Immunotherapy in lung cancer
Introduction
Lung cancer is the leading cause of cancer mortality in the United States and worldwide (1). An estimated 226,160 new cases of lung cancer will be diagnosed in 2012 in the United States alone, and 160,340 lung cancer deaths are estimated to occur (2). Broadly classified as non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), the five year survival for all lung cancer patients is a dismal 15%. NSCLC is the most common type of lung cancer, accounting for about 85% of all cases (3). In recent years, the recognition that NSCLC does not represent a single disease entity, but rather a collection of distinct molecularly-driven neoplasms has shifted the landscape of NSCLC therapy to a personalized approach based on the molecular alterations of a patient’s tumor; a paradigm typified by targeted therapies in epidermal growth factor receptor (EGFR) mutant and ALK translocation driven adenocarcinomas of the lung. Despite these therapeutic advances, metastatic NSCLC in the absence of an EGFR mutation or ALK translocation is still associated with a disappointing median overall survival (OS) of about one year (4). The remaining 15% of lung cancer cases represent SCLC (3). SCLC is associated with an aggressive clinical course characterized by rapid growth and a tendency to metastasize early (5). While often initially highly sensitive to chemotherapy and radiation therapy, the majority of patients with SCLC will relapse and long-term survival is rare (5).
While the role of immunotherapy in the treatment of melanoma and renal cell carcinoma is well established, immunotherapies in lung cancer have historically been associated with disappointing results (6-9). Lung cancer’s ability to evade the immune system is characterized by cytokine alterations, cellular immune dysfunction, and antigen presentation defects (10). Decrease in the function of the tumor suppressor cytokine TGFβ in lung cancer has been linked to the downregulation of the TGFβ type II receptor (11). Patients with advanced lung cancer have been shown to have both T- and B-cell peripheral blood lymphopenias, and T-cell subset alterations in lung cancer are characterized by decreased naïve T-cells and increased effector/memory CD4+ and CD8+ T-cell subsets (12-14). Studies in early stage disease have demonstrated an increased proportion of CD4+ regulatory T-cells (CD4+ Tregs) in NSCLC, which have a constitutive high level expression of cytotoxic T-lymphocyte antigen-4 (CTLA-4) and mediate potent inhibition of autologous T-cell proliferation (15). This has important consequences as CD4+ Tregs suppress cytotoxic T-lymphocytes (CD8+ T-cells), which are responsible for tumor cell cytotoxicity, immunosurveillance and immune memory. Recently, there have been several novel immunotherapeutic strategies that have been evaluated in lung cancer with early evidence of activity. This review will highlight two approaches of particular interest: immune checkpoint inhibition, which aims to counteract the physiologic mechanisms of immune tolerance co-opted by some tumors, and vaccine therapy, which enables enhanced exposure to tumor antigen.
Endpoint considerations in clinical trials evaluating immunotherapy
Traditional response criteria, such as RECIST, have relied on measurable changes in tumor size to indicate response to cytotoxic therapies (16). These response criteria operate under the assumption that an increase in tumor growth or the appearance of new lesions is indicative of progressive disease (PD). In contrast to cytotoxic chemotherapy, immunotherapeutic agents produce their antitumor effects by modifying the native immune process or by inducing a cancer-specific immune response (17). These lead to response patterns that extend beyond those of cytotoxic agents and often occur after an initial increase in tumor burden or the appearance to new lesions. The precedent for the immune-related response criteria (irRC) is the distinct patterns of response to anti-CTLA4 immunotherapy seen in melanoma. Ipilimumab is associated with an OS advantage of ten months in patients with advanced metastatic melanoma and is also associated with four distinct response patterns; shrinkage in baseline lesions without new lesions; durable stable disease; a response after an initial increase in the total tumor burden; and a response in the presence of new lesions (17,18). While these response patterns were all associated with favorable survival, the latter two represent PD by traditional RECIST. In contrast to traditional response criteria, new lesions using the irRC do not necessarily define disease progression; rather, new lesions are incorporated into the overall tumor burden, and a determination of PD necessitates a 25% increase in tumor burden by measurements taken four weeks apart. While these criteria more adequately reflect the response patterns of immunotherapeutic agents, prospective evaluation is needed to validate their association with survival endpoints.
Immune checkpoint therapy
Complex regulatory pathways maintain the balance between the appropriate recognition and destruction of pathogens and tumors and the inappropriate overstimulation of immune responses, which leads to autoimmunity. These regulatory pathways involve both costimulatory and coinhibitory factors which fine-tune the antigen specific T-cell response after stimulation of the T-cell receptor (19,20). The 1st step (signal 1) in the T-cell specific response occurs when the T-cell receptor recognizes antigenic peptides in the context of the major histocompatibility complex on the surface of the antigen presenting cell (APC) (Figure 1). This 1st step requires a 2nd costimulatory signal (signal 2) for full T-cell activation, which occurs when the costimulatory receptor on the surface of the T-cells, CD28, binds to the B7 ligand subtypes CD80 and CD86 on the surface of the APC. Costimulation through CD28 and other such molecules including CD134 and CD137 augments the excitatory antigenic stimulation that leads to T-cell activation and potentiation of the immune response.
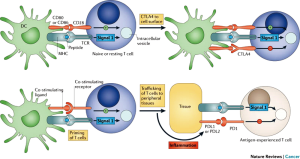
Immune checkpoint proteins are coinhibitory factors which diminish the antigen-specific immune response by limiting their magnitude and duration and include CTLA-4, PD-1, B7-H3, B7x, T-cell immunoglobulin and mucin-domain-containing molecule-3 (Tim-3), and B- and T-cell lymphocyte attenuator (BTLA). CTLA-4 is expressed on activated T-cells and is upregulated upon stimulation of the T-cell through the T-cell receptor. It then competes with CD28 for binding to the B7 ligand subtypes CD80 and CD86 on the APC (Figure 1). CTLA-4 has a higher affinity for the B7 ligands allowing for competitive inhibition of CD28 mediated T-cell activation, thereby limiting the subsequent T-cell response (21,22). This is an essential component of immune tolerance. The monoclonal antibody to CTLA-4, ipilimumab, blocks the interaction between CTLA-4 and its ligands CD80 and CD86, thereby promoting T-cell activation.
PD-1 is named for its involvement in programmed cell death. While it is undetectable on the surface of the resting T-cell, it is found on the cell surface within 24 hours of T-cell activation (23). The PD-1 receptor binds its known ligands PD-L1, also known as B7-H1, and PD-L2, also known as B7-DC (Figure 1) (24,25). Binding of PD1 to its ligands causes T-cell inhibition and down regulation of the T-cell response. Both PD-L1 and PD-L2 have been observed on cancer cells. This pathway in particular is co-opted by tumors through tumor expression of PD-L1 on the tumor cell surface and on cells within the tumor microenvironment, allowing for direct suppression of anti-tumor cytolytic T-cell activity by the tumor. Indeed, induction of the PD1/PD-L1 pathway represents an adaptive immune resistance mechanism exerted by tumor cells in response to endogenous anti-tumor activity (26). Monoclonal antibodies that block both PD1 (nivolumab and lambrolizumab) and PD-L1 (BMS936559, Medi-4736 and MPDL3280A) abrogate the immune tolerance exerted by tumors through the PD1/PD-L1 pathway (26). It is important to note that anti-PD-1 and anti-PD-L1 antibodies block distinct inhibitory pathways, possibly resulting in different clinical outcomes. While anti-PD-1 antibodies block PD-1 binding to PD-L1 and PD-L2, they do not affect the inhibitory signal occurring upon the PD-L1/B7.1 interaction. On the other hand, while anti-PD-L1 antibodies block PD-L1 binding to B7.1 and PD-1, they do not impede the inhibitory signaling provided by the interaction of PD-1 and PD-L2 (27).
A growing number of inhibitory receptors have been identified which may have important therapeutic implications in the future. Upregulation of PD-1 and Tim-3 expression has been associated with tumor-antigen-specific CD8+ T-cell dysfunction (28). Tim-3/Tim-3L blockade enhances cytokine production and proliferation of CD8+ T-cells upon prolonged antigen stimulation and acts in synergy with PD-1/PD-L1 blockade (28,29). BTLA blockade has also been shown to enhance the expansion, proliferation and cytokine production of CD8+ T-cells. Therefore, targeting multiple inhibitory receptors in future clinical trials may further improve the efficacy of anti-PD-1/PD-L1 antibody blockade in NSCLC.
Anti-CTLA-4 blockade
Chemo-immunotherapy is an approach to the treatment of cancer that combines the cytotoxic effects of chemotherapy with drugs that modulate the host immune response to the tumor. Tumor cell death triggered by chemotherapy or radiotherapy initiates an immunoadjuvant pathway that contributes to the success of cytotoxic treatments (30). The induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor specific CD8+ T-cells (31). Chemotherapy increases the susceptibility of tumor cells to the cytotoxic effects of CTLs. When combined with chemotherapy, CTLs raised against specific antigens are able to induce apoptosis in neighboring cells not expressing these antigens, suggesting that a small number of CTLs can mediate a potent antitumor effect when combined with chemotherapy (32). In preclinical mouse models, CTLA-4 blockade in combination with various chemotherapeutic agents was synergistic in inducing tumor regression and elicited prolonged anti-tumor effects and induction of a memory immune response (33,34).
It is with this rationale that ipilimumab was studied in combination with carboplatin and paclitaxel as 1st line treatment in advanced NSCLC and extensive stage (ES) SCLC in a randomized phase II clinical trial (35,36). In order to address if the sequencing of chemotherapy and immunotherapy affects outcome, two dosing schedules were tested: concurrent ipilimumab with carboplatin and paclitaxel, allowing ipilimumab to be present at the earliest phase of chemotherapy-induced antigen presentation, and a phased regimen in which carboplatin and paclitaxel were given prior to ipilimumab, which allowed antigen release to occur before ipilimumab administration. Patients were randomized 1:1:1 to receive carboplatin/paclitaxel/placebo or to receive carboplatin/paclitaxel with either concurrent or phased ipilimumab.
In the 204 chemotherapy-naïve patients with NSCLC, phased ipilimumab with carboplatin/paclitaxel was associated with a statistically significant improvement in immune related progression-free survival (irPFS) compared with chemotherapy alone (HR 0.72; P=0.05), while concurrent ipilimumab and chemotherapy was not associated with improved irPFS compared with chemotherapy alone (HR 0.81; P=0.13) (36). Phased ipilimumab was also associated with a PFS benefit as determined by modified WHO criteria (HR 0.69; P=0.02), while concurrent was not (HR 0.88; P=0.25). There was no difference in survival between the three treatment arms. What is striking is that the immune related best overall response rate (irBORR) was nearly doubled in patients treated with phased ipilimumab compared with patients treated with chemotherapy alone (32% versus 18%, respectively). Patients treated with concurrent ipilimumab had an irBORR of 21%. In an unplanned subset analysis, phased ipilimumab compared with chemotherapy alone was associated with a greater trend in improved irPFS in patients with squamous cell histology (HR 0.55; 95% CI, 0.27-1.12), relative to patients with non-squamous cell histology (HR 0.82; 95% CI, 0.52-1.28). The addition of ipilimumab to chemotherapy in NSCLC did not significantly increase the incidence of treatment-related grade 3 and four adverse events (AEs) across arms (37% for chemotherapy alone, 41% for phased ipilimumab and 39% of concurrent ipilimumab). Other common AEs were rash, pruritis and diarrhea, which were identified as immune related AEs (irAEs) and tended to occur in greater frequency in the ipilimumab containing arms. The overall incidence of grade 3 and 4 irAEs was 6% with chemotherapy alone, 20% with concurrent ipilimumab and 15% for phased ipilimumab. In addition to grade 3 diarrhea and grade 3 rash, two cases of grade 3 colitis were noted in the phased ipilimumab arm. One case each of grade 3 hypophysitis and grade 3 hypopituitarism were noted in the concurrent ipilimumab arm. There is an ongoing phase III clinical trial evaluating the role of phased carboplatin/paclitaxel/ipilimumab versus carboplatin/paclitaxel/placebo in patients with recurrent metastatic NSCLC of squamous cell histology with the primary endpoint of OS (NCT01285609).
In the 130 patients with chemotherapy naïve ES-SCLC who were randomized to receive either carboplatin/paclitaxel/placebo or phased or concurrent carboplatin/paclitaxel/ipilimumab, phased ipilimumab was associated with an improved irPFS compared with chemotherapy/placebo (HR 0.64; P=0.03), while concurrent ipilimumab was not (HR 0.75; P=0.11) (35). There were no differences in PFS as determined by modified WHO criteria for the phased ipilimumab schedule (HR 0.93; P=0.37), nor the concurrent ipilimumab schedule (HR 0.93; P=0.38). There was a trend toward improved OS in the patients treated with phased ipilimumab (HR 0.75; P=0.13). The irBORR for the phased ipilimumab schedule was 71%, which compared favorably with the irBORR of 53% with chemotherapy alone and 49% with concurrent ipilimumab. It is important to note that this trial was not formally powered for efficacy of ipilimumab in the ES-SCLC cohort. Nevertheless, these data suggest that a phased chemo-immunotherapeutic regimen with ipilimumab may be associated with a clinical benefit in patients with ES-SCLC. The overall incidence of treatment-related grade 3 and 4 AEs was higher in the ipilimumab-containing arms (43% concurrent, 50% phased) compared with chemotherapy alone (30%). Other common AEs of rash, pruritis, and diarrhea were more common in the ipilimumab-containing arms compared with chemotherapy alone. The overall incidence of grade 3 and 4 irAEs was 21% for concurrent ipilimumab, 17% for phased ipilimumab and 9% for chemotherapy. All events of severe diarrhea were grade 3, with the exception of one event of grade 4 diarrhea in the concurrent ipilimumab arm; one patient on the phased ipilimumab schedule experienced grade 3 colitis. There were two cases of grade 4 hepatitis, one in each of the ipilimumab-containing arms. There was one death in the concurrent ipilimumab arm which was attributed to treatment-related hepatotoxicity. A phase III clinical trial is currently ongoing to evaluate the efficacy of ipilimumab in addition to chemotherapy with platinum/etoposide in patients with ES-SCLC with the primary endpoint of OS (NCT01450761).
Anti-PD1 blockade
A phase I dose-escalation study of single agent nivolumab (BMS-936558; ONO-4538; MDX-1106), a monoclonal antibody to PD1, enrolled 39 patients with refractory solid tumors, of whom six (15.4%) patients had NSCLC (37). In this study nivolumab was administered as a single dose in dose-escalating cohorts ranging from 0.3 to 10 mg/kg, followed by a 15-patient dose expansion cohort at 10 mg/kg. Nivolumab was associated with a favorable toxicity profile with one serious AE of inflammatory colitis. One of the six NSCLC patients on this trial had significant lesional tumor regression, which did not meet formal response (PR) criteria. This pilot study established the basis for a larger phase I clinical trial of nivolumab in 296 patients with advanced melanoma, NSCLC, castration resistant prostate cancer and colorectal cancer with the endpoints of safety, activity and immune correlates of nivolumab response (38). Nivolumab was given at doses of 0.1 to 10 mg/kg every 2 weeks in 8-week treatment cycles for up to 12 cycles until PD or complete response (CR).
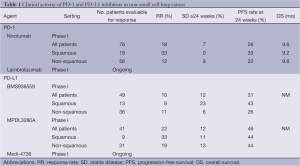
A total of 122 NSCLC patients were enrolled, of whom 76 were evaluable for response (38). The majority of these patients with NSCLC were heavily pretreated (55% had received three or more prior lines of therapy). In this patient population, nivolumab (all doses) was associated with an 18% cumulative response rate (RR), a 7% stable disease rate ≥24 weeks, and a PFS rate at 24 weeks of 26%, indicative of a durable clinical benefit (Table 1). Responses in NSCLC were seen at all dose levels; 6% at 1 mg/kg, 32% at 3 mg/kg, and 18% at 10 mg/kg and in patients with both squamous cell histology (RR 33%, 6 out of 18 patients) and non-squamous cell histology (RR 12%, 7 out of 56 patients).
Full table
The overall incidence of grade 3 and 4 treatment-related AEs was 14%. Other common treatment-related AEs were fatigue, rash, diarrhea, pruritis, anorexia, and nausea (38). Drug related serious AEs occurred in 11% of patients and these included the likely immune-related phenomena of pneumonitis, vitiligo, colitis, hepatitis, hypophysitis, and thyroiditis. Hepatic or gastrointestinal AEs (including three patients with grade 3 or 4 diarrhea and two patients with alanine aminotransferase elevations) were managed with treatment interruption and glucocorticoids as necessary and were reversible in all cases. Drug-related pneumonitis occurred in 3% patients, and grade 3 and 4 pneumonitis occurred in 1% patients. Early-grade pneumonitis was reversible in six patients with treatment discontinuation, glucocorticoids, or both. There were three drug-related deaths (1%) due to pneumonitis, two of whom had NSCLC.
Pretreatment specimens from 42 patients (including ten patients with NSCLC) were analyzed for surface PD-L1 expression by immunohistochemistry (38). Of the 25 patients whose tumors were PD-L1 positive, nine (36%) had objective responses, while none of the 17 patients with PD-L1 negative tumors had an objective response. Of the five NSCLC patients with PD-L1 positive NSCLC, one patient had a PR, and the remaining five NSCLC patients with PD-L1 negative tumors had no responses. Given the small number of tumor samples tested for PD-L1 on this clinical trial, these results should be interpreted with caution. The long-term follow-up and survival of the NSCLC patients treated on this clinical trial was recently updated and indicated a median OS across all dose cohorts of 9.6 months (9.2 months in patients with squamous cell histology and 9.6 months in patients with non-squamous cell histology) (39). Median OS was not reached at the 3 mg/kg dose level for either histology. At one year, nivolumab was associated with one-year survival rates of 44% and 41% in patients with squamous and non-squamous histology, respectively, and two-year survival rates of 44% and 17% in patients with squamous and non-squamous histology, respectively.
Given these unprecedented RR seen in patients with heavily pretreated NSCLC, there are ongoing phase III clinical trials evaluating the role of nivolumab compared with docetaxel in patients with advanced or metastatic NSCLC of both squamous cell histology (NCT01642004) and non-squamous cell histology (NCT01673867) in the 2nd line setting. A multi-arm phase I clinical trial is also ongoing evaluating the safety of nivolumab in combination with platinum doublet chemotherapy, bevacizumab maintenance therapy, erlotinib, ipilimumab or as monotherapy in the 1st line setting or as switch maintenance therapy in patients with advanced or metastatic NSCLC (NCT01454102). Lambrolizumab (MK-3475) is another monoclonal antibody to PD1 that is being evaluated in a phase I clinical trial involving multiple tumor types including NSCLC (Table 1; NCT01295827).
Anti PD-L1 blockade
A phase I clinical trial evaluated the safety and activity of BMS936559, a PD-L1-specific monoclonal antibody which inhibits binding of PD-L1 to PD-1 (40). In a total of 207 patients with advanced solid organ malignancies, BMS936559 was given every two weeks in 6-week treatment cycles for up to 16 cycles or until CR or confirmed disease progression. Of the 75 patients with NSCLC treated on this trial, 49 patients were evaluable for response, in which five objective responses were observed (four patients with non-squamous NSCLC and one patient with squamous NSCLC) at the 3 mg/kg and the 10 mg/kg dose levels (Table 1). The corresponding RRs were 8% and 16% at the 3 mg/kg and the 10 mg/kg dose levels, respectively. Across dose levels, BMS936559 was associated with a 12% stable disease rate at 24 weeks and 31% PFS rate at 24 weeks.
The maximum tolerated dose was not reached (40). The most common drug-related AEs were fatigue, infusions reactions, diarrhea, arthralgia, rash, nausea, pruritus and headache and were predominantly low-grade with grade 3 and 4 events noted in 9% of patients. Drug-related AEs which were potentially immune related occurred in 39% of patients and included rash, hypothyroidism, hepatitis, and one case each of sarcoidosis, endophthalmitis, diabetes mellitus, and myasthenia gravis. These predominantly grade 1 and 2 AEs were managed with treatment interruption or discontinuation. In nine patients, treatment with glucocorticoids resolved the AEs, four of whom maintained disease control despite glucocorticoid therapy. Infusion reactions were observed in 10% of patients, occurring predominantly at the 10 mg/kg dose level and were generally rapidly reversible with antihistamines, antipyretics and in some cases, glucocorticoids.
MPDL3280A is another monoclonal antibody to PD-L1. As previously mentioned, PD1 is present on the surface of the T-cell within 24 hours of activation (23). MPDL3280A is specifically engineered to block the PD1/PD-L1 interaction while avoiding the antibody-dependent cell-mediated cytotoxicity of activated T-cells that express PD1 and PD-L1 (41). Data from a phase Ia clinical trial assessing the safety, activity, and biomarkers of response to MPDL3280A in patients with locally advanced or metastatic tumors was recently presented at the 2013 Annual Meeting of the American Society of Clinical Oncology (ASCO) (41). At the time of the analysis, 53 patients with NSCLC were evaluable for safety and were treated at doses of MPDL3280A ranging from 1 to 20 mg/kg every three weeks. Sixty-two percent of these patients had been treated with three or more lines of prior therapy. The maximum tolerated dose was not met, and there were no dose-limiting toxicities. The majority of the AEs were grade 1 and 2, with no events of grade 3 to 5 pneumonitis. Six patients experienced grade 3 or 4 treatment-related AEs, which included pericardial effusion, dehydration, dyspnea and fatigue.
In the 41 patients evaluable for response, MPDL3208A was associated with a 22% overall RR (19% in non-squamous histology and 33% in squamous histology), with a stable disease rate at 24 weeks of 12% in all patients (13% in non-squamous histology and 11% in squamous histology) and a remarkable 24-week PFS rate of 46% (44% in non-squamous histology and 44% in squamous histology) (Table 1). In the subset of patients in whom PD-L1 status was known, PD-L1-positive tumors were associated with a RR of 80% (4 of 5 patients) with MPDL3208A therapy, and PD-L1 negative tumors were associated with a RR of 14% (4 of 28). Although PD-L1 expression by tumor cells appears to correlate with greater likelihood of a clinical response to anti-PD-1 and anti PD-L1 blockade, it is important to note there have been clinical responses in patients with PD-L1 negative tumors. Indeed, there is growing evidence that PD-L1 expression by tumor cells is induced by tumor-infiltrating T-cells upon production of cytokines like IFN-γ. Therefore, cancer immunotherapy might be preferentially beneficial for patients with a pre-existing T-cell inflamed tumor microenvironment (42). The expansion cohorts of this clinical trial are currently accruing, as is another phase I clinical trial of the PD-L1 inhibitor Medi-4736 in patients with advanced melanoma, NSCLC, renal cell carcinoma, and colorectal cancer (Table 1; NCT01693562).
Vaccine therapy
There are two major modalities that allow de novo development of antitumor immunity. Antigen-specific immunotherapy utilizes vaccines to induce specific antitumor immunity against relevant tumor-associated antigens that have been incorporated into the vaccine formulation (43). Tumor vaccines (whole vaccines) are immunologically active agents that are either autologous or allogeneic in origin and influence the patient’s immune system to allow recognition of the tumor as foreign and create de novo immunity towards the tumor cells (43,44). Several clinical trials are currently underway in NSCLC that incorporate both antigen specific and tumor specific vaccines.
Antigen specific immunotherapy
Melanoma-associated antigen-A3 (MAGE-A3)
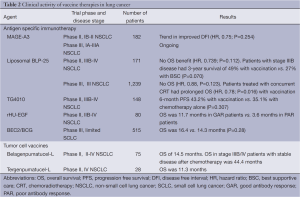
MAGE-A3 is present in numerous tumors including melanoma and lung cancer and is absent in normal adult tissue, with the exception of the testes and placenta. For this reason, MAGE-A3 is felt to be a true selective target for tumor-specific active immunotherapy. MAGE-A3 is presented to specific T cells as a tumor-specific antigen by HLA molecules at the cell surface (45). A phase II proof-of-concept clinical trial evaluated the MAGE-A3 immunotherapeutic as a postoperative adjuvant therapy in patients with completely resected stage IB or II MAGE-A3-positive NSCLC. MAGE-A3 combined with an immunostimulant was administered as 13 doses over 27 months with the primary endpoint of disease free interval (DFI). Overall, 122 patients were randomized to the MAGE-A3 vaccine arm and 60 to the placebo arm. The authors found that recurrence was observed in 35% of patients in the MAGE-A3 arm and 43% in the placebo arm (45). There was a non-statistically significant trend toward improved DFI (HR 0.75; P=0.254), disease free survival (DFS; HR 0.76; P=0.248), and OS (HR 0.81; P=0.454) (45) (Table 2). Only three patients from this study had grade 3 or 4 toxicities, emphasizing the tolerability of this vaccine. It should be noted that this study was performed at a time when adjuvant chemotherapy for NSCLC was not standard therapy for resected tumors.
Full table
Recently, an 84-gene expression signature was developed in MAGE-A3 antigen specific patients in two phase II studies involving metastatic melanoma and validated in resected NSCLC patients treated on the phase II MAGE-A3 clinical trial (46). Gene signature (GS) positive patients demonstrated an improved DFI with vaccination compared with placebo (HR 0.42; P=0.06), while GS negative had no difference in DFI (HR 1.17; P=0.65). The genes identified involve the interferon gamma pathways and specific chemokines, suggesting that clinical response is influenced by alterations in the tumor microenvironment (46). The phase III MAGRIT study (MAGE-A3 as Adjuvant Non-Small-Cell Lung Cancer Immunotherapy trial) is underway evaluating the role of the MAGE-A3 vaccine in patients with Stage IB through Stage IIIA MAGE-A3 positive NSCLC who have and have not received chemotherapy with the primary endpoint of DFS.
Membrane-associated glycoprotein (MUC-1)
MUC-1 is normally expressed on epithelial cells. MUC-1 expression is greatly increased in cancer cells, including NSCLC, breast, colorectal, prostate, and multiple myeloma. Whilst the precise role is not clear, MUC1 expression in tumors promotes growth and survival and is associated with disease progression and poor prognosis (47). More than 80% of tumor cells express MUC1 and greater than 60% of NSCLCs express MUC-1 (47). Liposomal BLP-25, a peptide based vaccine targeting exposed core peptide of MUC-1, was evaluated in a phase II trial of stage IIIB and IV NSCLC patients who were stable following chemotherapy and/or radiation. Of the 171 patients enrolled, 88 patients in the L-BLP25 arm received a single intravenous dose of cyclophosphamide 300 mg/m2 followed by eight weekly subcutaneous immunizations with L-BLP25 (1,000 μg). Subsequent immunizations were administered at 6-week intervals. Results indicated a median survival time of 17.4 versus 13 months in the best supportive care arm (HR 0.739; P=0.112; Table 2) (48). The 3-year survival rate was 31% in patients receiving L-BLP25 plus best supportive care and 17% in those receiving best supportive care (P=0.035) (49,50). A post hoc analysis suggested the greatest benefit in survival with the vaccine was in patients with Stage IIIB disease (49,50). In this subgroup, 3-year survival was 49% in patients receiving L-BLP25 plus best supportive care versus 27% in those receiving best supportive care alone (P=0.070; Table 2).
With these results, the phase III Stimulating Targeted Antigenic Responses to NSCLC Trial START trial (START trial) was initiated, with results recently presented at the 2013 Annual Meeting of ASCO. Patients with unresectable Stage III NSCLC following treatment with chemoradiation were randomized to receive cyclophosphamide plus vaccine or placebo with the primary endpoint of OS. A total of 1,239 patients were randomized with a median OS of 25.6 months in the L-BLP25 arm vs. 22.3 months in the placebo arm (HR 0.88; P=0.123; Table 2) (51). A predefined subgroup analysis demonstrated a benefit in patients treated with concurrent chemoradiotherapy with L-BLP25 versus placebo (HR 0.78; P=0.016; Table 2), while a benefit in patients treated with sequential chemoradiotherapy was not seen (HR 1.12; P=0.38) (51). A phase III trial (INSPIRE) assessing L-BLP25 plus best supportive care compared with placebo plus best supportive care in East-Asian patients with unresectable stage III NSCLC in ongoing.
TG4010 is a targeted immunotherapy based on a poxvirus (modified vaccine virus Ankara) encoding for MUC1 tumor-associated antigen and interleukin 2. In a phase II study, TG4010 was given at a dose of 108 plaque-forming units in combination with cisplatin and vinorelbine as first-line chemotherapy in Stage IIIB/IV MUC-1 positive NSCLC patients. Sixty five patients were randomly assigned to receive the vaccine concurrent with chemotherapy (n=44) versus sequentially (TG4010 alone until disease progression then combined with chemotherapy; n=21) (52). No responses were seen with the first stage of the Simon design in the sequential arm. In the concurrent arm, a RR of 35% was observed with an OS of 12.7 months and median time to progression of 4.8 months. There were 17 patients who continued onto maintenance therapy in the concurrent arm. This was very well tolerated with the most common side effects being fatigue, injection site reaction, and fever.
More recently, a phase IIB study compared 148 patients with MUC-1 positive Stage IIIB/IV NSCLC treated with chemotherapy (cisplatin/gemcitabine) combined with the vaccine versus chemotherapy alone. TG4010 was given weekly for six weeks then once every three weeks till progression. Six-month PFS rate was 43.2% in the TG4010 group and 35.1% in the chemotherapy alone group (P=0.307; Table 2). TG4010 was associated with a RR of 41.9% compared with 28.4% in the chemotherapy arm (P=0.82) (53). The two groups had similar OS; 10.7 months for the TG4010 group vs. 10.3 months for chemotherapy arm (53). The toxicities were similar to the previous study with injection side reaction, fatigue, and fever. An exploratory analysis demonstrated a benefit in the TG4010 group compared with the chemotherapy group in patients with normal levels of NK cells at baseline arm (median OS 17.1 vs. 11.3 months, respectively). Additionally, patients with high levels of NK cells at baseline had an increased incidence of AEs with TG4010 (53). The combination of TG4010 with first-line chemotherapy in Stage IV MUC-1 positive NSCLC is currently being tested in a phase IIB/III study.
Recombinant human epidermal growth factor (CIMAvax EGF)
Approximately 40-80% of NSCLC express the EGFR. Recently, a recombinant human growth factor (rHU-EGF) was developed that combines recombinant EGF fused to a carrier protein, P64K, with Montanide ISA 51 (54). In a phase II study, 80 patients with advanced NSCLC following first-line chemotherapy were randomly assigned to CIMAvax vs. best supportive care (55). Patients received cyclophosphamide three days prior to the vaccine. CIMAvax was then administered weekly for four doses and then monthly till disease progression. Good anti-EGF antibody response (GAR) was obtained in 51.3% of vaccinated patients compared with none in the best supportive care group. Median OS was 11.7 months in patients with GAR compared with 3.6 months in patients with poor antibody response (Table 2). A 64.3% decrease in EGF concentrations was seen in vaccinated patients. In addition, an EGF concentration of less than 168 pg/mL was associated with significant improvement in survival (13 months with EGF concentration vs. 5.6 months with EGF concentration >168 pg/mL) (55). There currently is a phase III trial underway comparing the rHU-EGF-P64K/Montanide ISA 51 vaccine with best supportive care in advanced NSCLC patients.
Bec2 combined with Bacillus Calmette-Guerin (BCG)
Bec2 is an anti-idiotypic antibody that mimics GD3, a ganglioside antigen expressed on the outer surface of cells involved in cell-cell signaling (56). While the ganglioside GD3 is a cell surface glycosphingolipid antigen with limited expression in normal tissues, it is expressed in up to 60% of small cell lung cancers at twice the concentration as that of normal lung tissue. The combination of Bec2 and BCG produces detectable anti-GD3 antibodies in approximately 20% to 33% of patients (56-58). BeC2/BCG was tested in SCLC in a randomized phase III study of 515 patients with limited stage SCLC, with stable disease after induction therapy (56). The study demonstrated that there was no improvement in survival, PFS, or quality of life improvement in the vaccination arm compared with observation (median survival 16.4 vs. 14.3 months in the vaccine and observation arms, respectively; P=0.28; Table 2).
Tumor cell vaccines
Belagenpumatucel-L
TGF-β2 elevations are associated with immunosuppression in cancer patients and the level of TGF-β2 is inversely correlated with prognosis in patients with NSCLC (59). Belagenpumatucel-L (Lucanix®) is a therapeutic vaccine comprised of 4 TGF-β2 antisense gene-modified allogeneic NSCLC cell lines. A single arm phase II clinical trial of 21 patients demonstrated an OS of 562 days with belagenpumatucel-L with a suggestion that the level of circulating tumor cells (CTC) at baseline correlates with survival; patients with less than two CTC at baseline had a OS of 660 versus 150 days in patients with greater than or equal to two CTCs (59). An updated survival analysis showed a median survival for all subjects was 14.5 months and one, two, and five-year survivals were respectively 55%, 35% and 20% (Table 2). Patients with stages IIIB/IV subjects had a median survival of 15.9 months and one, two and five-year survivals were respectively 61%, 41% and 18%. For stage IIIB/IV patients with non-PD following frontline chemotherapy, median survival was 44.4 months and five-year survival was 50% (Table 2) (60). With these encouraging results, a Phase III trial is underway to determine the efficacy of belagenpumatucel-L in the maintenance setting in stage III/IV NSCLC with stable disease following chemotherapy.
Tergenpumatucel-L
With the success of sipuleucel-T for prostate cancer recently, tergenpumatucel-L (HyperAcute-Lung immunotherapy, HAL) was investigated in NSCLC. Tergenpumatucel-L consists of genetically modified NSCLC cells that provoke a strong, targeted attack from the immune system. It is thought to function by mediating a potent innate immunity response. Normally through innate immunity, cells bearing the xenoantigen (αGal) are killed. HAL consists of genetically modified allogeneic NSCLC tumor cells with the αGal moiety on the cell surfaces. This then generates an innate immune reaction, killing the foreign NSCLC tumor cells. The results of a phase II study were presented at the 2013 Annual Meeting of ASCO by Morris and colleagues, on which 28 patients with metastatic NSCLC or recurrent disease received 300×106 HAL cells every two weeks for eight doses (61). Median OS was 11.3 months (Table 2). Eight of 28 patients had stable disease for greater than or equal to 16 weeks. Nine of the 16 patients who progressed went onto receive additional chemotherapy of whom 31% achieved a PR and 25% had SD, alluding to a chemosensitization effect from HAL. The potential chemosensitization effect is being evaluated in a phase III study that is underway (61).
Conclusions
Historically considered a non-immunogenic disease, lung cancer is the latest oncologic disease to enter the immunotherapeutic landscape. This review underscores the early evidence of effectiveness of targeting the immune checkpoint proteins, CTLA-4, PD-1 and PD-L1, antigen specific vaccination strategies against MAGE-A3, MUC-1 and EGFR, and whole tumor vaccines. Moving forward, confirmatory studies are necessary to validate the role of immune therapy in the treatment of both NSCLC and SCLC. It is also necessary to delineate where immunotherapy fits in the landscape of cytotoxic therapies for lung cancer; should these therapies be given in a concurrent or sequential manner; does the sequencing of immunotherapy with other therapies matter; does the role of immunotherapy vary based on the molecular subtype of the lung carcinoma? Of particular interest is the role of targeted therapies in enhancing the anti-tumor immune response and expression of inhibitory receptors by T-cells. For example, in melanoma, BRAF inhibitors are associated with increased expression of melanoma antigens, an increase in CD8+ T-cell infiltrate and increased expression of PD-1, PD-L1 and Tim-3 (62). Such findings would support combinatorial approaches of targeted therapies and immune checkpoint blockade in patients with NSCLC. Of utmost importance is the elucidation of biomarkers of response to better select patients who will benefit the most from immunotherapies, while limiting toxicity to those who are unlikely to respond. With these reservations in mind, the clinical data to date suggests that immunotherapies may hold the key to the next frontier of treatment of lung cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER stat fact sheets: lung and bronchus. Available online: http://seer.cancer.gov/statfacts/html/lungb.html
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [PubMed]
- Villaruz LC, Burns TF, Ramfidis VS, et al. Personalizing therapy in advanced non-small cell lung cancer. Semin Respir Crit Care Med 2013;34:822-36. [PubMed]
- Stupp R, Monnerat C, Turrisi AT 3rd, et al. Small cell lung cancer: state of the art and future perspectives. Lung Cancer 2004;45:105-17. [PubMed]
- Eton O, Legha SS, Bedikian AY, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol 2002;20:2045-52. [PubMed]
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103-11. [PubMed]
- Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688-96. [PubMed]
- Coppin C, Porzsolt F, Awa A, et al. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev 2005;CD001425. [PubMed]
- Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opin Biol Ther 2012;12:923-37. [PubMed]
- Halder SK, Cho YJ, Datta A, et al. Elucidating the mechanism of regulation of transforming growth factor β Type II receptor expression in human lung cancer cell lines. Neoplasia 2011;13:912-22. [PubMed]
- Sato Y, Mukai K, Watanabe S, et al. Lymphocyte subsets in pulmonary venous and arterial blood of lung cancer patients. Jpn J Clin Oncol 1989;19:229-36. [PubMed]
- Wesselius LJ, Wheaton DL, Manahan-Wahl LJ, et al. Lymphocyte subsets in lung cancer. Chest 1987;91:725-9. [PubMed]
- Zikos TA, Donnenberg AD, Landreneau RJ, et al. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother 2011;60:819-27. [PubMed]
- Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002;168:4272-6. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006;6:295-307. [PubMed]
- Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006;90:297-339. [PubMed]
- Collins AV, Brodie DW, Gilbert RJ, et al. The interaction properties of costimulatory molecules revisited. Immunity 2002;17:201-10. [PubMed]
- van der Merwe PA, Bodian DL, Daenke S, et al. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med 1997;185:393-403. [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [PubMed]
- Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-9. [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37.
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [PubMed]
- Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010;207:2175-86. [PubMed]
- Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010;207:2187-94. [PubMed]
- Apetoh L, Tesniere A, Ghiringhelli F, et al. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008;68:4026-30. [PubMed]
- Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 2003;170:4905-13. [PubMed]
- Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010;120:1111-24. [PubMed]
- Jure-Kunkel M, Masters G, Girit E, et al. Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunol Immunother 2013;62:1533-45. [PubMed]
- Lee F, Jure-Kunkel MN, Salvati ME. Synergistic activity of ixabepilone plus other anticancer agents: preclinical and clinical evidence. Ther Adv Med Oncol 2011;3:11-25. [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Brahmer JR, Horn L, Antonia SJ, et al. Survival and long-term follow-up of the phase I trial of nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in patients (pts) with previously treated advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8030.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Spigel DR, Gettinger SN, Horn L, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8008.
- Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013;5:200ra116.
- Holt GE, Podack ER, Raez LE. Immunotherapy as a strategy for the treatment of non-small-cell lung cancer. Therapy 2011;8:43-54. [PubMed]
- Brahmer JR. Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol 2013;31:1021-8. [PubMed]
- Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013;31:2396-403. [PubMed]
- Ulloa-Montoya F, Louahed J, Dizier B, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 2013;31:2388-95. [PubMed]
- Sharma S, Srivastava MK, Harris-White M, et al. MUC1 peptide vaccine mediated antitumor activity in non-small cell lung cancer. Expert Opin Biol Ther 2011;11:987-90. [PubMed]
- Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 2005;23:6674-81. [PubMed]
- Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 2011;137:1337-42. [PubMed]
- Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res 2007;13:s4652-4. [PubMed]
- Butts CA, Socinski MA, Mitchell P, et al. START: a phase III study of L-BLP25 cancer immunotherapy for unresectable stage III non-small cell lung cancer. J Clin Oncol 2013;31:abstr 7500.
- Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol 2008;3:735-44. [PubMed]
- Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol 2011;12:1125-33. [PubMed]
- Rodríguez PC, Rodríguez G, González G, et al. Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small-cell lung cancer therapy. MEDICC Rev 2010;12:17-23. [PubMed]
- Neninger Vinageras E, de la Torre A, Osorio Rodríguez M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol 2008;26:1452-8. [PubMed]
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [PubMed]
- McCaffery M, Yao TJ, Williams L, et al. Immunization of melanoma patients with BEC2 anti-idiotypic monoclonal antibody that mimics GD3 ganglioside: enhanced immunogenicity when combined with adjuvant. Clin Cancer Res 1996;2:679-86. [PubMed]
- Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guérin. Clin Cancer Res 1999;5:1319-23. [PubMed]
- Nemunaitis J, Nemunaitis M, Senzer N, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 2009;16:620-4. [PubMed]
- Bazhenova L, Carrier E, Shawler D, et al. Long-term survival in a phase II study of belagenpumatucel-L (TGF-β antisense modified tumor cell vaccine) in non-small cell lung cancer (NSCLC). In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31-Apr 4; Chicago, IL. Abstract nr 5367.
- Morris JC, Rossi GR, Harold N, et al. Potentaial chemo-sensitization effect of tergenpumatucel-L immunotherapy in treated patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8094.
- Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225-31. [PubMed]


