Pembrolizumab in advanced pretreated small cell lung cancer patients with PD-L1 expression: data from the KEYNOTE-028 trial: a reason for hope?
Small cell lung cancer (SCLC) is an aggressive subtype of lung cancer, representing around 15% of all lung cancer cases. SCLC is characterized by neuroendocrine pathological features, strong association with tobacco exposure, rapid widespread, high mutational rates and no oncogenic drivers (1).
At diagnosis, around 70% of cases present with extensive disease (ED-SCLC). Platinum-etoposide doublet is the standard of care, offering response rates of 70–80%. However, despite this initial significant chemosensitivity, progression of the disease will occur after completion of chemotherapy, with median progression-free survival (PFS) of only 2–3 months. In the refractory setting, topotecan offers modest benefit, with response rates of 10% to 20%, and significant toxicity. Consequently, the overall prognosis for patients with ED-SCLC is poor, with median overall survival (OS) of 8–13 months and 5-year OS rate of 1–2% (2).
In contrast with non-small cell lung cancer (NSCLC), progress in SCLC has been minimal in the last decades, being topotecan, in 1996, the last approved agent worldwide for the treatment of SCLC (3). For these reasons, SCLC is now considered a recalcitrant cancer.
One of the hallmarks of cancer is immune evasion, in which the immune system is not capable of generating an effective antitumor response (4). Programmed cell death 1 (PD-1) is a negative costimulatory receptor expressed on the surface of activated T cells. The binding of PD-1 to one of its ligands, PD-L1 or PD-L2, can inhibit a cytotoxic T-cell response. Blockade of the interaction of PD-1 or PD-L1 with monoclonal antibodies has led to durable objective responses and survival benefit in several cancer types, such as melanoma, renal cancer, bladder cancer or NSCLC (5). Nivolumab and pembrolizumab, as PD-1 inhibitors, and atezolizumab, durvalumab and avelumab, as PD-L1 drugs, have received approval by FDA for different indications across a wide range of tumor types (6).
Recent evidence supports that SCLC tumors are associated with increased immunogenicity. Firstly, it is known that patients with SCLC achieving a long-term survival have a higher T-effector to T-regulator cells ratio. Secondly, SCLC patients with autoantibodies and neurologic paraneoplastic syndromes are more likely to obtain a prolonged survival (7). Finally, due to strong association with tobacco exposure, SCLC is one of the tumor types with the highest rate of mutational burden. Rizvi et al. reported a correlation between higher objective response and higher mutational burden, in patients with advanced NSCLC treated with pembrolizumab (8). For all these reasons, investigation of immunotherapy strategies is warranted in SCLC.
The first immune checkpoint inhibitor evaluated in SCLC was ipilimumab, a fully human IgG1 anti-CTLA-4 monoclonal antibody. Several clinical trials were conducted, evaluating different doses and different schedules in combination with chemotherapy as front-line therapy for untreated ED-SCLC patients. Unfortunately, despite some signals of efficacy were observed with the addition of ipilimumab, no improvement of survival was documented for the overall population. Furthermore, the combination of chemotherapy plus ipilimumab was associated with significant toxicity (9).
Preclinical data suggested that the combination of dual blockade against PD-1 and CTLA-4 could improve antitumor activity. The combination of nivolumab plus ipilimumab has demonstrated deep and durable responses in several tumour types, independently of PD-L1 expression, and has received approval for use in advanced melanoma.
The CheckMate 032 trial was a phase 1b/2 study that evaluated single-agent nivolumab (n=98) and nivolumab in combination with ipilimumab (n=118 in three different dose cohorts) in patients ED-SCLC after failure to at least one regimen of therapy. Patients were not selected by PD-L1 expression criteria. Both the combination and the monotherapy arms showed activity and durable responses in some patients, with tolerable toxicity. Notably, responses were also documented in PD-L1 negative patients. For the nivolumab arm, the overall response rate (ORR) was 10% (10 of 98 patients), being higher, 21% and 19%, for the two cohorts of nivolumab and ipilimumab combinations, respectively. Treatment-related adverse events (TRAEs) were higher in the combination arms than in the nivolumab monotherapy arm (10).
In a recent update, presented at the annual ASCO congress 2017, it was reported a median OS of 4.1 months for the nivolumab arm and 7.9 months for the ipilimumab 3 mg/kg plus nivolumab 1 mg/kg (11). Considering the efficacy data and the favorable and manageable safety profile, nivolumab alone or in combination with ipilimumab is recommended (category 2A) in the National Comprehensive Cancer Network guidelines as an option of treatment for ED-SCLC patients after failure to platinum-etoposide chemotherapy (12).
In the article accompanying this editorial, Ott et al. report the data from the ED-SCLC cohort, included in the multicohort KEYNOTE-028 trial (13). This is an open-label, phase Ib study of pembrolizumab in patients with advanced solid tumors, selected as PD-L1 positive by immunohistochemistry. Pembrolizumab is a humanized IgG4 PD-1 blocking antibody that binds to the PD-1 receptor on T cells, releasing inhibition of the antitumor immune response. In this trial, twenty-four ED-SCLC patients with PD-L1 positive expression finally received pembrolizumab at a dose of 10 mg/kg every 2 weeks for up to 2 years. The confirmed ORR was 33.3%, and responses were rapid and durable as demonstrates the median duration of response of 19.4 months. The median OS was 9.7 months and the OS rate at 12 months was 37.7%, higher than the historical cohorts. Last, pembrolizumab was well tolerated and safety was consistent with the reported toxicity profile in other tumor types.
Some important questions arise after the review of this article and must be analyzed:
Could PD-L1 expression be considered as a predictive biomarker in SCLC?
One of the major controversies at present time in the field of immunotherapy is the lack of standardized predictive biomarkers.
PD-L1 expression by immunohistochemistry and mutational tumor burden appear to be significant determinants of higher benefit to anti-PD(L)1 inhibitors in some tumor types. However, important limitations do exist regarding cut-off thresholds, technical concerns and tumor heterogeneity.
The role of selection of SCLC patients for anti-PD1 therapies according to PD-L1 is currently unknown and under study. In contrast to CheckMate 032, the present study only included patients with expression of PD-L1.
Tumor PD-L1 expression was evaluated at a central laboratory by using a prototype assay and the 22C3 antibody. Both archival and fresh tumor samples were valid but only those with at least 50 viable neoplastic cells were considered adequate for analysis. PD-L1 positivity was defined by membranous PD-L1 expression in at least 1% of tumor and associated inflammatory cells or positive staining in stroma.
Of a total of 145 evaluable patients, only 46 (31.7%) were considered PD-L1 positive and eligible for the study. This information is in contrast with other previous reports that showed PD-L1 expression was present in 50% to 80% of tumor specimens. However, different tumor samples and different antibodies were used, limiting the interpretation of the results (14,15).
In the present study, all treated patients did express PD-L1, but there was no correlation between higher PD-L1 expression and frequency of response (P=0.235).
In the CheckMate 032 trial, PD-L1 expression was assessable in 148 (69%) of 216 patient samples. Only 25 (17%) of 148 did express PD-L1 in at least 1% of tumor cells. Notably, responses were observed irrespective of PD-L1 expression status.
The KEYNOTE-158 (NCT02628067) is an ongoing single-arm study of pembrolizumab at a flat dose of 200 mg every 3 weeks, in several tumor types, unselected according to PD-L1 status. This trial aims to improve the knowledge on the role of PD-L1 expression by immunohistochemistry and gene expression profiling by RNA analysis as predictors of benefit from pembrolizumab.
At present time, whether PD-L1 expression or other biomarkers are predictive of benefit from anti-PD(L)1 drugs in SCLC must await further analysis.
What is the real impact of pembrolizumab in the refractory setting in ED-SCLC?
In the present trial, the confirmed ORR (investigator assessed per RECIST v1.1) with pembrolizumab was 33.3% (95% CI, 15.6–55.3%), including one complete response (4.2%) and seven partial responses (29.2%), which is higher than the ORR of 10% reported for the nivolumab monotherapy arm in the CheckMate 032 study. This data suggests that enrichment by PD-L1 expression, as mentioned before, may have a role for selecting patients.
After a median duration of follow-up of 9.8 months (95% CI, 0.5–24.0), the median PFS was 1.9 months (95% CI, 1.7–5.9) and the PFS rate at 6 and 12 months, was 28.6% and 23.8%, respectively. In the CheckMate 032 the median PFS obtained with nivolumab was 1.4 months (95% CI, 1.4–1.9), and the PFS rate at 12 months was 11%, lower than the observed in the present study.
The documented median OS with pembrolizumab was 9.7 months (95% CI, 4.1– not reached) and the 6- and 12-month OS rates were 66.0% and 37.7%, respectively. As mentioned before, in the recent update on the CheckMate 032 trial, it was reported a median OS of 4.1 months for the nivolumab arm, and the 12- and 24-month OS rates were 30% and 17%, respectively. Of significant interest, for the ipilimumab 3 mg/kg plus nivolumab 1 mg/kg arm, the median OS was 7.9 months and the 12- and 24-month OS rates were 42% and 30%, respectively, very impressive and similar to the long-term survival rates observed in other tumor types.
One major concern in a disease with such a rapid progression condition, like SCLC, is the median time to objective response. In the present trial, time to response with pembrolizumab was 2.0 months, exactly the same that was observed in the nivolumab arm in the CheckMate 032. For those patients who achieved an objective response, the median duration of response was 19.4 months (range, 3.6–20.0 months), and notably, at the time of data cutoff, three patients were still on treatment. It is important to notice that the median duration of response achieved with pembrolizumab in the KEYNOTE-001 trial was 10.4 months (range, 1.0–10.4 months) in previously treated NSCLC patients.
Regarding the exposure to previous therapies, the population of the study was heavily pretreated, as 21 out of 24 patients (87.5%) had been treated with two or more previous lines, including 9 patients of them (37.5%) who had been exposed to three or more regimens.
Data about platinum sensitivity were not collected in this trial, making difficult to generate any inferences about the activity of pembrolizumab in both platinum-resistant and platinum-sensitive disease. In the CheckMate 032 trial, although the numbers of patients in both nivolumab monotherapy and ipilimumab plus nivolumab combination subgroups were quite small, no relevant different in antitumor activity were observed regarding platinum-sensitivity status and exposure to previous lines of therapy.
Comparing all these results with historical data, the pivotal trials of topotecan in second-line for ED-SCLC reported ORR in range of 7% to 24% and median OS of around 6 months. For that reasons, despite the very limited number of patients included in this phase 1 trial, pembrolizumab seems to be a better option for the PD-L1 positive ED-SCLC population after failure to standard therapy.
Is there any safety concern with pembrolizumab in the SCLC population?
The safety profile of pembrolizumab in the present study is similar to the previously reported and no new safety signal has been documented. This is important as SCLC is associated to higher prevalence of autoimmune disorders then other neoplasms and it treatment with immune checkpoint inhibitors could exacerbate the autoimmunity phenomena. In this trial, TRAEs were seen in 16 (66.7%) of 24 patients, being arthralgia, asthenia, rash, diarrhea and fatigue the most common. Eight patients (33.3%) had grade 3 to 5 adverse events, two of whom were considered as TRAEs (one case of grade 3 bilirubin elevation and one case of grade 5 colitis/intestinal ischemia). No endocrine toxicity and no toxic pneumonitis were reported in this trial. This report is similar to the toxicity documented from the CheckMate 032 study, with 60% of adverse events related to nivolumab in the monotherapy arm, being 14% of them grade 3–4 and with a discontinuation rate of only 5%.
It is well known that toxicity is more significant with the combination strategies, as previously reported in other tumor types. In the ipilimumab 3 mg/kg plus nivolumab 1 mg/kg arm from the CheckMate 032 trial, TRAEs were reported in 82% of cases, 33% of them grade 3–4 with 7 (11%) patients discontinuing permanently the treatment because of toxicity. Limbic encephalitis as a TRAE was seen in three patients treated with the combination of ipilimumab plus nivolumab, in two of them was considered as grade 2 and resolved with immunosuppressive therapy and in the third case was grade 4 and did not respond to intravenous immunoglobulin and corticosteroid treatment.
In conclusion, pembrolizumab, as single agent, has a favorable safety profile and toxicity is mild and manageable for this heavily pretreated SCLC population. Toxicity is remarkable less significant than the associated with topotecan and seems to be also lower than the observed with combination of nivolumab plus ipilimumab.
Further studies have been designed in order to validate the role of pembrolizumab in SCLC. PembroPlus trial (NCT02331251) is a phase 1b-2 study that evaluated the role of pembrolizumab in combination with different cytotoxic agents (16). In the cohort of ED-SCLC, after progression to standard treatment, patients received pembrolizumab at a dose of 2 mg/kg plus irinotecan at a dose of 300 mg/m2 both administered every three weeks. Early efficacy analysis showed that 2 out of 3 patients obtained partial responses that were long-lasting. Recently, at ASCO annual meeting 2017, a phase II trial of maintenance pembrolizumab in ED-SCLC was presented (17). Forty-five patients with advanced ED-SCLC after 4–6 cycles of platinum-etoposide were enrolled, and received pembrolizumab at 200 mg flat dose every 3 weeks, for a maximum of 2 years. PD-L1 expression was assessed in 35 patients and was positive (at least 1% of tumor cells expressing PD-L1) only in 1 case (3%). Of a total of 35 patients with measurable disease, 4 of them (ORR 11.4%) achieved responses (3 PR and 1 CR). The median PFS was 1.4 months (90% CI, 1.3–4.0) and the median OS was 9.2 months (90% CI, 6.1–15.2). After a median follow up of 6 months, eleven patients were still ongoing (3–20 cycles). No new safety signals were documented. Overall, this study suggests, as denoted by the favorable median OS, that some patients could derive significant benefit from maintenance treatment with pembrolizumab.
In conclusion, despite the promising signal of efficacy of pembrolizumab in advanced ED-SCLC disease, as highlighted in this small size clinical trial, there is an urgent need for a better understanding. First, finding a better predictive biomarker for a more accurate identification of patients most likely to benefit from anti-PD1 inhibition is of crucial relevance. Other factors than PD-L1 expression by immunohistochemistry, such as tumor mutational burden and gene expression profiles are being evaluated in the ongoing KEYNOTE-158 trial. Second, as shown in the NSCLC scenario, the addition of pembrolizumab to standard front-line platinum-etoposide chemotherapy, could lead to an increase of response rate, PFS and probably OS. The ongoing KEYNOTE-604 trial is aimed to solve this relevant question. Other studies evaluating anti-PD(L)1 plus anti-CTLA4 in combination with chemotherapy (Table 1) and also with other approaches such as radiation and new agents like rovalpituzumab, are also of great interest.
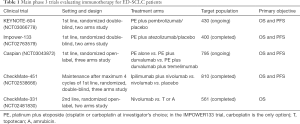
Full table
Therefore, despite some lights appear to be more evident in the shadows, still major efforts must be done in clinical and translational research until SCLC is not considered as a recalcitrant disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106-11. [Crossref] [PubMed]
- Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now? -a review. Transl Lung Cancer Res 2016;5:26-38. [PubMed]
- Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 1997;15:2090-6. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-9. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non−small cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Reck M, Heigener D, Reinmuth N. Immunotherapy for small-cell lung cancer: emerging evidence. Future Oncol 2016;12:931-43. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016;34:3740-8. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in re- current small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): first report of a randomized expansion cohort from CheckMate 032. J Clin Oncol 2017;35:8503.
- National Comprehensive Cancer Network Guidelines: Small-cell lung cancer, 2017.
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol 2017;35:3823-9. [Crossref] [PubMed]
- Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015;10:426-30. [Crossref] [PubMed]
- Schultheis AM, Scheel AH, Ozretić LL, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015;51:421-6. [Crossref] [PubMed]
- Weiss GJ, Waypa J, Blaydorn L, et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer 2017;117:33-40. [Crossref] [PubMed]
- Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts). J Clin Oncol 2017;35:8504.


