Repurposing established cyclic adenosine monophosphate reducing agents for the prevention and therapy of epidermal growth factor receptor inhibitor resistance in non-small cell lung cancer
Introduction
Non-small cell lung cancer (NSCLC) is a family of cancers comprised of four different histological subtypes: adenocarcinoma, squamous cell carcinoma, large cell carcinoma and carcinoids. Among the NSCLC subtypes, adenocarcinoma is the most common with incidence rising (1). Adenocarcinomas often express activating mutations of the epidermal growth factor receptor (EGFR) and these mutations are predictive of responsiveness to cancer therapy with EGFR tyrosine kinase inhibitors (2). Unfortunately, resistance to these agents always develops due to the formation of additional mutations, of which only one type (EGFR T790M mutation) responds to newer generation EGFR inhibitors (3).
Beta-adrenergic receptors (β1, β2, β3-ARs) are members of the seven-transmembrane receptor family coupled to the stimulatory G-protein Gs that activates adenylyl cyclase (AC), a required step for the formation of intracellular cyclic adenosine monophosphate (cAMP) (4). The activation of AC is inhibited by receptors coupled to the inhibitory G-protein Gi and balanced Gs versus Gi signaling ensures cAMP homeostasis (5).
The regulatory role of β-ARs in the cardiovascular system is well established and has provided the rational for the use of β-AR antagonists (β-blockers) as cardiovascular therapeutics (6). The growth stimulating function of this receptor family on lung adenocarcinomas was first reported by our laboratory and involved the cAMP-driven release of arachidonic acid (AA) that activates the AA-cascade, resulting in the activation of cAMP response element binding protein (CREB) and extracellular signal-regulated kinase (ERK) (7) downstream of Gs-coupled receptors of the prostaglandin E2 (PGE2) family while simultaneously transactivating the EGFR (8). In addition, it has been shown that the downstream effectors of β-ARs, cAMP and activated protein kinase A (PKA), cause the release of EGF (9), interleukin-6 (IL-6) (10) as well as vascular endothelial growth factor (VEGF) (11), all of which stimulate NSCLC development and progression at the levels of cell proliferation and angiogenesis. Moreover, we have identified the potent nicotine derived tobacco carcinogen N-nitroso-nicotine ketone (NNK) as a high affinity agonist for β1 and β2-ARs with strong pro-proliferative activity in human lung adenocarcinoma cell lines via β-adrenergic signaling (7), a mechanism that may contribute to the development of this cancer in smokers. NNK additionally induced NSCLC of the adenocarcinoma subtype in Syrian golden hamsters, and the development of these lung tumors was prevented by the general β-blocker propranolol whereas treatment with epinephrine had tumor promoting effects (12).
The inhibitory neurotransmitter γ-aminobutyric acid (GABA) as well as opioids and endogenous opioid peptides inhibited the growth of adenocarcinoma cell lines in vitro and in mouse xenografts by blocking cAMP formation via their respective Gi-coupled receptors (13-16). In accord with the function of the stress neurotransmitters epinephrine and norepinephrine as physiological β-AR agonists, social stress significantly promoted the development and progression of NSCLC xenografts in mice (17). This effect was accompanied by increases in the systemic levels of epinephrine, norepinephrine, cortisol and cAMP, with increased tumor levels of cAMP, p-CREB and p-ERK while tumor levels of GABA and its two synthesizing enzymes GAD65 and GAD67 were reduced (17). All of these cancer promoting responses of social stress were inhibited by treatment of the mice with GABA (17).
Cancer stem cells enriched from lung adenocarcinoma cell lines by selective culture conditions in spheroid formation assays responded with a significant increase in stem cell self-renewal to epinephrine, an effect accompanied by significant increases in the levels of the NSCLC cancer stem cell markers sonic hedgehog (SHH) and aldehyde dehydrogenase-1 (ALDH-1) (16). The stimulating in vivo effects of stress neurotransmitters on the cancer stem cell driven progression of NSCLC was corroborated by findings that systemic reductions in epinephrine and norepinephrine as determined in serum samples in a mouse model of stress reduction significantly reduced the development and progression of NSLC xenografts. while simultaneously reducing the tumor levels of cAMP, p-CREB, p-ERK, P-AKT, p-Src, VEGF, SHH and ALDH-1 whereas the expression of pro-apoptotic proteins increased (16). Interestingly, stress reduction also significantly increased serum levels of GABA and the endogenous opioid peptides met-enkephalin, dynorphin A and dynorphin B, indicating that the observed inhibitory effects of stress reduction on NSCLC were mediated by Gi-coupled GABAB receptors (GABAB-Rs) and delta and kappa-opioid receptors (DORs, KORs) that are also coupled to Gi . In vitro spheroid formation assays with NSCLC cancer stem cells identified strong reductions in intracellular cAMP and the cancer stem cell markers SHH, Notch-1 and ALDH-1 in response to each of these agents accompanied by complete inhibition of cancer stem cell self renewal (16). Moreover, it has been shown that inhibition of SHH signaling or depletion of its downstream transcription factor GLi1 enhanced the therapeutic effects of EGFR tyrosine kinase inhibitors in cancer stem cells from NSCLC cell lines (18), suggesting an important role of SHH signaling in the development of EGFR inhibitor resistance. Collectively, these findings underline the key role of increased intracellular cAMP in the activation of multiple pathways that drive the development, progression and EGFR inhibitor resistance of NSCLC and suggest that agents that inhibit the formation of intracellular cAMP, including β-blockers as well as agonists of Gi-coupled receptors such as GABA, endogenous opioid peptides and opioid drugs may be useful for the prevention and therapy of NSCLC.
Novel findings
A recent publication in Sci Transl Med (19) has reported a novel regulatory function of β-ARs in EGFR mutant NSCLC cell lines exposed to epinephrine and in mouse xenografts under psychological stress conditions: the β2-AR-mediated, cAMP-dependent promotion of EGFR inhibitor resistance via inactivation of tumor suppressing liver kinase B1 (LKB1), an effect caused by the β2-AR-stimulated release of interleukin-6 (IL-6) and abrogated by the general β-blocker propranolol or IL-6 antibodies. The investigators additionally found a positive association of high IL-6 levels with the development of EGFR inhibitor resistance in NSCLC patients and improved responsiveness to EGFR kinase inhibitor therapy in NSCLC patients with incidental β-blocker therapy (19). These findings are of immediate high clinical relevance as they suggest the off-label use of not only general β-blockers as concluded by the authors (19) but also other cAMP-inhibiting agents (Table 1) as promising adjunct therapy in NSCLC patients for the prevention and treatment of EGFR inhibitor resistance. The selective role of the β2-AR without contribution of β1-AR signaling to the observed promotion of EGFR resistance is of particular importance as they indicate that newer generation cardiovascular therapeutics that selectively block β1-ARs would not be suitable for this strategy.

Full table
Agents and lifestyle factors that reduce cAMP levels (Table 1)
There are numerous therapeutics for non-neoplastic diseases that inhibit cAMP formation by activating Gi-coupled receptors and these agents can be rapidly re-purposed for the adjuvant therapy of lung adenocarcinomas, including the prevention and treatment of EGFR inhibitor resistance. Nutritional over the counter (OTC) GABA supplements are widely used because of their anxiolytic and muscle relaxing effects. The level of endogenous GABA is also increased by valerian extract, a botanical OTC sleep aid that induces the GABA synthesizing enzymes GAD65 and GAD67 (20). Opioid drugs that signal through Gi-coupled opioid receptors (15) are used as analgesics and cough suppressants. The levels of endogenous opioid peptides that are agonists for the same receptors can be significantly increased by stress reduction/happiness (16). Medical marihuana reduces cAMP by binding to Gi-coupled cannaboid receptors (CB1, CB2) and the CB2 receptor additionally stimulates the release of endogenous β-endorphin (21). However, chronic treatment with such Gi-coupled receptor agonists can potentially de-sensitize the receptors, leading to tumor promoting effects. Positive allosteric modulators (PAMs) of Gi-coupled receptors circumvent this problem as they do not change receptor sensitivity. PAMs for the GABAB-Receptor are currently used for the treatment of drug addiction (22) while PAMs for opioid receptors are currently being developed (23).
Agents and lifestyle factors that increase cAMP levels (Table 2)
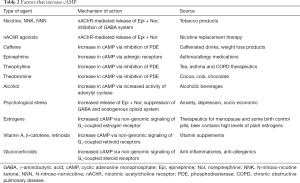
Full table
Caffeine contained in numerous beverages and in many weight loss products increases cAMP by inhibiting the phosphodiesterase (PDE) responsible for the enzymatic breakdown of cAMP and stimulates the in vitro growth of lung adenocarcinomas (24). Theophylline contained in tea, and additionally used for the treatment of asthma and chronic obstructive pulmonary disease (COPD) as well as theobromine contained in cocoa, chocolate and cola increase cAMP via the same mechanism as caffeine. Both, theophylline and green tea have been shown to promote the growth of lung adenocarcinoma in preclinical studies whereas they inhibited the development of neuroendocrine lung cancer (12,25).
Tobacco products and nicotine replacement therapy increase cAMP in response to the nicotinic acetylcholine receptor-mediated release of epinephrine and norepinephrine and simultaneous impairment of the endogenous GABA system (26). Alcohol consumption increases cAMP levels by inducing AC activity (27). Epinephrine, the physiological agonist for β-ARs, is contained in asthma inhalers and in many allergy medications and decongestants. The levels of endogenous epinephrine and norepinephrine are also increased in response to psychological stress (28) while simultaneously the levels of endogenous GABA and opioid peptides are decreased (29,30).
Estrogens and glucocorticoids previously thought to bind only to nuclear receptors increase cAMP levels by binding to non-genomic Gs-coupled cell membrane receptors (31). Vitamin A, β-carotene and retinoids initially believed to act through nuclear receptors increase cAMP via similar non-genomic signaling (32,33). In fact, this mechanism of action likely triggered the increase in lung adenocarcinoma incidence that lead to the discontinuation of a lung cancer prevention trial by β-carotene and retinoid supplementation (34). This interpretation is supported by observations that β-carotene stimulated the growth of lung adenocarcinoma cells in vitro via cAMP signaling (32) and promoted the development of NNK-induced lung adenocarcinomas in hamsters via this mechanism (33).
Conclusions
Clinical observations have corroborated the preclinical findings that cAMP signaling activates multiple pathways which drive the development and progression of NSCLC. It has thus been shown that incidental β-blocker therapy significantly improved survival in NSCLC patients (35) while overexpression of the Gi-coupled GABAB-R was associated with a better prognosis (36). In light of the prominent role of β2-ARs in the reported regulation of EGFR inhibitor resistance (19) the correct choice of β-blockers for this approach is of key importance. General beta-blockers such as propranolol are appropriate whereas selective β1-blockers are contra-indicated as they not only leave β2-ARs uninhibited but would even cause reactive sensitization of β2-ARs, resulting in enhanced responses to agonists (37). It is therefore no surprise that there are also studies that found no significant survival benefits in lung cancer patients who had received pre- or post-diagnostic treatment with beta-blockers (38). Alternatives to β-blocker therapy summarized in this editorial should be explored with preference given to PAMs of Gi-coupled receptors or agents and lifestyles that increase the levels of endogenous Gi-receptor agonists as these are less likely to desensitize their respective receptors than high affinity synthetic agonists. The powerful influence of the mood on cAMP signaling cannot be over-emphasized and any pharmacological attempts to reduce tumor promoting cAMP signaling have to be accompanied by careful avoidance of psychological stress, active stress reduction and targeted activities that convey feelings of happiness. In addition, the cAMP enhancing agents summarized in Table 2 have to be avoided as well and any cohort studies for the assessment of cAMP-reducing therapy have to be corrected for the potential influence of these agents on clinical outcomes. Finally, the goal of these strategies has to be the restoration of cAMP homeostasis and not its arbitrary blockage/elimination. In turn, this requires careful monitoring of systemic cAMP levels (e.g., in blood lymphocytes) analogous to the monitoring of blood sugar levels in diabetics.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Wynder EL, Muscat JE. The changing epidemiology of smoking and lung cancer histology. Environ Health Perspect 1995;103 Suppl 8:143-8. [Crossref] [PubMed]
- Levy M, Lyon L, Barbero E, et al. Histologic grade is predictive of incidence of epidermal growth factor receptor mutations in metastatic lung adenocarcinoma. Med Sci (Basel) 2017;5. [Crossref] [PubMed]
- Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Lefkowitz RJ. The superfamily of heptahelical receptors. Nat Cell Biol 2000;2:E133-6. [Crossref] [PubMed]
- Ferré S. The GPCR heterotetramer: Challenging classical pharmacology. Trends Pharmacol Sci 2015;36:145-52. [Crossref] [PubMed]
- Frishman WH. beta-Adrenergic blockade in cardiovascular disease. J Cardiovasc Pharmacol Ther 2013;18:310-9. [Crossref] [PubMed]
- Schuller HM, Tithof PK, Williams M, et al. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res 1999;59:4510-5. [PubMed]
- Laag E, Majidi M, Cekanova M, et al. NNK activates ERK1/2 and CREB/ATF-1 via beta-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int J Cancer 2006;119:1547-52. [Crossref] [PubMed]
- Grau M, Soley M, Ramirez I. Interaction between adrenaline and epidermal growth factor in the control of liver glycogenolysis in mouse. Endocrinology 1997;138:2601-9. [Crossref] [PubMed]
- Bernabe DG, Tamae AC, Biasoli ER, et al. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun 2011;25:574-83. [Crossref] [PubMed]
- Madden KS, Szpunar MJ, Brown EB. beta-Adrenergic receptors (beta-AR) regulate VEGF and IL-6 production by divergent pathways in high beta-AR-expressing breast cancer cell lines. Breast Cancer Res Treat 2011;130:747-58. [Crossref] [PubMed]
- Schuller HM, Porter B, Riechert A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J Cancer Res Clin Oncol 2000;126:624-30. [Crossref] [PubMed]
- Schuller HM, Al-Wadei HA, Majidi M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis 2008;29:1979-85. [Crossref] [PubMed]
- Al-Wadei HA, Al-Wadei MH, Ullah MF, et al. Gamma-amino butyric acid inhibits the nicotine-imposed stimulatory challenge in xenograft models of non-small cell lung carcinoma. Curr Cancer Drug Targets 2012;12:97-106. [Crossref] [PubMed]
- Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci U S A 1990;87:3294-8. [Crossref] [PubMed]
- Banerjee J, Papu John AM, Schuller HM. Regulation of non-small cell lung cancer stem cell like cells by neurotransmitters and opioid peptides. Int J Cancer 2015;137:2815-24. [Crossref] [PubMed]
- Al-Wadei HA, Plummer HK 3rd, Ullah MF, et al. Social stress promotes and gamma-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer Prev Res (Phila) 2012;5:189-96. [Crossref] [PubMed]
- Bora-Singhal N, Perumal D, Nguyen J, et al. Gli-Mediated regulation of SOX2 facilitates self-renewal of stem-like cells and confers resistance to EGFR inhibitors in non-small cell lung cancer. Neoplasia 2015;17:538-51. [Crossref] [PubMed]
- Nilsson MB, Sun H, Diao L, et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: Implications for combinations with β-blockers. Sci Transl Med 2017;9. [Crossref] [PubMed]
- Kakehashi A, Kato A, Ishii N, et al. Valerian inhibits rat hepatocarcinogenesis by activating GABA(A) receptor-mediated signaling. PLoS One 2014;9:e113610. [Crossref] [PubMed]
- Gao F, Zhang LH, Su TF, et al. Signaling mechanism of cannaboid receptor-2 activation-induced β-endorphin release. Mol Neurobiol 2016;53:3616-25. [Crossref] [PubMed]
- Filip M, Frankowska M, Sadakierska-Chudy A, et al. GABAB receptors as a therapeutic strategy in substance use disorders: focus on positive allosteric modulators. Neuropharmacology 2015;88:36-47. [Crossref] [PubMed]
- Livingston KE, Traynor JR. Allostery at opioid receptors: modulation with small molecule ligands. Br J Pharmacol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Al-Wadei HA, Takahashi T, Schuller HM. Caffeine stimulates the proliferation of human lung adenocarcinoma cells and small airway epithelial cells via activation of PKA, CREB and ERK1/2. Oncol Rep 2006;15:431-5. [PubMed]
- Schuller HM, Porter B, Riechert A, et al. Neuroendocrine lung carcinogenesis in hamsters is inhibited by green tea or theophylline while the development of adenocarcinomas is promoted: implications for chemoprevention in smokers. Lung Cancer 2004;45:11-8. [Crossref] [PubMed]
- Haass M, Kubler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther 1997;10:657-65. [Crossref] [PubMed]
- Qualls-Creekmore E, Gupta R, Yoshimura M. The effect of alcohol on recombinant proteins derived from mammalain adenylyl cyclase. Biochem Biophys Rep 2017;10:157-64. [Crossref] [PubMed]
- Robinson JD, Cinciripini PM. The effects of stress and smoking on catecholaminergic and cardiovascular response. Behav Med 2006;32:13-8. [Crossref] [PubMed]
- Hu W, Zhang M, Czeh B, et al. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 2010;35:1693-707. [Crossref] [PubMed]
- Knoll AT, Carlezon WA Jr. Dynorphin, stress, and depression. Brain Res 2010;1314:56-73. [Crossref] [PubMed]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 2012;153:2953-62. [Crossref] [PubMed]
- Al-Wadei HA, Takahashi T, Schuller HM. Growth stimulation of human pulmonary adenocarcinoma cells and small airway epithelial cells by beta-carotene via activation of cAMP, PKA, CREB and ERK1/2. Int J Cancer 2006;118:1370-80. [Crossref] [PubMed]
- Al-Wadei HA, Schuller HM. beta-Carotene promotes the development of NNK-induced small airway-derived lung adenocarcinoma. Eur J Cancer 2009;45:1257-64. [Crossref] [PubMed]
- Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst 2004;96:1743-50. [Crossref] [PubMed]
- Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 2013;24:1312-9. [Crossref] [PubMed]
- Zhang X, Zhang R, Zheng Y, et al. Expression of gamma-aminobutyric acid receptors on neoplastic growth and prediction of prognosis in non-small cell lung cancer. J Transl Med 2013;11:102. [Crossref] [PubMed]
- Hall JA, Ferro A, Dickerson JE, et al. Beta adrenoreceptor subtype cross regulation in the human heart. Br Heart J 1993;69:332-7. [Crossref] [PubMed]
- Weberpals J, Jansen L, Haefeli WE, et al. Pre-and post-diagnostic β-blocker use and lung cancer survival: A population-based cohort study. Sci Rep 2017;7:2911. [Crossref] [PubMed]


