review. Radiother Oncol 2016;119:371-80. [Crossref] [PubMed]
- Warren S, Panettieri V, Panakis N, et al. Optimizing collimator margins for isotoxically dose-escalated conformal radiation therapy of non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;88:1148-53. [Crossref] [PubMed]
- van Elmpt W, De Ruysscher D, van der Salm A, et al. The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol 2012;104:67-71. [Crossref] [PubMed]
- Matuszak MM, Xiao Y, Presley J, et al. The Importance of Dry Run Credentialing for RTOG 1106/ACRIN 6697: A Trial of Individualized Adaptive Radiation Therapy for Patients with Locally Advanced Non-small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2012;84:S26-7. [Crossref]
- Li C, Lu HJ, Na FF, et al. Prognostic role of hypoxic inducible factor expression in non-small cell lung cancer: a meta-analysis. Asian Pac J Cancer Prev 2013;14:3607-12. [Crossref] [PubMed]
- Ren W, Mi D, Yang K, et al. The expression of hypoxia-inducible factor-1α and its clinical significance in lung cancer: A systematic review and meta-analysis. Swiss Med Wkly 2013;143:w13855. [PubMed]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393-410. [Crossref] [PubMed]
- Egeland TAM, Gulliksrud K, Gaustad JV, et al. Dynamic contrast-enhanced-MRI of tumor hypoxia. Magn Reson Med 2012;67:519-30. [Crossref] [PubMed]
- Øvrebø KM, Hompland T, Mathiesen B, et al. Assessment of hypoxia and radiation response in intramuscular experimental tumors by dynamic contrast-enhanced magnetic resonance imaging. Radiother Oncol 2012;102:429-35. [Crossref] [PubMed]
- Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 2006;82:699-757. [Crossref] [PubMed]
- Young IR, Clarke GJ, Bailes DR, et al. Enhancement of relaxation rate with paramagnetic contrast agents in NMR imaging. J Comput Tomogr 1981;5:543-7. [Crossref] [PubMed]
- Linnik IV, Scott ML, Holliday KF, et al. Noninvasive tumor hypoxia measurement using magnetic resonance imaging in murine U87 glioma xenografts and in patients with glioblastoma. Magn Reson Med 2014;71:1854-62. [Crossref] [PubMed]
- O’Connor JP, Naish JH, Parker GJ, et al. Preliminary Study of Oxygen-Enhanced Longitudinal Relaxation in MRI: A Potential Novel Biomarker of Oxygenation Changes in Solid Tumors. Int J Radiat Oncol Biol Phys 2009;75:1209-15. [Crossref] [PubMed]
- O’Connor JP, Boult JK, Jamin Y, et al. Oxygen-enhanced MRI accurately identifies, quantifies, and maps tumor hypoxia in preclinical cancer models. Cancer Res 2016;76:787-95. [Crossref] [PubMed]
- Peeters SG, Zegers CM, Lieuwes N, et al. A comparative study of the hypoxia PET tracers [18F]HX4, [18F]FAZA, and [18F]FMISO in a preclinical tumor model. Int J Radiat Oncol Biol Phys 2015;91:351-9. [Crossref] [PubMed]
- Menten MJ, Wetscherek A, Fast MF. MRI-guided lung SBRT: Present and future developments. Phys Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ménard C, van der Heide U. Introduction: Systems for Magnetic Resonance Image Guided Radiation Therapy. Semin Radiat Oncol 2014;24:192. [Crossref] [PubMed]
- Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, et al. MRI/linac integration. Radiother Oncol 2008;86:25-9. [Crossref] [PubMed]
- Tadic T, Fallone BG. Design and optimization of superconducting MRI magnet systems with magnetic materials. IEEE Transactions on Applied Superconductivity 2012;22. [Crossref]
- Keall PJ, Barton M, Crozier S. The Australian Magnetic Resonance Imaging-Linac Program. Semin Radiat Oncol 2014;24:203-6. [Crossref] [PubMed]
- Mutic S, Dempsey JF. The ViewRay System: Magnetic Resonance-Guided and Controlled Radiotherapy. Semin Radiat Oncol 2014;24:196-9. [Crossref] [PubMed]
- Fallone BG, Murray B, Rathee S, et al. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system. Med Phys 2009;36:2084-8. [Crossref] [PubMed]
- Wooten HO, Green O, Yang M, et al. Quality of Intensity Modulated Radiation Therapy Treatment Plans Using a (60)Co Magnetic Resonance Image Guidance Radiation Therapy System. Int J Radiat Oncol Biol Phys 2015;92:771-8. [Crossref] [PubMed]
- Saenz DL, Paliwal BR, Bayouth JE. A dose homogeneity and conformity evaluation between ViewRay and pinnacle-based linear accelerator IMRT treatment plans. J Med Phys 2014;39:64-70. [Crossref] [PubMed]
- Merna C, Rwigema JC, Cao M, et al. A treatment planning comparison between modulated tri-cobalt-60 teletherapy and linear accelerator-based stereotactic body radiotherapy for central early-stage non−small cell lung cancer. Med Dosim. 2016;41:87-91. [Crossref] [PubMed]
- Raaijmakers AJ, Raaymakers BW, Lagendijk JJ. Magnetic-field-induced dose effects in MR-guided radiotherapy systems: dependence on the magnetic field strength. Phys Med Biol 2008;53:909-23. [Crossref] [PubMed]
- Oborn BM, Ge Y, Hardcastle N, et al. Dose enhancement in radiotherapy of small lung tumors using inline magnetic fields: A Monte Carlo based planning study. Med Phys 2016;43:368. [Crossref] [PubMed]
- Boda-Heggemann J, Lohr F, Wenz F, et al. kV cone-beam CT-based IGRT: a clinical review. Strahlenther Onkol 2011;187:284-91. [Crossref] [PubMed]
- Guckenberger M, Krieger T, Richter A, et al. Potential of image-guidance, gating and real-time tracking to improve accuracy in pulmonary stereotactic body radiotherapy. Radiother Oncol 2009;91:288-95. [Crossref] [PubMed]
- Sweeney RA, Seubert B, Stark S, et al. Accuracy and inter-observer variability of 3D versus 4D cone-beam CT based image-guidance in SBRT for lung tumors. Radiat Oncol 2012;7:81. [Crossref] [PubMed]
- Galerani AP, Grills I, Hugo G, et al. Dosimetric impact of online correction via cone-beam ct-based image guidance for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1571-8. [Crossref] [PubMed]
- Liang J, Li M, Zhang T, et al. The effect of image-guided radiation therapy on the margin between the clinical target volume and planning target volume in lung cancer. J Med Radiat Sci 2014;61:30-7. [Crossref] [PubMed]
- Oelfke U. Magnetic Resonance Imaging-guided Radiation Therapy: Technological Innovation Provides a New Vision of Radiation Oncology Practice. Clin Oncol (R Coll Radiol) 2015;27:495-7. [Crossref] [PubMed]
- Mickevicius NJ, Paulson E. Investigation of undersampling and reconstruction algorithm dependence on respiratory correlated 4D-MRI for online MR-guided radiation therapy. Phys Med Biol 2017;62:2910-21. [Crossref] [PubMed]
- Jan N, Balik S, Hugo GD, et al. Interfraction displacement of primary tumor and involved lymph nodes relative to anatomic landmarks in image guided radiation therapy of locally advanced lung cancer. Int J Radiat Oncol Biol Phys 2014;88:210-5. [Crossref] [PubMed]
- Schaake EE, Rossi MM, Buikhuisen WA, et al. Differential motion between mediastinal lymph nodes and primary tumor in radically irradiated lung cancer patients. Int J Radiat Oncol Biol Phys 2014;90:959-66. [Crossref] [PubMed]
- Britton KR, Starkschall G, Tucker SL, et al. Assessment of Gross Tumor Volume Regression and Motion Changes During Radiotherapy for Non-Small-Cell Lung Cancer as Measured by Four-Dimensional Computed Tomography. Int J Radiat Oncol Biol Phys 2007;68:1036-46. [Crossref] [PubMed]
- Ramella S, Fiore M, Silipigni S, et al. Local Control and Toxicity of Adaptive Radiotherapy Using Weekly CT Imaging: Results from the LARTIA Trial in Stage III NSCLC. J Thorac Oncol 2017;12:1122-30. [Crossref] [PubMed]
- Kontaxis C, Bol GH, Lagendijk JJ, et al. Towards adaptive IMRT sequencing for the MR-linac. Phys Med Biol 2015;60:2493-509. [Crossref] [PubMed]
- Kontaxis C, Bol GH, Lagendijk JJ, et al. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol 2015;60:7485-97. [Crossref] [PubMed]
- Takao S, Miyamoto N, Matsuura T, et al. Intrafractional Baseline Shift or Drift of Lung Tumor Motion During Gated Radiation Therapy With a Real-Time Tumor-Tracking System. Int J Radiat Oncol Biol Phys 2016;94:172-80. [Crossref] [PubMed]
- Nuyttens JJ, Prévost JB, Praag J, et al. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: Marker placement and early results. Acta Oncol 2006;45:961-5. [Crossref] [PubMed]
- van der Voort van Zyp NC, Prévost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol 2009;91:296-300. [Crossref] [PubMed]
- Depuydt T, Poels K, Verellen D, et al. Treating patients with real-time tumor tracking using the Vero gimbaled linac system: Implementation and first review. Radiother Oncol 2014;112:343-51. [Crossref] [PubMed]
- Patel A, Khalsa B, Lord B, et al. Planting the seeds of success: CT-guided gold seed fiducial marker placement to guide robotic radiosurgery. J Med Imaging Radiat Oncol 2013;57:207-11. [Crossref] [PubMed]
- Yan H, Tian Z, Shao Y, et al. A new scheme for real-time high-contrast imaging in lung cancer radiotherapy: a proof-of-concept study. Phys Med Biol 2016;61:2372-88. [Crossref] [PubMed]
- Keall PJ, Joshi S, Vedam SS, et al. Four-dimensional radiotherapy planning for DMLC-based respiratory motion tracking. Med Phys 2005;32:942-51. [Crossref] [PubMed]
- Yun J, Yip E, Wachowicz K, et al. Evaluation of a lung tumor autocontouring algorithm for intrafractional tumor tracking using low-field MRI: A phantom study. Med Phys 2012;39:1481. [Crossref] [PubMed]
- Cerviño LI, Du J, Jiang SB. MRI-guided tumor tracking in lung cancer radiotherapy. Phys Med Biol 2011;56:3773-85. [Crossref] [PubMed]
- Crijns SP, Raaymakers BW, Lagendijk JJ. Proof of concept of MRI-guided tracked radiation delivery: tracking one-dimensional motion. Phys Med Biol 2012;57:7863-72. [Crossref] [PubMed]
- Yip E, Yun J, Wachowicz K, et al. Prior data assisted compressed sensing: A novel MR imaging strategy for real time tracking of lung tumors. Med Phys 2014;41:082301. [Crossref] [PubMed]
- Haslett K, Franks K, Hanna GG, et al. Protocol for the isotoxic intensity modulated radiotherapy (IMRT) in stage III non-small cell lung cancer (NSCLC): a feasibility study. BMJ Open 2016;6:e010457. [Crossref] [PubMed]
- George ML, Dzik-Jurasz AS, Padhani AR, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg 2001;88:1628-36. [Crossref] [PubMed]
- Kim S, Loevner LA, Quon H, et al. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol 2010;31:262-8. [Crossref] [PubMed]
- Zahra MA, Tan LT, Priest AN, et al. Semiquantitative and Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging Measurements Predict Radiation Response in Cervix Cancer. Int J Radiat Oncol Biol Phys 2009;74:766-73. [Crossref] [PubMed]
- Weiss E, Ford JC, Olsen KM, et al. Apparent diffusion coefficient (ADC) change on repeated diffusion-weighted magnetic resonance imaging during radiochemotherapy for non-small cell lung cancer: A pilot study. Lung Cancer 2016;96:113-9. [Crossref] [PubMed]
- Chang Q, Wu N, Ouyang H, et al. Diffusion-weighted magnetic resonance imaging of lung cancer at 3.0 T: a preliminary study on monitoring diffusion changes during chemoradiation therapy. Clin Imaging 2012;36:98-103. [Crossref] [PubMed]
- Ohno Y, Koyama H, Yoshikawa T, et al. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol 2012;198:75-82. [Crossref] [PubMed]
- Yabuuchi H, Hatakenaka M, Takayama K, et al. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology 2011;261:598-604. [Crossref] [PubMed]
- Askoxylakis V, Dinkel J, Eichinger M, et al. Multimodal hypoxia imaging and intensity modulated radiation therapy for unresectable non-small-cell lung cancer: the HIL trial. Radiat Oncol 2012;7:157. [Crossref] [PubMed]
- Iwasawa T, Saito K, Ogawa N, et al. Prediction of postoperative pulmonary function using perfusion magnetic resonance imaging of the lung. J Magn Reson Imaging 2002;15:685-92. [Crossref] [PubMed]
- Ogasawara N, Suga K, Karino Y, et al. Perfusion characteristics of radiation-injured lung on Gd-DTPA-enhanced dynamic magnetic resonance imaging. Invest Radiol 2002;37:448-57. [Crossref] [PubMed]
- Mahasittiwat P, Yuan S, Xie C, et al. Metabolic Tumor Volume on PET Reduced More than Gross Tumor Volume on CT during Radiotherapy in Patients with Non-Small Cell Lung Cancer Treated with 3DCRT or SBRT. J Radiat Oncol 2013;2:191-202. [Crossref] [PubMed]
- Kong FM, Ten Haken RK, Schipper M, et al. Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non-Small-Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
祁峰
江苏省肿瘤医院(更新时间:2021.7)
李潇
江苏省肿瘤医院(更新时间:2021.7)
廖林虹
江西省赣州市妇幼保健院(更新时间:2021.7)
(本译文仅供学术交流,实际内容请以英文原文为准。)