Is there a role for prophylactic radiotherapy to intervention tract sites in patients with malignant pleural mesothelioma?
Introduction
Malignant pleural mesothelioma is an aggressive malignancy arising from the pleural lining, with a strong causal link to prior asbestos use (1). Despite a significant reduction in its use in many countries worldwide, the long latency period between asbestos exposure and disease has led to an increased incidence over the last 20 years, with over 2,500 deaths in the UK alone in 2015 (2,3). Mesothelioma causes significant morbidity to patients, including dyspnoea (often related to malignant pleural effusion), systemic symptoms and chest pain (4,5). It is invariably fatal, with a median survival of approximately 1 year from diagnosis (6).
Mesothelioma can be difficult to diagnose. Imaging techniques are often unable to accurately distinguish the disease from other malignant and benign pleural conditions and the diagnostic yield from cytological analysis of pleural fluid is notoriously poor (7). Therefore, most patients will undergo more invasive pleural procedures to facilitate diagnosis (8), which could include: image guided pleural biopsy, local anaesthetic thoracoscopy (LAT), video-assisted thoracoscopic surgery (VATS) or open pleural biopsy. Many also require pleural interventions to manage recurrent symptomatic pleural effusions.
Procedure tract metastasis (PTM) are a recognised complication of pleural interventions in mesothelioma. Tumour cells seed along the intervention sites resulting in the formation of subcutaneous metastasis, which can be painful and unsightly (9). Possible mechanisms of this phenomenon include disruption of the tumour sheet during the procedure, stimulating tumour growth along the plane of the intervention or leakage of pleural fluid containing tumour cells along the newly formed tract into the subcutaneous tissues (10,11).
Mesothelioma is known to be radiosensitive in vitro (12), but due to the large treatment volumes required to encompass all disease and consequent toxicity to other thoracic organs, radical radiotherapy as treatment for mesothelioma is currently not routinely used. However, it has been hypothesised that delivering low dose, targeted prophylactic radiotherapy to procedural tracts immediately after pleural interventions could destroy local tumour cells thereby reducing the subsequent incidence of PTM.
Conflicting results from three historical randomised controlled trials (RCT) of prophylactic radiotherapy in mesothelioma (13-15) and multiple retrospective observational studies (11,16) led to uncertainty about its efficacy in reducing PTM’s in mesothelioma. This caused variations in recommendations for prophylactic radiotherapy amongst historical international guidelines (3,17,18) and substantial variation in clinical practice (16). In order to resolve this clinical equipoise, two large, robust, multicentre RCTs have recently been conducted which have expanded the body of evidence considerably (6,19).
This review article critically summarises the literature on prophylactic radiotherapy in mesothelioma and highlights areas that warrant further evaluation.
How common are PTMs in those not receiving prophylactic radiotherapy?
The reported incidence of PTM in the literature has ranged from 0–48% (16), with much of the evidence derived from small retrospective case series (11,16). Two of the largest series report similar PTM incidence in those patients not receiving prophylactic radiotherapy of around 13% [Ruffie et al., 45/332 patients (13.6%) (20); Metintas et al. 28/212 patients (13.2%)] (20,21). However other retrospective studies found higher PTM rates of 40/123 (32.5%) in patients not receiving prophylactic radiotherapy (9). This variation may be explained by a lack of consistent definitions of a PTM, particularly in retrospective series where follow-up may not be as robust. It is also feasible that scar tissue from a procedure could mimic a small subcutaneous tumour nodule.
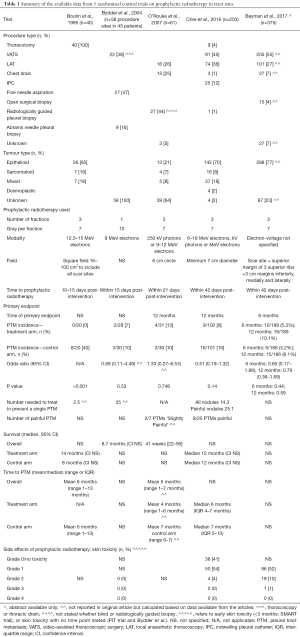
Five randomised trials have now been reported, which on the whole provide more reliable estimations. The first published RCT reported a high rate of PTM in the control arm of 8/20 (40%) (14). However, the subsequent larger RCTs, including 2 trials with over 100 patients in their control arm, consistently showed a PTM incidence of 10–16% in the control arms which is felt to be more reflective of that seen in clinical practice (Table 1) (6,13,15,19).
Full table
Are there any risk factors for PTM development?
Mesothelioma is a heterogenous disease, with different histological subtypes with varying prognoses (4,5). Diagnostic and treatment pathways often vary between individuals and hence identifying those at greatest risk of PTM development could be beneficial.
There is data to show that larger-bore procedures confer a higher risk of tract metastasis than small bore interventions. In a retrospective cohort of 100 consecutive patients, surgical biopsy (either thoracotomy or thoracoscopy) had a PTM rate of 15/69 (22%) whereas image guided biopsy had a lower PTM rate of 1/22 (5%) (8). A subsequent retrospective study showed a similar pattern, with a PTM incidence of 8/31 (26%) in patients undergoing thoracotomy compared with 20/181 (11%) in those who had undergone a thoracoscopy or pleural biopsy (P=0.025) (21).
The role of other factors in increasing PTM risk is less clear. Clive et al. showed that the epithelioid subtype of mesothelioma had higher PTM rates, with a PTM incidence of 15/72 (21%) in epithelioid versus 1/29 (3%) in other histological subtypes in patients not receiving prophylactic radiotherapy (6). This was hypothesised to be due to the poorer prognosis of the other subtypes, resulting in many patients dying before a PTM had time to develop. However, these findings have not been consistently replicated by other retrospective studies (9,21).
The efficacy of chemotherapy in mesothelioma has improved in the last decade. Previous unproven treatment combinations have been replaced by pemetrexed-based regimes which have been shown to prolong survival in clinical trials (22). Historical retrospective data published prior to the routine use of pemetrexed-based chemotherapy showed no difference in PTM incidence in those receiving chemotherapy and those who did not [19/157 (12.1%) and 3/29 (10.3%) respectively] (21). However, it is conceivable that newer, more effective chemotherapy regimens may reduce the risk of PTM due to loco-regional effects of chemotherapy preventing tumour implantation. This may explain the findings of a subgroup analysis of a recently published RCT, which found that in those patients not receiving prophylactic radiotherapy, fewer patients who had chemotherapy developed PTMs than those not receiving chemotherapy [8/64 (13%) and 8/37 (22%) respectively] (6).
Does prophylactic radiotherapy reduce the risk of PTM—evidence from initial randomised control trials
The seminal randomised control trial investigating the use of prophylactic radiotherapy to prevent PTM was published by Boutin et al. (14). In this study 40 patients were randomised to either 7 Gray in three fractions over three consecutive days, 10–15 days after thoracoscopy, or no radiotherapy. They found a significant difference in PTM incidence between the treatment arms [0/20 (0%) in the prophylactic radiotherapy group versus 8/20 (40%) in the control group (P<0.001)]. Despite this seemingly convincing evidence, uncertainly remained, given the small sample size of the study and the very high incidence of PTM in those not receiving prophylactic radiotherapy, which was not perceived to reflect that seen in usual clinical practice and has not been replicated in subsequent RCTs (6,13,15,19).
Due to these uncertainties following the Boutin trial, another two small randomised control trials were subsequently conducted (13,15). Bydder et al. randomised 58 procedure sites from 43 patients to receive either a single fraction of 10 Gy radiotherapy using 9 MeV electrons or no prophylactic radiotherapy (13). The study found no difference between groups, with a PTM incidence of 2/28 (7%) in the treatment arm versus 3/30 (10%) in the control arm (P=0.53). The authors speculated that their baseline incidence of PTM may have been lower than that reported by Boutin et al. due to their inclusion of small-bore interventions as opposed to only thoracoscopies (14). There may also have been some inherent bias in the study as they randomised procedure sites rather than patients, when multiple intervention sites in the same individual may not behave independently (16). However, despite the lack of efficacy of prophylactic radiotherapy in their trial, they concluded that the radiotherapy dose used was too small and therefore advocated the use of prophylactic radiotherapy for high risk procedures at the dose used by Boutin et al. (14).
Another study, using the same radiation dose as the Boutin et al. paper, was conducted to provide more clarification on the utility of prophylactic radiotherapy. O’Rourke et al. randomised 61 patients to receive 21 Gy in three fractions using either 250 kV photons or 9–12 MeV electrons (15). Over a 12-month follow-up period, the PTM incidence was 4/31 (13%) in the treatment arm and 3/30 (10%) in the control arm, with no significance between the two groups (P=0.748). However, the sample size calculation, which utilised the high PTM rate found in the control arm of Boutin et al.’s prior study (14) meant the study was criticized for being underpowered.
Therefore despite three randomised control trials, there was still significant uncertainty about the efficacy of prophylactic radiotherapy in mesothelioma. Due to the conflicting trial findings, there was still widespread variation in the use of prophylactic radiotherapy. A survey of UK practice conducted in 2009 showed that 75% of responding centres used prophylactic radiotherapy but the timings and dose fractionation delivered varied widely (16).
Additionally, in recent years the management options for patients with mesothelioma have improved. Non-evidence-based chemotherapy has been replaced by pemetrexed-based regimens which have been shown to prolong survival and time to progression in mesothelioma and are now frequently offered to suitable patients (22). Also, the use of indwelling pleural catheters (IPCs) in the management of malignant pleural effusion has dramatically increased in the last decade. Hence the role of prophylactic radiotherapy in modern mesothelioma management was unclear and there was a desire for a suitably powered RCT to conclusively address the issue (16).
The surgical and large bore procedures in malignant pleural mesothelioma and radiotherapy trial (SMART trial)
The SMART trial is a recently published RCT that evaluated the use of prophylactic radiotherapy to reduce PTMs in malignant pleural mesothelioma (6). Its primary endpoint was the incidence of PTM within 12 months, but it also evaluated pain, analgesia requirements, health-related quality of life and included health economic analysis. This large multi-centre trial included 22 centres and randomised 203 patients to ‘immediate’ radiotherapy with 21 Gy over 3 working days within 42 days of pleural intervention, or active surveillance with deferred radiotherapy if a PTM developed during follow-up. This study focussed on large bore pleural procedures, including: open pleural biopsy, surgical thoracotomy, VATS, LAT, large bore chest drains and IPCs. The definition of a PTM was clearly defined and required independent clinical examination by 2 people and the trial follow-up was rigorous for 12 months after randomisation.
The SMART trial found a PTM incidence rate of 9/102 (9%) in the treatment arm compared to 16/101 (16%) in the control arm, which was not statistically significant using their pre-defined intention to treat analysis [odds ratio (OR) 0.51; 95% confidence interval (CI), 0.19–1.32; P=0.14; number needed to treat (NNT) to prevent one PTM =14.3]. Interestingly 17/25 PTMs were asymptomatic (NNT to prevent one painful PTM =25.1) and PTMs tended to develop in the latter stages of the disease, with 14/25 (56%) PTMs developing after 6 months in this cohort who had a median survival of 12 months. Therefore, even if the trial had recruited more patients to increase the study’s power (23,24), the patient derived benefit of delivering prophylactic radiotherapy is likely to be small.
The pre-specified per-protocol analysis, whereby patients with major protocol violations were excluded, did show a statistically significant difference in PTM incidence, with a rate of 5/84 (6%) in the intervention arm versus 16/99 (16%) in the control arm [OR 0.33; (95% CI, 0.09–1.00), P=0.037]. This highlights that if prophylactic irradiation is to be used, it is vital that it is given within protocol, both in terms of timing and field size. Although whether this is realistic or feasible in routine clinical practice is not clear, particularly given the protocol violations in the context of this large, robustly conducted RCT.
Therefore, in view of a lack of statistical significance in the intention to treat cohort, and the tendency for PTMs to be asymptomatic and develop late, the authors concluded the routine use of prophylactic radiotherapy to prevent PTM in all patients with mesothelioma was not justified. Potential signals were identified in certain patient subgroups, which will be discussed in more detail below.
The Prophylactic Irradiation of Tracts in patients with pleural mesothelioma (PIT) trial
The PIT trial recruited concurrently with the SMART trial and also adds important data regarding the role of prophylactic radiotherapy in mesothelioma. Three hundred seventy-five patients from 54 UK centres were randomised to either receive 21 Gy in three fractions of electrons over three consecutive days or no prophylactic radiotherapy (19,25). They used a wide field with at least a 3 cm lateral, medial and inferior margin, and a superior margin extended to include 3 superior ribs (19,25). Included procedures were: VATS, open surgical biopsy, LAT or chest drains of any calibre. Needle biopsies, large thoracotomies and IPC’s were excluded (25). The primary endpoint was chest wall metastasis at 6 months. Full results are eagerly awaited but a summary abstract of their findings has recently been presented (19).
The abstract reported no significant difference in PTM incidence between the treatment arms at 6 months [6/186 (3.2%) in the treatment arm and 10/189 (5.3%) in the control arm (OR 0.60; 95% CI, 0.17–1.86; P=0.44)]. There was also no significant difference in their intention to treat analysis at 12 months, with a PTM incidence of 15/186 (8.1%) in the treatment arm versus 19/189 (10.1%) in the control arm (OR 0.79; 95% CI, 0.36–1.69; P=0.59) (19).
The full results from the PIT trial are awaited with great interest; particularly those looking at the important patient-centred secondary outcome of chest pain. However, the provisional primary endpoint data is consistent with the results of the SMART trial, adding weight to both trials conclusions. Thus 2 large, robustly conducted RCTs have shown no compelling evidence that prophylactic radiotherapy to tract sites is effective in preventing PTMs in all-comers with mesothelioma.
The impact of prophylactic radiotherapy on symptoms and quality of life
With its poor life expectancy, a significant consideration for any treatment in mesothelioma is the impact on patients remaining quality of life. Prophylactic radiotherapy to procedure sites is a palliative procedure, to reduce the potentially troublesome symptoms PTMs cause. Any such treatment should ideally be efficacious, minimally invasive, have limited side effects and most importantly impact positively on the patient’s symptoms and quality of life.
Prophylactic radiotherapy is well tolerated, with minimal side effects. In the SMART trial, the commonest adverse effects related to radiotherapy were grade 1 skin toxicity and lethargy (6). However, 3 consecutive hospital attendances to receive radiotherapy can be burdensome for patients, particularly so soon after a terminal diagnosis, with 28% of patients in SMART at least a little inconvenienced by this radiotherapy regimen (6).
Traditionally PTM’s were thought to be painful and affect quality of life, however trial data suggests for the majority of patients this may not be the case. O’Rourke et al. were the first RCT to comment on any patient-related outcome measures, investigating metastasis symptomology and depression and anxiety scores between cohorts (15). Only 2 of the 7 metastasis associated with tract sites were noted to be ‘slightly painful’, with the other five deemed ‘not painful’ (15). However, with regard to depression and anxiety scores the low rate of questionnaire completion led the authors to conclude little weight can be given to their findings (15).
As discussed previously, the SMART trial found only 8/25 (32%) of PTMs were painful and the NNT to prevent a symptomatic nodule was 25.1 (6). In those who developed a PTM in the SMART trial, there was no difference in chest pain visual analogue scale (VAS) scores or analgesia use between the treatment groups. Additionally, there was no significant differences in symptom scores, analgesia use or any validated quality of life score in those receiving prophylactic radiotherapy compared to delayed radiotherapy if a PTM developed. This provides support for ongoing active surveillance of patients with mesothelioma after diagnosis to ensure symptoms are promptly addressed.
Cost-effectiveness of radiotherapy
The SMART trial is the only trial to date to investigate the cost effectiveness and economic impact of prophylactic radiotherapy. They report that prophylactic radiotherapy was not cost-effective, with no discernible clinically or statistically significant differences identified for either mean costs, survival or the quality of life between the two groups (6,26). Those patients who did develop a PTM had neither a reduction in their quality of life or an increase in healthcare system costs. With a willingness to pay threshold of £30,000 per quality adjusted life year (QALY), there was only a 24% chance that prophylactic radiotherapy, as opposed to deferred radiotherapy, was cost-effective (26). With many feeling that £30,000 is an overestimation, and a cost per QALY of £13,000 would be more appropriate (27), in this context prophylactic radiotherapy would be even less cost-effective.
Are there any specific sub-groups that may benefit from prophylactic radiotherapy?
Whilst no trials have been powered to specifically investigate specific subpopulations, pre-defined secondary per-protocol analysis was undertaken in SMART, which identified potential effects of prophylactic radiotherapy in certain subgroups (28).
The SMART trial identified that prophylactic radiotherapy may reduce the incidence of PTMs in the subgroup of patients with epithelioid-only histology, with 6/71 (8%) developing a PTM in the prophylactic radiotherapy arm compared to 15/72 (21%) in deferred radiotherapy arm in patients with epithelioid histology (OR 0.35; 95% CI, 0.11–1.04, P=0.057) (6). Again, this may explained by the improved survival of this subgroup compared to other histological subtypes (4,5), meaning these patients live long enough to develop PTMs. Therefore, it is possible that prophylactic RT may confer more benefits in this subgroup.
When evaluating patients who did not receive chemotherapy in the SMART trial, prophylactic radiotherapy appeared to significantly reduce the incidence of PTM compared to those in the deferred arm, with an incidence of 2/46 (4%) versus 8/37 (22%) respectively, (P=0.021) (6). The reasons for this may be that systemic chemotherapy aids local disease control, negating the need for prophylactic radiotherapy, whereas if chemotherapy is not given, prophylactic radiotherapy may help destroy tumour cells in the subcutaneous tissues, thereby reducing the incidence of PTM.
The other subgroup analyses in the SMART trial, looking at procedure type and those patients with a follow up of >6 months, failed to show any effect of prophylactic radiotherapy in preventing PTM. However, whether prophylactic radiotherapy is beneficial in the context of the largest bore surgical interventions remains unanswered. Such small numbers of patients in the SMART trial were randomised having had a thoracotomy, that it is not possible to make a definitive conclusion regarding this.
Given the small sample size of all these subgroups, these data should be interpreted with extreme caution, as they certainly need to be replicated before delivering prophylactic radiotherapy to these populations should be advised. Further data from the PIT trial will be vital to explore these subgroups further. A planned patient-level meta-analysis using data from both SMART and PIT will help to inform the conclusions further, particularly in these smaller subgroups which are currently underpowered to reliably inform practice.
Conclusions
After years of debate, two recently conducted, large, robust randomised trials have finally added clarity to the role of prophylactic radiotherapy in mesothelioma. Both provide independent, consistent evidence that providing this treatment routinely to all patients following large bore pleural interventions is ineffective in preventing PTM in the context of modern mesothelioma management. Whilst the full results of the PIT trial are eagerly awaited and collaboration between PIT and SMART trial teams will help clarify potential subgroups that may derive benefit, such as patients not receiving chemotherapy or those with epithelioid histology, at present the routine use of prophylactic radiotherapy cannot be recommended.
The SMART trial has demonstrated that prophylactic radiotherapy is not cost effective and confers no patient-derived benefit in terms of symptomology, analgesia use or health-related quality of life parameters. By providing pragmatic, patient centred care, with vigilant clinical follow-up of patients with mesothelioma, patients within the deferred arm of the SMART trial were not disadvantaged in terms of symptom control.
It is refreshing after years of ambiguity and debate that two, high profile, international mesothelioma guidelines recently published in the UK and the USA now consistently advise, based on high quality evidence, that prophylactic irradiation of tracts should not be offered to patients to prevent procedure tract metastases (29,30). By conclusively resolving this issue, research attention can now be diverted to other areas of the investigation and management of this devastating disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Doll R, Peto J. Effects of health of exposure to asbestos. Health & Safety Commission 1985.
- Smittenaar CR, Petersen KA, Stewart K, et al. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer 2016;115:1147-55. [Crossref] [PubMed]
- British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007;62 Suppl 2:ii1-ii19. [PubMed]
- Meniawy TM, Creaney J, Lake RA, et al. Existing models, but not neutrophil-to-lymphocyte ratio, are prognostic in malignant mesothelioma. Br J Cancer 2013;109:1813-20. [Crossref] [PubMed]
- Borasio P, Berruti A, Bille A, et al. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg 2008;33:307-13. [Crossref] [PubMed]
- Clive AO, Taylor H, Dobson L, et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol 2016;17:1094-104. [Crossref] [PubMed]
- Renshaw AA, Dean BR, Antman KH, et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 1997;111:106-9. [Crossref] [PubMed]
- Agarwal PP, Seely JM, Matzinger FR, et al. Pleural mesothelioma: sensitivity and incidence of needle track seeding after image-guided biopsy versus surgical biopsy. Radiology 2006;241:589-94. [Crossref] [PubMed]
- Froment MA, Frechette E, Dagnault A. Prophylactic irradiation of intervention sites in malignant pleural mesothelioma. Radiother Oncol 2011;101:307-10. [Crossref] [PubMed]
- Davies HE, Musk AW, Lee YC. Prophylactic radiotherapy for pleural puncture sites in mesothelioma: the controversy continues. Curr Opin Pulm Med 2008;14:326-30. [Crossref] [PubMed]
- Arnold DT, Clive AO. Prophylactic radiotherapy for procedure tract metastases in mesothelioma: a review. Curr Opin Pulm Med 2017;23:357-64. [Crossref] [PubMed]
- Carmichael J, Degraff WG, Gamson J, et al. Radiation sensitivity of human lung cancer cell lines. Eur J Cancer Clin Oncol 1989;25:527-34. [Crossref] [PubMed]
- Bydder S, Phillips M, Joseph DJ, et al. A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer 2004;91:9-10. [Crossref] [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [Crossref] [PubMed]
- O'Rourke N, Garcia JC, Paul J, et al. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol 2007;84:18-22. [Crossref] [PubMed]
- Lee C, Bayman N, Swindell R, et al. Prophylactic radiotherapy to intervention sites in mesothelioma: a systematic review and survey of UK practice. Lung Cancer 2009;66:150-6. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016;14:825-36. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v31-9. [Crossref] [PubMed]
- Bayman N, Appel W, Ashcroft L, et al. Prophylactic Irradiation of Tracts (PIT) in Patients with Pleural Mesothelioma: Results of a Multicenter Phase III Trial. J Thorac Oncol 2017;12:S1747. [Crossref]
- Ruffie P, Feld R, Minkin S, et al. Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: a retrospective study of 332 patients. J Clin Oncol 1989;7:1157-68. [Crossref] [PubMed]
- Metintas M, Ak G, Parspour S, et al. Local recurrence of tumor at sites of intervention in malignant pleural mesothelioma. Lung Cancer 2008;61:255-61. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Landau D, Lim E. Prophylactic radiotherapy to prevent procedure-tract metastases. Lancet Oncol 2016;17. [Crossref] [PubMed]
- Zalcman G, Brosseau S, Scherpereel A. Prophylactic radiotherapy to prevent procedure-tract metastases. Lancet Oncol 2016;17. [Crossref] [PubMed]
- Bayman N, Ardron D, Ashcroft L, et al. PIT: A phase III trial of Prophylactic Irradiation of Tracts in patients with malignant pleural mesothelioma following invasive chest wall intervention. Lung Cancer 2014;83:S80. [Crossref]
- Stewart SA, Clive AO, Maskell NA, et al. Evaluating quality of life and cost implications of prophylactic radiotherapy in mesothelioma: Health economic analysis of the SMART trial. PLoS One 2018;13. [Crossref] [PubMed]
- Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19:1-503. v-vi. [Crossref] [PubMed]
- Clive AO, Wilson P, Taylor H, et al. Protocol for the surgical and large bore procedures in malignant pleural mesothelioma and radiotherapy trial (SMART Trial): an RCT evaluating whether prophylactic radiotherapy reduces the incidence of procedure tract metastases. BMJ Open 2015;5. [Crossref] [PubMed]
- Woolhouse I, Maskell NA. Introducing the new BTS guideline: the investigation and management of pleural malignant mesothelioma. Thorax 2018;73:210-2. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]


