Current standards for clinical management of small cell lung cancer
Introduction
Small cell lung cancer (SCLC) is an aggressive high-grade neuroendocrine malignancy with high metastatic potential and poor clinical outcomes. First characterized as a tumor of lung origin and described as “oat-celled sarcoma” in 1926 (1) (though SCLC is in fact a carcinoma), SCLC was recognized early as a cancer that is initially highly sensitive to cytotoxic chemotherapy. Indeed, there was initial hope that, with enough cytotoxic therapy, metastatic SCLC could be curable. However, despite over 30 years of clinical trials designed to improve therapies for SCLC, the vast majority of SCLCs remain incurable. The median overall survival (mOS) for patients with metastatic SCLC receiving standard chemotherapy has remained in range of 9–11 months over the past 20+ years, even in the most recent large randomized clinical trials (2-6). While there is tremendous hope for therapeutic breakthroughs to come from improved preclinical models, promising new therapeutic strategies, and innovative clinical trials, it is instructive to review the current clinical management strategies for patients diagnosed with SCLC today. Here, we provide an overview of current clinical standards in both medical oncology and radiation oncology, and we review the data supporting those strategies.
Diagnosis and staging of SCLC
SCLC has a classic radiographic presentation with bulky hilar and mediastinal lymph node involvement (Figure 1), which is frequently accompanied by metastatic spread, often involving liver, adrenals, bones and/or brain. Interestingly, SCLC can often present with a small or even unidentified primary lesion in the lung, despite bulky lymph node involvement. However, not all SCLCs fit this classic radiographic presentation. Ultimately, making the diagnosis requires tissue confirmation, with fine needle aspirate (FNA), biopsy, or resection. Histopathologically, SCLC is classified by the 2015 WHO criteria among a spectrum of neuroendocrine tumors (7). SCLC is a high-grade neuroendocrine tumor, and is characterized by small cells with scant cytoplasm and nuclear features of fine, dispersed chromatin without distinct nucleoli. While the majority of SCLCs express at least one neuroendocrine marker detectable by immunohistochemistry, neuroendocrine marker detection is not required for diagnosis (8).
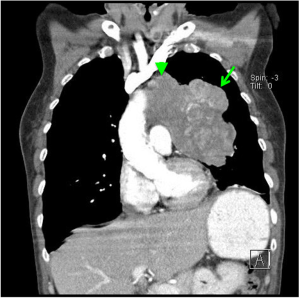
Clinical staging of any solid tumor is ultimately a question of “where is the cancer?” SCLC is typically staged using CT scans. In addition, the use of PET scan and brain MRI has increased the sensitivity for detection of distant metastases (9). Two staging systems are commonly used. The Veterans’ Administration Lung Study Group (VALSG) introduced a two-stage classification system in the 1950s which was later revised in 1989 [(10), and reviewed in (9)]. Briefly, this system classifies SCLC as limited-stage (LS), in which the disease is confined to an area within the thorax that can be encompassed within a radiation port, and extensive-stage (ES), in which disease cannot be classified as limited, and may include malignant pleural or pericardial effusions or metastases consistent with hematogenous spread.
The International Association for the Study of Lung Cancer (IASLC) subsequently proposed that the TNM lung cancer staging system be used in place of the VALSG system (11). In the TNM system, cancers are staged using tumor (T), nodal (N), and metastatic (M) parameters. An updated eighth edition of the TNM lung cancer staging system is anticipated within the next year (12), and again appears to have prognostic value (13). Although the TNM system is more precise, for practical purposes the VALSG system is often still used clinically, as further sub-classification by stage rarely impacts management.
Limited stage SCLC
Approximately 30% of patients with SCLC present with early stage disease, classified as limited stage by the VALSG system or as M0 by the TNM system. A small subset of these patients present without clinical or pathologic evidence of mediastinal lymph node involvement. For those T1–T2 N0 M0 SCLCs, surgical resection is recommended for patients with sufficient performance status (14,15). While there are no prospective studies assessing the benefit of adjuvant chemotherapy in this setting, a retrospective series of 1,574 cases in the National Cancer Database between 2003 and 2011 indicates that overall survival (OS) was improved among those patients who received adjuvant chemotherapy with or without adjuvant radiation (16). Furthermore, in a retrospective review of 82 patients at Johns Hopkins University who underwent surgical resection of SCLC, outcomes were improved for those who received platinum-based neoadjuvant or adjuvant therapy compared to non-platinum regimens (17). Thus, platinum-based adjuvant therapy is generally recommended following surgical resection of any early-stage, node-negative SCLC.
The vast majority of LS-SCLCs have mediastinal lymph node involvement at the time of diagnosis. The optimal treatment for these cancers is concurrent chemotherapy and radiation (15). This issue remained controversial until the early 1990s. Previously, numerous randomized trials had been conducted to assess the effect of thoracic radiotherapy in patients with LS-SCLC, but most were not sufficiently powered to detect differences in survival. Subsequently, two meta-analyses published in 1992 established that concurrent chemotherapy and RT improves survival and local control of disease compared to chemotherapy alone (18,19). In the larger of the two studies, which included 13 trials and 2,140 patients with limited disease, with a median follow up of 43 months, the relative risk of death in the concurrent therapy group compared to the chemotherapy alone group was 0.86, and the OS benefit at 3 years was 5.4% (19).
The optimal radiation schedule for LS-SCLC remains controversial. Turrisi et al. randomized 417 patients to receive a total of 45 Gy radiotherapy, either once-daily (1.8 Gy in 25 fractions) or twice-daily (1.5 Gy in 30 fractions) with concurrent cisplatin and etoposide (VP-16) (20). After a median follow up of nearly 8 years, there was a significant difference in median survival, 19 months for the once-daily group and 23 months for the twice-daily group (21). Widespread implementation of this method was limited (22) by the logistical difficulties associated with twice-daily treatment, as well as the concern that 45 Gy in once-daily fractions was not biologically equivalent to 45 Gy in twice-daily fractions. Other studies have examined outcomes in patients treated with a 60–70 Gy of once-daily radiation with concurrent chemotherapy (23-26). A dose-escalation study demonstrated that the maximum tolerated dose for once-daily treatment was 70 Gy, rather than 45 Gy (23). This regimen is considered safe and efficacious.
Recently, the CONVERT trial randomized 547 patients to receive either 45 Gy in 30 twice-daily fractions of 1.5 or 66 Gy in 33 once-daily fractions of 2 Gy, each with 4 to 6 cycles of concurrent cisplatin/etoposide (EP) (27). After a median follow up of 45 months, the mOS was 30 months in the twice-daily group at 25 months in the once-daily group (hazard radio for death in the once-daily group 1.18, 95% CI, 0.95–1.45, P=0.14). Toxicity did not significantly differ in each arm. This trial failed to demonstrate superiority of the once-daily regimen, and was insufficiently powered to demonstrate equivalence of the regimens. Thus, while twice-daily radiation to 45 Gy remains the standard of care in this setting, once-daily radiation to 60–70 Gy is often more feasible, and either regimen is generally considered acceptable for management of LS-SCLC. This question may be further clarified by the CALGB 30610/RTOG 0538 study comparing 45 Gy twice-daily to 70 Gy once-daily, which is underway with an estimated primary completion date of June 2023 (NCT00632853). The optimal timing of radiation is to start within the first two cycles of chemotherapy (28-31).
The preferred chemotherapy backbone for treatment of LS-SCLC is cisplatin and etoposide. A study published in 2002 randomized 436 patients to first-line systemic therapy with EP or cyclophosphamide, epirubicin, and vincristine (CEV) (32). Among the randomized patients, 214 had limited stage disease. In this cohort, the median survival was 14.5 versus 9.7 months and the 2-year OS was 25% versus 10%, in patient treated with EP vs. CEV, respectively. Four to six cycle of chemotherapy are recommended by the NCCN (15).
For patients who achieve a complete response, partial response or stable disease with chemoradiotherapy, subsequent prophylactic cranial irradiation (PCI) is generally recommended. A meta-analysis of PCI in patients with a complete response after induction chemotherapy analyzed seven trials involving 987 patients. Among a mix of limited and extensive stage patients, there was an improvement in 3-year survival of approximately 5.4% (15.3% in control arm vs. 20.7% in treatment arm). The incidence of future brain metastases was decreased from 58.6% to 33.3% (33). Twenty-five Gy is generally considered standard of care (34). There is also increasing interest in the role of hippocampal-avoiding radiotherapy, a technique which minimizes radiotherapy to the hippocampus, for PCI in LS-SCLC. Patients with SCLC were excluded from the initial trials of HA-WBRT (35), but randomized trials in SCLC are now ongoing.
Extensive stage SCLC: systemic therapy
Extensive stage SCLC is generally not considered curable, and is managed palliatively with therapies aimed at prolonging life and reducing symptoms associated with disease. The most commonly used first-line systemic therapy in the United States and Europe is platinum (cisplatin or carboplatin) combined with etoposide. Table 1 summarizes a selection of randomized studies, reported between 1991 and 2017, in which platinum/etoposide was compared to an investigational arm. The response rates, median PFS and median OS for the platinum/etoposide arm in each study are shown in Table 1.
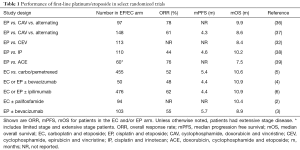
Full table
There are several notable points to make from reviewing these studies. First, none showed superiority of the comparator arm versus platinum/etoposide. Thus, platinum/etoposide has remained the standard of care for first-line management of SCLC for nearly 30 years. Second, the response rates to platinum/etoposide range from 44% to 78%. This reflects the clinical observation that many SCLCs are highly sensitive to platinum/etoposide in the first-line setting, and as a result, this is often the first choice for managing a patient with symptomatic disease. Indeed, the NCCN guidelines advise consideration of systemic therapy even for patients with poor performance status due to SCLC, reflecting the fact that many patients have clinical response and improvement of symptoms with initiation of systemic therapy. Third, the median PFS and median OS remain fairly consistent across studies, despite the fact that these studies span over 25 years. Even in the most recent randomized first-line trials, the mOS from the time of diagnosis is in the range of 9 to 11 months. Therefore, platinum/etoposide remains a reliable regimen for obtaining a rapid response (and often clinical improvement), but these responses are short-lived and long-term outcomes remain poor.
Irinotecan combined with carboplatin or cisplatin is also an acceptable regimen as first-line treatment of SCLC, and is commonly used in Japan. The Japan Clinical Oncology Group randomized 154 previously untreated SCLC patients to cisplatin/irinotecan (IP) or EP. The mOS in the IP arm was statistically superior than in the EP arm (12.8 vs. 9.4 months, P=0.002), and the 1-year survival rates were 58.4% versus 37.3% (40). However, it was unclear whether this difference would be seen in a larger study and in a population outside of Japan. A subsequent randomized study of 331 patients enrolled at sites in the United States, Canada and Australia failed to demonstrate a significant improvement in outcomes with IP compared to EP (38); response rate was 48% versus 43.6%, median time to progression was 4.1 versus 4.6 months, and mOS was 9.3 versus 10.2 months. Tolerability was similar in each arm, though the specific spectrum of adverse events varied depending on the treatment. Specifically, in the EP arm there were significantly higher rates of grade 3–4 neutropenia (86.5% with EP versus 36.2% with IP), febrile neutropenia (10.4% versus 3.7%) and anemia (11.5% versus 4.8%), whereas grade 3–4 diarrhea was more common in the IP arm (0% vs. 21.3%). Two other randomized trials in North America and Europe have further confirmed the equivalent efficacy of EP and IP in these populations, as well as their distinct toxicity profiles (41,42). Thus, outside of Japan, platinum/etoposide is generally considered standard of care, though platinum/irinotecan is also an acceptable regimen.
Several trials have compared cisplatin- to carboplatin-based regimens in ES-SCLC. A 2012 meta-analysis of four trials with 663 patients found no significant differences in response rates (67.1% vs. 66.0%), median PFS (5.5 vs. 5.3 months), or median OS (9.6 vs. 9.4 months) (43). Notably, rates of hematologic toxicity were higher among patients treated with carboplatin, whereas rates of nonhematologic toxicity were higher among patients treated with cisplatin. Therefore, either carboplatin or cisplatin can be used as a backbone of first-line therapy.
Topotecan is the only FDA- and EMA-approved second line therapy in SCLC. Intravenous topotecan was studied in the second-line setting in a phase 2 study published in 1997 (44). There were two groups of patients enrolled, refractory or sensitive, defined as having failed first-line therapy ≤3 or >3 months, respectively, after completion of first-line therapy. Patients were treated with IV topotecan 1.5 mg/m2 days 1–5 of each 21-day cycle. There were 47 refractory and 45 sensitive patients evaluable for response. The overall response rates (ORR) in the refractory and sensitive groups were 6.4% and 37.8%, respectively. The mOS in the groups was 4.7 and 6.9 months. A randomized study published subsequently compared IV topotecan to cyclophosphamide, doxorubicin and vincristine (CAV) (45). ORRs in the topotecan and CAV arms were 24.3% and 18.3%, respectively, though not statistically different. Median OS was also highly similar, 25.0 versus 24.7 weeks.
In addition to the IV form, oral topotecan (2.3 mg/m2/d days 1–5 of each 21-day cycle) has also gained FDA and EMA approval for second-line treatment of SCLC. Compared to best supportive care, oral topotecan leads to improvement in mOS (25.9 versus 13.9 weeks), a slower quality of life decline, and greater symptom control (46). Among patients with a treatment-free interval following completion of first-line therapy of at least 90 days, oral and IV topotecan demonstrated similar efficacy and tolerability (47).
To date, no randomized trial has demonstrated superiority of an experimental arm over topotecan for second-line treatment of SCLC (48,49). However, multiple other chemotherapies have shown activity comparable to topotecan in the second-line setting, including irinotecan (50), paclitaxel (51,52), docetaxel (53), gemcitabine (54,55), vinorelbine (56,57), and temozolomide (58,59). Regardless of the cytotoxic regimen used, patients with “sensitive” disease (relapse >90 days after completion of first line therapy) overall have better outcomes than patients with “refractory” disease. An analysis of 21 studies published between 1984 and 2001 demonstrated that among 921 patients with sensitive disease and 780 patients with refractory disease, response rates to second-line therapy were significantly higher in the sensitive group (27.7% vs. 14.8%) (60). mOS was also improved, 7.7 versus 5.4 months. The specific selection of cytotoxic second-line and subsequent regimens varies widely among clinicians (38), and likely depends on patient specific characteristics and physician preference. There is no FDA approved therapy in the third-line setting or beyond.
Recent data demonstrate that immune checkpoint inhibitors can also have activity in a subset of patients with relapsed SCLC. The most extensive data presented to date are from the Checkmate-032 study, in which patients received the PD-1 nivolumab either alone or in combination with the CTLA-4 inhibitor ipilimumab (61). A recent update was presented at the 2017 ASCO annual meeting (62). Patients with previously treated SCLC were enrolled to either a non-randomized cohort or a randomized cohort, and were treated with either nivolumab alone 3 mg/kg Q2 weeks or nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3 weeks ×4 cycles, followed by nivolumab monotherapy 3 mg/kg Q2 weeks, until progression of unacceptable toxicity. Among all patients treated (245 with nivolumab alone, and 156 with nivolumab plus ipilimumab) the ORRs were 11% and 22%, respectively. The frequencies of grade 3–4 toxicities were 12% and 37%. Responses were observed regardless of platinum sensitivity, line of therapy or PD-L1 status. Nivolumab with or without ipilimumab are now among the NCCN-recommended regimens for relapsed SCLC (15).
Interestingly, a recent study indicates that tumor mutational burden (TMB) may be a useful biomarker to predict likelihood of response to immune checkpoint inhibitors in SCLC (63). An exploratory analysis of patients on CheckMate 032 whose tumors were evaluable for TMB (n=133 in the nivolumab arm and n=78 in the nivolumab plus ipilimumab arm) found improved outcomes among patients with high TMB compared to those with medium or low TMB in both arms. The results were most striking in the nivolumab plus ipilimumab arm, where among patients with TMB high vs. medium vs. low, response rates were 46.2% vs. 16.0% vs. 22.2%; median progression free survival (mPFS) was 7.8 vs. 1.3 vs. 1.5 months; and median OS was 22.0 vs. 3.6 vs. 3.4 months. While these data are exploratory, further prospective assessment of TMB as a biomarker of sensitivity to immune checkpoint inhibitors in SCLC is certainly warranted.
Extensive stage SCLC: radiation therapy
While PCI is generally recommended in patients with LS SCLC after chemoradiotherapy, the role of PCI in patients with ES SCLC is an area of debate. The initial data in support of PCI in ES-SCLC were based on patients who did not undergo intracranial staging with MRI or CT. EORTC 22993 (64) randomized 286 patients with ES-SCLC and a response to 4–6 cycles of chemotherapy to PCI versus observation. There was a significant reduction in the risk of symptomatic brain metastases (HR 0.27, 95% CI, 0.16–0.44, P<0.001) and an increase in median OS (6.7 vs. 5.4 months, P=0.003) with PCI. However, brain imaging was not required for staging or follow-up unless patients were symptomatic, and only 29% of patients had brain imaging as time of diagnosis, raising concern that the survival benefit from PCI was driven in part by patients with asymptomatic brain metastases at the time of study enrollment. Moreover, PCI is also associated with a decline in both short-term quality of life (64) and long-term cognitive functioning (65). Recently, a multi-institutional Japanese trial (66) assessed the impact of PCI in patients with MRI staging and surveillance. This trial randomized 224 patients with ES-SCLC, response to chemotherapy and negative brain MRI to PCI versus observation. All patients had MRIs prior to study enrollment, at 3-month intervals for 1 year and then at 18 and 24 months. The study was terminated early due to futility on interim analysis. While there was a reduction in brain metastases with PCI, this did not translate to an improvement in survival, with a mOS of 11.6 months with PCI vs. 13.7 months with observation (HR 1.27, 95% CI, 0.96–1.68, P=0.094). Therefore, while PCI has been shown to decrease the rate of brain metastases, given the potential associated toxicities of PCI and lack of survival benefit in recent trials, careful consideration should be given before proceeding with PCI. Those patients who do not receive PCI must continue with regular MRI surveillance to ensure prompt initiation of salvage whole brain radiotherapy in the event of intracranial progression. As the emergence of new brain metastases can also be seen following PCI, surveillance of this group is also warranted, though salvage options may be limited.
Despite initial response to chemotherapy, most patients with ES-SCLC will ultimately develop intrathoracic disease progression. For those patients with symptomatic disease progression, radiotherapy is often offered for palliation. However, given the risk of local disease progression, questions have arisen as to the role of consolidative thoracic radiotherapy in ES-SCLC, particularly in those patients with minimal extrathoracic disease burden. A single-institution randomized trial (67) demonstrated an improvement in both local control and OS in patients with a complete distant response and at least partial local response to chemotherapy who received chemotherapy with concurrent thoracic radiotherapy and PCI versus continued chemotherapy and PCI. A single-arm phase II trial (68) and retrospective series also demonstrated an improvement in local disease control with thoracic radiotherapy in carefully selected populations of patients with limited extra-thoracic disease burden (69,70). The CREST Trial/NTR1527 (71) randomized 498 patients with ES-SCLC and response to chemotherapy to thoracic radiotherapy to 30 Gy in 10 fractions and PCI versus PCI alone. While the primary endpoint of an improvement in 1-year OS was not met, there was a significant improvement in 2-year OS with the use of thoracic radiotherapy (13% vs. 3%, P=0.004). A meta-analysis of the Jeremic trial and the CREST trial showed a 20% improvement in OS and 25% improvement in progression-free survival with consolidative thoracic radiotherapy (72). Careful consideration should be given to consolidative thoracic radiotherapy in patients with response to chemotherapy and primarily intrathoracic disease burden.
Consolidative radiotherapy to multiple sites of metastasis has also been explored, specifically in RTOG 0937, a phase II trial which randomized 97 patients with complete or partial response to chemotherapy to PCI with or without consolidative radiotherapy to the thorax and up to 4 extracranial metastases (73). While there was an improvement in progression-free survival with the use of consolidative radiotherapy, there was no associated improvement in OS. Survival was higher than predicted for both study arms. Of note, more patients in the consolidative radiotherapy arm had 2–4 metastases as opposed to 1 metastasis, as well as a higher rate of partial response as opposed to a complete response after chemotherapy. Further study is needed to determine if there is a favorable group which would benefit from consolidative radiotherapy to multiple sites, but based on the current data, we would not recommend consolidative radiotherapy to multiple sites of metastasis in ES-SCLC.
Future directions
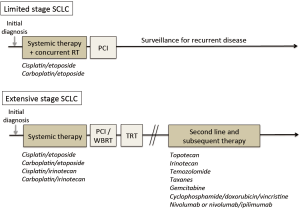
A simplified overview to SCLC clinical management is shown in Figure 2. Although the standard clinical management of SCLC has changed little in the past 20 years, there are emerging reasons to remain hopeful that significant improvements in outcomes are attainable. Newer preclinical model systems and comprehensive molecular profiling studies have begun to uncover new targets in SCLC, and at least some of these are now maturing into clinical trials. Furthermore, rather than the older approach of adding more cytotoxic agents, newer strategies are focusing on different pathways within cancer cells (such as targeting DLL3 with an antibody-drug conjugate, inhibiting PARP, modulating transcription, and others) as well as now modulating the immune system. We anticipate that the next 20 years will see far more progress in SCLC than the prior, and that outcomes for patients with this disease will improve substantially.
Acknowledgements
We thank our colleagues in thoracic oncology at Massachusetts General Hospital.
Footnote
Conflicts of Interest: AF Farago acts consulting or advisory role for Abbive, Pharmamar, Merrimack Pharmaceuticals, Takeda, Intervention Insights, Honorarium from Foundation Medicine. FK Keane has no conflicts of interest to declare.
References
- Barnard WG. The nature of the “oat-celled sarcoma” of the mediastinum. J Pathol 1926;29:241-4. [Crossref]
- Jalal SI, Lavin P, Lo G, et al. Carboplatin and Etoposide With or Without Palifosfamide in Untreated Extensive-Stage Small-Cell Lung Cancer: A Multicenter, Adaptive, Randomized Phase III Study (MATISSE). J Clin Oncol 2017;35:2619-23. [Crossref] [PubMed]
- Tiseo M, Boni L, Ambrosio F, et al. Italian, Multicenter, Phase III, Randomized Study of Cisplatin Plus Etoposide With or Without Bevacizumab as First-Line Treatment in Extensive-Disease Small-Cell Lung Cancer: The GOIRC-AIFA FARM6PMFJM Trial. J Clin Oncol 2017;35:1281-7. [Crossref] [PubMed]
- Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215-22. [Crossref] [PubMed]
- Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol 2009;27:4787-92. [Crossref] [PubMed]
- Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:3740-8. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Guinee DG Jr, Fishback NF, Koss MN, et al. The spectrum of immunohistochemical staining of small-cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am J Clin Pathol 1994;102:406-14. [Crossref] [PubMed]
- Kalemkerian GP. Staging and imaging of small cell lung cancer. Cancer Imaging 2012;11:253-8. [Crossref] [PubMed]
- Stahel RA, Ginsberg R, Havermann K, et al. Staging and prognostic factors in small cell lung cancer: a consensus report. Lung Cancer 1989;5:119-26. [Crossref]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:300-11.
- Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9:1132-9. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology, Small Cell Lung Cancer. 2017. Available online: https://www.tri-kobe.org/nccn/guideline/lung/english/small.pdf
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. [Crossref] [PubMed]
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890-5. [Crossref] [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [Crossref] [PubMed]
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [Crossref] [PubMed]
- Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;59:943-51. [Crossref] [PubMed]
- Farrell MJ, Yahya J, Degnin C, et al. OA01.05 Radiation Dose and Fractionation for Limited-Stage Small Cell Lung Cancer: A Survey of US Radiation Oncologists on Practices. J Thorac Oncol 2017;12:S1550. [Crossref]
- Choi NC, Herndon JE 2nd, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol 1998;16:3528-36. [Crossref] [PubMed]
- Miller KL, Marks LB, Sibley GS, et al. Routine use of approximately 60 Gy once-daily thoracic irradiation for patients with limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:355-9. [Crossref] [PubMed]
- Roof KS, Fidias P, Lynch TJ, et al. Radiation dose escalation in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;57:701-8. [Crossref] [PubMed]
- Bogart JA, Herndon JE 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys 2004;59:460-8. [Crossref] [PubMed]
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116-25. [Crossref] [PubMed]
- Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol 2004;22:4837-45. [Crossref] [PubMed]
- Huncharek M, McGarry R. A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist 2004;9:665-72. [Crossref] [PubMed]
- De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 2006;17:543-52. [Crossref] [PubMed]
- De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol 2006;24:1057-63. [Crossref] [PubMed]
- Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol 2002;20:4665-72. [Crossref] [PubMed]
- Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Le Pechoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467-74. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855-61. [Crossref] [PubMed]
- Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282-91. [Crossref] [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [Crossref] [PubMed]
- Baka S, Califano R, Ferraldeschi R, et al. Phase III randomised trial of doxorubicin-based chemotherapy compared with platinum-based chemotherapy in small-cell lung cancer. Br J Cancer 2008;99:442-7. [Crossref] [PubMed]
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91. [Crossref] [PubMed]
- Schmittel A, Fischer von Weikersthal L, Sebastian M, et al. A randomized phase II trial of irinotecan plus carboplatin versus etoposide plus carboplatin treatment in patients with extended disease small-cell lung cancer. Ann Oncol 2006;17:663-7. [Crossref] [PubMed]
- Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530-5. [Crossref] [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [Crossref] [PubMed]
- Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 1997;15:2090-6. [Crossref] [PubMed]
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658-67. [Crossref] [PubMed]
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [Crossref] [PubMed]
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086-92. [Crossref] [PubMed]
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012-9. [Crossref] [PubMed]
- Evans TL, Cho BC, Udud K, et al. Cabazitaxel Versus Topotecan in Patients with Small-Cell Lung Cancer with Progressive Disease During or After First-Line Platinum-Based Chemotherapy. J Thorac Oncol 2015;10:1221-8. [Crossref] [PubMed]
- Masuda N, Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 1992;10:1225-9. [Crossref] [PubMed]
- Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998;77:347-51. [Crossref] [PubMed]
- Yamamoto N, Tsurutani J, Yoshimura N, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res 2006;26:777-81. [PubMed]
- Smyth JF, Smith IE, Sessa C, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. The Early Clinical Trials Group of the EORTC. Eur J Cancer 1994;30A:1058-60. [Crossref] [PubMed]
- van der Lee I, Smit EF, van Putten JW, et al. Single-agent gemcitabine in patients with resistant small-cell lung cancer. Ann Oncol 2001;12:557-61. [Crossref] [PubMed]
- Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol 2003;21:1550-5. [Crossref] [PubMed]
- Jassem J, Karnicka-Mlodkowska H, van Pottelsberghe C, et al. Phase II study of vinorelbine (Navelbine) in previously treated small cell lung cancer patients. EORTC Lung Cancer Cooperative Group. Eur J Cancer 1993;29A:1720-2. [Crossref] [PubMed]
- Furuse K, Kubota K, Kawahara M, et al. Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Japan Lung Cancer Vinorelbine Study Group. Oncology 1996;53:169-72. [Crossref] [PubMed]
- Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012;18:1138-45. [Crossref] [PubMed]
- Zauderer MG, Drilon A, Kadota K, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 2014;86:237-40. [Crossref] [PubMed]
- Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866-72. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo) +- ipilimumab (ipi) in advanced small-cell lung cancer: First report of randomized expansion cohort from CheckMate 032. J Clin Oncol 2017;35:abstr 8503.
- Rizvi N, Antonia S, Callahan MK, et al. Impact of tumor mutation burden on the efficacy of nivolumab or nivolumab plus ipilimumab in small cell lung cancer: An exploratory analysis of CheckMate 032. 2017 World Conference on Lung Cancer. Abstract OA 07.03a.
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [Crossref] [PubMed]
- Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys 2013;86:656-64. [Crossref] [PubMed]
- Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663-71. [Crossref] [PubMed]
- Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol 1999;17:2092-9. [Crossref] [PubMed]
- Yee D, Butts C, Reiman A, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol 2012;102:234-8. [Crossref] [PubMed]
- Zhu H, Zhou Z, Wang Y, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer 2011;117:5423-31. [Crossref] [PubMed]
- Giuliani ME, Atallah S, Sun A, et al. Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy. Clin Lung Cancer 2011;12:375-9. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- Palma DA, Warner A, Louie AV, et al. Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Meta-Analysis. Clin Lung Cancer 2016;17:239-44. [Crossref] [PubMed]
- Gore EM, Hu C, Sun AY, et al. Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. J Thorac Oncol 2017;12:1561-70. [Crossref] [PubMed]


