10th Congress on Lung Cancer—updates on clinical trials: goal
Introduction
Lung cancer is a major cause of cancer death worldwide, largely because most patients have advanced stage disease at the time of diagnosis (1). During the last five years due to the rapid and exciting discovery of many “driver mutations” in non-small cell lung cancer (NSCLC), novel paradigms of treatment have emerged in our daily clinical practice. This oncogene addiction model, where key genetic alterations control cell survival and proliferation has led to an explosion of knowledge in the field of lung cancer biology, targeted therapies, and resistance mechanisms. Epidermal growth factor receptor (EGFR) has surfaced as one of those important targets for therapy in different solid tumors, including NSCLC. The EGFR is a member of a family of closely related receptors, including EGFR (ErbB1), human EGFR-2 HER2/neu (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). EGFR is overexpressed in the majority of NSCLC and its expression is inversely related to survival outcome (2). The activation of this tyrosine kinase (TK) receptor leads to autophosphorylation of the intracellular domain of EGFR. Improper activation of EGFR TK results in increased malignant cell survival, proliferation, invasion, and metastasis (3). Mutations of the EGFR gene have been identified in specimens from NSCLC patients who have dramatic responses to treatment with the reversible EGFR TKIs gefitinib and erlotinib (4-6). Randomized phase III clinical trials have demonstrated that personalizing first-line therapy by EGFR mutation status with these reversible TKIs leads to improvement in progression-free survival (PFS) compared to conventional chemotherapy (7-10). But despite the impressive initial response to treatment, disease progression generally occurs after 9 to 14 months of erlotinib or gefitinib therapy, driven in approximately 60% of cases by a second site EGFR point mutation that results in the substitution of threonine with methionine at amino acid position 790 (T790M) (11-13), so novel approaches, including new EGFR TKIs and new combinations are needed and are being evaluated in the field of NSCLC.
Combination therapy for EGFR mutated patients: the goal trial rationale
Despite initial and dramatic efficacy of EGFR TKIs in NSCLC patients harboring EGFR activating mutations, all patients eventually acquire resistance. There are two main mechanisms of resistance to EGFR TKI, including the lack of initial response to therapy, also known as de novo or primary resistance to TKI, and resistance that develops after an initial response to EGFR TKIs. In order to improve patients outcome, a better knowledge of resistance mechanisms seem to be a significant challenge to develop more effective targeted therapies alone or in combination with EGFR TKI for advanced EGFR mutated NSCLC patients.
Pretreatment T790M generally has been described with another EGFR sensitizing mutations and has been related to decreased sensitivity to EGFR TKIs (14), but also the presence of additional genetic alterations that can occur concomitantly with EGFR mutations can be related to resistance mechanisms.
The Spanish experience reported by Rosell et al., included data from a total of 2,015 patients who had either stage IIIB NSCLC with pleural effusion or stage IV NSCLC and were prospectively screened for EGFR mutations (15). The overall response rate was 70.6%, median PFS was 14 months (95% CI, 11.3-16.7), and median overall survival (OS) was 27 months. PFS was not significantly affected by performance status, age, first-line versus second- or third-line therapy, or smoking history. The Spanish Lung Cancer Group (SLCG) has also analyzed the presence of the T790M mutation in pretreatment tumor samples of NSCLC patients prospectively treated with erlotinib, and correlated the presence of the T790M mutation with clinical outcomes (16). The T790M mutation was found in 35% of 129 patients at baseline. PFS was 12 months in patients with T790M and 18 months for those without T790M (P=0.05). Additionally, it was found that low BRCA1 expression levels could neutralize the negative effect of the T790M mutation; PFS was 27 months in patients with low BRCA1 mRNA levels, 18 months in those with intermediate levels, and 10 months in those with high levels (P=0.02). In the multivariate analysis, the presence of the T790M mutation [hazard ratio (HR), 4.35; P=0.001], intermediate BRCA1 levels (HR, 8.19; P<0.0001), and high BRCA1 levels (HR, 8.46; P<0.0001) emerged as markers of shorter PFS. These findings suggest that baseline assessment of the T790M mutation and BRCA1 expression could be used to predict outcome and provide alternative individualized treatment to patients based on T790M mutations and BRCA1 expression.
Very recently, it has also been evaluated the impact of pretreatment somatic EGFR T790M mutations, TP53 mutations and BIM mRNA expression in 95 patients with EGFR-mutant NSCLC included in the EURTAC trial (NCT00446225). T790M mutations were detected in 65.26% of patients using our highly sensitive method based on laser microdissection and PNA-clamping PCR. PFS to erlotinib was 9.7 months for those patients with T790M mutations and 15.8 months for those without, while among patients receiving chemotherapy, it was 6 and 5.1 months, respectively (P<0.0001). PFS to erlotinib was 12.9 months for those with high and 7.2 months for those with low/intermediate BIM expression levels, while among chemotherapy-treated patients, it was 5.8 and 5.5 months, respectively (P=0.0003). OS was 28.6 months for patients with high BIM expression and 22.1 months for those with low/intermediate BIM expression (P=0.0364). Multivariate analyses showed that erlotinib was a marker of longer PFS (HR =0.35; P=0.0003), while high BIM expression was a marker of longer PFS (HR=0.49; P=0.0122) and OS (HR=0.53; P=0.0323). These results suggest that low-level pretreatment T790M mutations can frequently be detected and also that BIM mRNA expression could a biomarker of survival in EGFR-mutant NSCLC (17).
Low levels of BRCA1 are related to sensitivity to PARP inhibitors and also PARP inhibitors can downregulate levels of BRCA1 and RAD51 (18). The main molecular hypothesis for this trial, is that levels of BRCA1 can influence response to gefitinib and the combination of gefitinib plus olaparib (a PARP1 inhibitor, that has demonstrated significant response rate in pre-treated breast and ovarian cancer patients with BRCA1 and BRCA2 mutations) (19,20) could be superior to gefitinib alone based on the above mentioned pre-clinical (18) and clinical evidence (17). The proposed treatment model testing the combination of gefitinib and olaparib, in the GOAL trial could affect those patients with EGFR mutations (independent of the presence of T790M) who could benefit with longer overall PFS when treated with the combination therapy compared to gefitinib alone. For patients treated with gefitinib alone, BIM mRNA levels could be the most predictive marker for PFS and OS. Also the influence of T790M could be modulated by the addition of olaparib, although the molecular understanding remains to be clarified. BRCA1 (low/high) could be as well the best biomarker assay for predicting the effect of olaparib. And finally, PARP1 levels warrant examination as a potential predictive biomarker of sensitivity to olaparib.
GOAL trial
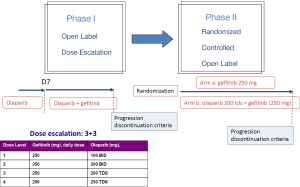
The GOAL trial, a phase Ib/II trial is testing this hypothesis comparing gefitinib vs. gefitinib vs. olaparib in EGFR mutated NSCLC patients. The primary endpoint of the phase I trial was to evaluate the safety [dose limiting toxicity (DLT) and maximum tolerated dose (MTD)] of orally administered olaparib in combination with gefitinib. Twenty-two patients have been included across four dose levels of olaparib in the phase I. Most common toxicities were G1-2, including anemia, leucopenia, nausea, diarrhea, asthenia, rash and anorexia. Durable PR and SD were observed in both EGFR TKI-naïve and previously treated patients. MTD of olaparib was 200 mg TDS (21). For the phase IIb (NCT01513174), that has been already started, the primary endpoint will be to assess the efficacy in terms of PFS to gefitinib in combination with olaparib, compared with gefitinib alone, in first line advanced NSCLC patients with EGFR mutations (Figure 1).
Conclusions
In the future of lung cancer therapy, a better understanding of the biology of the disease is critical, including the prognostic or predictive effect of the different EGFR mutations as well as how to overcome acquired or primary resistance to EGFR TKIs. The GOAL trial is a novel and appealing opportunity to improve the outcome of EGFR mutant patients treated with EGFR TKIs.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Brabender J, Danenberg KD, Metzger R, et al. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin Cancer Res 2001;7:1850-5. [PubMed]
- Kumar A, Petri ET, Halmos B, et al. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol 2008;26:1742-51. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [PubMed]
- Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 2011;17:3812-21. [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [PubMed]
- Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res 2011;17:1160-8. [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [PubMed]
- Hegan DC, Lu Y, Stachelek GC, et al. Inhibition of poly(ADP-ribose) polymerase down-regulates BRCA1 and RAD51 in a pathway mediated by E2F4 and p130. Proc Natl Acad Sci U S A 2010;107:2201-6. [PubMed]
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245-51. [PubMed]
- Tutt A, Robson M, Garber JE, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010;376:235-44. [PubMed]
- Campelo RG, Felip E, Massuti B, et al. Phase IB study of olaparib (AZD2281) plus gefitinib in EGFR-mutant patients (p) with advanced non-small-cell lung cancer (NSCLC) (NCT01513174/GECP-GOAL). J Clin Oncol 2013;31:abstr 2581.