The use of patient-reported outcome measures (PROMs) in the management of malignant pleural mesothelioma: a descriptive literature survey
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive malignancy arising from the mesothelial surfaces of the pleural cavity. This tumor was once rare, but its incidence is increasing worldwide (1). Overall survival is poor with an median survival of seven to 11 months after diagnosis (2).
Whereas most patients experience symptoms, the disease is already at an advanced stage. Up to 60% present with dyspnea, chest wall pain and pleural effusion. Other frequent symptoms are coughing, night sweats, weight loss, fatigue and a mass on the chest wall, all which have a significant impact on the health-related quality of life (hrQoL) (3). The treatment options are for most patients limited to palliative chemotherapy and best supportive care (BSC) (1).
Therefore, it is recommended to evaluate and preserve the symptoms and hrQoL. This can be achieved with patient-reported outcome measures (PROMs), which measure outcomes regarding the health of the patient and are directly reported by the patient. They can range from simple symptomatic to more complex concepts, such as hrQoL (4).
The aim of this literature survey is to provide an up to date review of the use of PROMs in mesothelioma. In line with a former review of PROMs in lung cancer (5), a concise comparison is made of the identified instruments.
Methods
This survey was conducted in accordance with the guideline Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (6). The latest database search is conducted on 02 January 2018 in PubMed, Web of Science and Google scholar using the following search terms: (((“patient reported“ OR “patient related” OR “patient based” OR “patient centered” OR “self-reported”) AND (outcome OR outcomes OR measure*)) OR (prom OR proms OR pro OR pros) OR quality of life [MeSH Terms]) AND mesothelioma [MeSH Terms]. The Risk of Bias in included studies was assessed using the appraisal tools recommended by the Cochrane Netherlands (7). PROMs were included if they showed good psychometric properties (validity, reliability and responsiveness).
Results
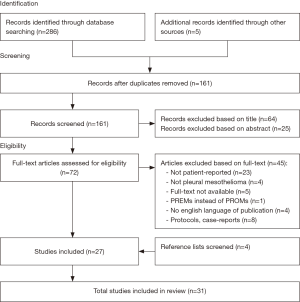
The search yielded a total of 286 hits. After removing the duplicates, screening the titles and abstracts 216 articles were excluded. The remaining 72 articles were evaluated for full text, which led to the exclusion of an additional 45 articles. Therefore, a total of 31 articles on PROMs in MPM were identified that met the inclusion criteria (Figure 1).
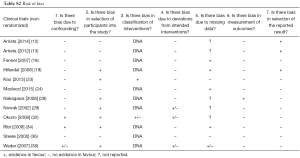
Most of these reports (Table 1) present the results of phase II (n=12) or III (n=8) clinical trials. PROMs are the primary outcome in 11 (34%) articles, and a secondary endpoint in the remaining 21 (66%). Of all 31 studies’ interventions, 22 (71%) assessed chemotherapy alone, 8 (26%) surgery with or without chemo/radiotherapy and 2 (7%) radiotherapy alone. Tables S1-S4 shows the risk of bias with poor quality of data in the phase II studies and descriptive series.

Full table

Full table
Full table

Full table

Full table
PROMs need good psychometric properties to be accepted as a scientific measure. Overall, 14 instruments were identified and included in this survey (in total online: http://tlcr.amegroups.com/public/system/tlcr/supp-tlcr.2018.07.08-6.pdf) (20,21,39-56). The instruments can be categorized in generic (n=2), cancer-specific (n=4), lung cancer-specific (n=3), mesothelioma-specific (n=2) and symptom-specific (n=3). The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-core module (EORTC QLQ-C30) was the most frequently used [in 19 (61%) of 31 studies]. In nine of the 19 studies, the EORTC QLQ-C30 was supplemented with the EORTC QLQ-lung cancer module (EORTC QLQ-LC13). The Rotterdam Symptom Checklist (RSCL) was used in four studies, like the Lung Cancer Symptom Scale (LCSS) of which in three studies the modified version for mesothelioma was used (LCSS-meso) (57-65).
Additional instruments used included Brief Pain Inventory (BPI), European Quality of Life-five dimensions (EQ-5D), Fatigue Severity Scale (FSS), Functional Assessment of Cancer Therapy-Lung (FACT-L), Hospital Anxiety and Depression Scale (HADS), Quality of Life Questionnaire for Cancer Patients Treated with Anti-Cancer Drugs (QOL-ACD), Medical Outcome Study 36-item Short-Form Health Survey (SF-36) and Symptom Distress Scale (SDS). Seventeen studies (55%) used more than one instrument. Furthermore, seven (23%) studies combined generic with disease-specific instruments.
Discussion
MPM remains a highly symptomatic and aggressive malignancy. The PROMs are of great importance for the improvement of the quality of care. PROMs were mostly included in clinical trials assessing chemotherapy, which is encouraged by the Food and Drug Administration (FDA) for labeling claims (66). Although the popularity of PROMs is still growing, they were already the primary endpoint in one third of all included studies. If PROMs were not the primary endpoint then they have become an important secondary endpoint in numerous studies. Since the clinical effectiveness of treatments in mesothelioma is still limited, their impact on the patient is considered crucial.
The phase II studies and descriptive series showed poor quality of data, which are the majority of the papers included in this review. The high rate of drop-outs was not even mentioned. Furthermore, the interpretation of the PROMs has not been described in the majority of the studies as reflected by Tables S1-S4. Based on these data it seems justified not to use PROMs in single arm studies.
In general, PROMs were measured by using well-known instruments with adequate psychometric properties. However, preference was given to disease-specific instruments as they are more sensitive for subtle changes. The EORTC QLQ-C30 in conjunction with the QLQ-LC13 is most frequently used. Besides the dominant EORTC instruments, a broad variety of other instruments were used (in total online: http://tlcr.amegroups.com/public/system/tlcr/supp-tlcr.2018.07.08-6.pdf). Despite being the only instrument available specific for the mesothelioma population, the LCSS-Meso was not used as frequently.
Because this malignancy is similar to lung cancer in terms of symptoms and survival, an entirely new instrument specific for mesothelioma is not considered necessary. Most lung cancer-specific instruments (EORTC QLQ-LC13, FACT-L and LCSS) have been validated in MPM showing good results (20,26,30). Still a new mesothelioma-specific instrument, the MD Anderson Symptom Inventory Malignant Pleural Mesothelioma (MDASI-MPM), is under development and has not yet been psychometrically validated. So there is a wide range of options for assessing PRO’s in MPM.
With no established instrument for measuring PROMs in MPM there are several aspects one should consider when choosing an instrument. The specific or more comprehensive instruments are more suited for routine use in the clinical practice. Brief and generic instruments such as the EQ-5D on the other hand put less of a burden on the patient. But the coarseness of the system with only three levels per item limits the responsiveness. In studies of patients undergoing therapy, ceiling effect problems may not be serious. In long-term follow up ceiling effect issues may be more problematic (67). Although most included instruments are suited for both routine care as clinical trials. The clinician/researcher should consider the domains, comprehensiveness/sensitivity/burden, psychometric properties, cost and aim when choosing the right instrument.
Conclusions
PROMs should not be used in single arm studies (grade 2C).
PROMs have the potential to improve the management of MPM. No particular instrument is specifically recommended, although there is a preference for patient-reported disease-specific instruments encompassing the concept of hrQoL and relevant symptoms. Such instruments are the EORTC QLQ-LC13, LCSS-Meso and FACT-L, which measure the impact of malignant mesothelioma and its treatment on patients (grade 1C).
Assessments should be made on baseline and post-treatment. The frequency of assessments should be further evaluated in this population (grade 2C).
Appendix: inclusion criteria
Studies that meet all inclusion criteria, without any exclusion criterion, were included. The criteria are: English language of publication; participants are MPM patients regardless of stage or treatment; PROMs are the primary or secondary endpoint of the study; evidence is available for the validity, reliability and responsiveness of PROMs. Exclusion criteria are: full-text not available; data is not patient-reported; studies about patient-reported experience measures (PREMs) instead of PROMs; protocols or case-reports.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Robinson BWS, Lake RA. Advances in Malignant Mesothelioma. N Engl J Med 2005;353:1591-603. [Crossref] [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Chen SE, Pace MB. Malignant pleural mesothelioma. Am J Health Syst Pharm 2012;69:377-85. [Crossref] [PubMed]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [Crossref]
- Bouazza YB, Chiairi I, El Kharbouchi O, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: A systematic review. Lung Cancer 2017;113:140-51. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Offringa M, Assendelft WJJ, Scholten RJPM. editors. Inleiding in Evidence-Based Medicine. Klinisch handelen gebaseerd op bewijsmateriaal. Vierde herziene druk ed. Houten: Bohn, Stafleu, Van Loghum, 2013.
- Ambrogi V, Baldi A, Schillaci O, et al. Clinical impact of extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Surg Oncol 2012;19:1692-9. [Crossref] [PubMed]
- Ambrogi V, Mineo D, Gatti A, et al. Symptomatic and quality of life changes after extrapleural pneumonectomy for malignant pleural mesothelioma. J Surg Oncol 2009;100:199-204. [Crossref] [PubMed]
- Arnold DT, Hooper CE, Morley A, et al. The effect of chemotherapy on health-related quality of life in mesothelioma: results from the SWAMP trial. Br J Cancer 2015;112:1183-9. [Crossref] [PubMed]
- Arnold DT, Rowen D, Versteegh MM, et al. Testing mapping algorithms of the cancer-specific EORTC QLQ-C30 onto EQ-5D in malignant mesothelioma. Health Qual Life Outcomes 2015;13:6. [Crossref] [PubMed]
- Arrieta O, Lopez-Macias D, Mendoza-Garcia VO, et al. A phase II trial of prolonged, continuous infusion of low-dose gemcitabine plus cisplatin in patients with advanced malignant pleural mesothelioma. Cancer Chemother Pharmacol 2014;73:975-82. [Crossref] [PubMed]
- Arrieta Ó, Medina LA, Estrada-Lobato E, et al. First-line chemotherapy with liposomal doxorubicin plus cisplatin for patients with advanced malignant pleural mesothelioma: phase II trial. Br J Cancer 2012;106:1027-32. [Crossref] [PubMed]
- Bottomley A, Coens C, Efficace F, et al. Symptoms and patient-reported well-being: do they predict survival in malignant pleural mesothelioma? A prognostic factor analysis of EORTC-NCIC 08983: randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma. J Clin Oncol 2007;25:5770-6. [Crossref] [PubMed]
- Bottomley A, Gaafar R, Manegold C, et al. Short-term treatment-related symptoms and quality of life: results from an international randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an EORTC Lung-Cancer Group and National Cancer Institute, Canada, Intergroup Study. J Clin Oncol 2006;24:1435-42. [Crossref] [PubMed]
- Burkholder D, Hadi D, Kunnavakkam R, et al. Effects of extended pleurectomy and decortication on quality of life and pulmonary function in patients with malignant pleural mesothelioma. Ann Thorac Surg 2015;99:1775-80. [Crossref] [PubMed]
- Clive AO, Taylor H, Dobson L, et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol 2016;17:1094-104. [Crossref] [PubMed]
- Fennell DA, Steele JP, Shamash J, et al. Efficacy and safety of first- or second-line irinotecan, cisplatin, and mitomycin in mesothelioma. Cancer 2007;109:93-9. [Crossref] [PubMed]
- Hillerdal G, Sorensen JB, Sundstrom S, et al. Treatment of malignant pleural mesothelioma with liposomized doxorubicine: prolonged time to progression and good survival. A Nordic study. Clin Respir J 2008;2:80-5. [Crossref] [PubMed]
- Hollen PJ, Gralla RJ, Liepa AM, et al. Adapting the Lung Cancer Symptom Scale (LCSS) to mesothelioma: using the LCSS-Meso conceptual model for validation. Cancer 2004;101:587-95. [Crossref] [PubMed]
- Hollen PJ, Gralla RJ, Liepa AM, et al. Measuring quality of life in patients with pleural mesothelioma using a modified version of the Lung Cancer Symptom Scale (LCSS): psychometric properties of the LCSS-Meso. Support Care Cancer 2006;14:11-21. [Crossref] [PubMed]
- Jassem J, Ramlau R, Santoro A, et al. Phase III Trial of Pemetrexed Plus Best Supportive Care Compared With Best Supportive Care in Previously Treated Patients With Advanced Malignant Pleural Mesothelioma. J Clin Oncol 2008;26:1698-704. [Crossref] [PubMed]
- Kao SC, Vardy J, Harvie R, et al. Health-related quality of life and inflammatory markers in malignant pleural mesothelioma. Support Care Cancer 2013;21:697-705. [Crossref] [PubMed]
- MacLeod N, Chalmers A, O'Rourke N, et al. Is Radiotherapy Useful for Treating Pain in Mesothelioma?: A Phase II Trial. J Thorac Oncol 2015;10:944-50. [Crossref] [PubMed]
- Mollberg NM, Vigneswaran Y, Kindler HL, et al. Quality of life after radical pleurectomy decortication for malignant pleural mesothelioma. Ann Thorac Surg 2012;94:1086-92. [Crossref] [PubMed]
- Muers MF, Rudd RM, O'Brien ME, et al. BTS randomised feasibility study of active symptom control with or without chemotherapy in malignant pleural mesothelioma: ISRCTN 54469112. Thorax 2004;59:144-8. [Crossref] [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [Crossref] [PubMed]
- Nakagawa K, Yamazaki K, Kunitoh H, et al. Efficacy and safety of pemetrexed in combination with cisplatin for malignant pleural mesothelioma: a phase I/II study in Japanese patients. Jpn J Clin Oncol 2008;38:339-46. [Crossref] [PubMed]
- Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 2002;87:491-6. [Crossref] [PubMed]
- Nowak AK, Stockler MR, Byrne MJ. Assessing quality of life during chemotherapy for pleural mesothelioma: feasibility, validity, and results of using the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire and Lung Cancer Module. J Clin Oncol 2004;22:3172-80. [Crossref] [PubMed]
- O'Brien ME, Watkins D, Ryan C, et al. A randomised trial in malignant mesothelioma (M) of early (E) versus delayed (D) chemotherapy in symptomatically stable patients: the MED trial. Ann Oncol 2006;17:270-5. [Crossref] [PubMed]
- Okuno SH, Delaune R, Sloan JA, et al. A phase 2 study of gemcitabine and epirubicin for the treatment of pleural mesothelioma: a North Central Cancer Treatment Study, N0021. Cancer 2008;112:1772-9. [Crossref] [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Ribi K, Bernhard J, Schuller JC, et al. Individual versus standard quality of life assessment in a phase II clinical trial in mesothelioma patients: feasibility and responsiveness to clinical changes. Lung Cancer 2008;61:398-404. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Steele JP, Shamash J, Evans MT, et al. Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol 2000;18:3912-7. [Crossref] [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized Phase III Study of Cisplatin With or Without Raltitrexed in Patients With Malignant Pleural Mesothelioma: An Intergroup Study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [Crossref] [PubMed]
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202. [Crossref] [PubMed]
- König HH, Ulshöfer A, Gregor M, et al. Validation of the EuroQol questionnaire in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2002;14:1205-15. [Crossref] [PubMed]
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. [Crossref] [PubMed]
- Coons SJ, Rao S, Keininger DL, et al. A Comparative Review of Generic Quality-of-Life Instruments. Pharmacoeconomics 2000;17:13-35. [Crossref] [PubMed]
- Nicklasson M, Bergman B. Validity, reliability and clinical relevance of EORTC QLQ-C30 and LC13 in patients with chest malignancies in a palliative setting. Qual Life Res 2007;16:1019-28. [Crossref] [PubMed]
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30A:635-42. [Crossref] [PubMed]
- Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy—lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. [Crossref] [PubMed]
- Butt Z, Webster K, Eisenstein AR, et al. Quality of Life in Lung Cancer: The Validity and Cross-Cultural Applicability of the Functional Assessment of Cancer Therapy–Lung Scale. Hematol Oncol Clin North Am 2005;19:389-420. [Crossref] [PubMed]
- Hollen PJ, Gralla RJ, Kris MG, et al. Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS). Eur J Cancer 1993;29A Suppl 1:S51-8. [Crossref] [PubMed]
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Psychometric assessment of the lung cancer symptom scale. Cancer 1994;73:2087-98. [Crossref] [PubMed]
- de Haes JCJM, Olschewski M, Fayers P, et al. Measuring the quality of life of cancer patients with the Rotterdam Symptom Checklist: a manual. 2nd ed, 2012.
- McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med 1983;17:431-8. [Crossref] [PubMed]
- McCorkle R, Cooley ME, She JA. A user's manual for the Symptom Distress Scale. Philadelphia: University of Pennsylvania, 1998.
- Kurihara M, Shimizu H, Tsuboi K, et al. Development of quality of life questionnaire in Japan: quality of life assessment of cancer patients receiving chemotherapy. Psychooncology 1999;8:355-63. [Crossref] [PubMed]
- Matsumoto T, Ohashi Y, Morita S, et al. The quality of life questionnaire for cancer patients treated with anticancer drugs (QOL-ACD): validity and reliability in Japanese patients with advanced non-small-cell lung cancer. Qual Life Res 2002;11:483-93. [Crossref] [PubMed]
- Cleeland CS. The Brief Pain Inventory User Guide, 2009.
- Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121-3. [Crossref] [PubMed]
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. J Psychosom Res 2002;52:69-77. [Crossref] [PubMed]
- Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res 1997;42:17-41. [Crossref] [PubMed]
- Dorman P, Slattery J, Farrell B, et al. Qualitative Comparison of the Reliability of Health Status Assessments With the EuroQol and SF-36 Questionnaires After Stroke. Stroke 1998;29:63-8. [Crossref] [PubMed]
- Ware JE Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998;51:903-12. [Crossref] [PubMed]
- Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160-4. [Crossref] [PubMed]
- Monras P, Gralla RJ, Burke MT, et al. Development of specific instruments for subjective evaluation of patients with lung cancer: Comparison of observer assessment with patient generated visual analogue scales (VAS). Proceedings of the American Society of Clinical Oncology 1985;4:251.
- de Haes JC, van Knippenberg FC, Neijt JP. Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer 1990;62:1034-8. [Crossref] [PubMed]
- Pelayo-Alvarez M, Perez-Hoyos S, Agra-Varela Y. Reliability and Concurrent Validity of the Palliative Outcome Scale, the Rotterdam Symptom Checklist, and the Brief Pain Inventory. J Palliat Med 2013;16:867-74. [Crossref] [PubMed]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38. [PubMed]
- Radbruch L, Loick G, Kiencke P, et al. Validation of the German Version of the Brief Pain Inventory. J Pain Symptom Manage 1999;18:180-7. [Crossref] [PubMed]
- Brazier JE, Harper R, Munro J, et al. Generic and condition-specific outcome measures for people with osteoarthritis of the knee. Rheumatology (Oxford) 1999;38:870-7. [Crossref] [PubMed]
- U.S. Department of Health and Human Service Food and Drug Administration (FDA). Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [Crossref] [PubMed]
- Lipscomb J, Gotay CC, Snyder C. Outcomes Assessment in Cancer: Measures, Methods and Applications: Cambridge University Press, 2004.