Targeting IL22: a potential therapeutic approach for Kras mutant lung cancer?
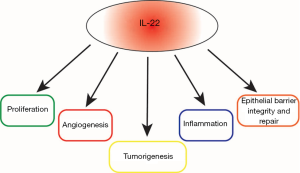
It is now well accepted that inflammation plays a pivotal role in tumorigenesis. Multiple cytokines have been implicated in tumor promoting inflammation. One of these cytokines, interleukin 22 (IL22) plays an important role in tumorigenesis (Figure 1). IL22 producing cells are known to exist in a variety of human diseases (1). Interestingly, a number of malignancies, including lung, liver, gastric, colon and pancreatic, demonstrate elevated levels of IL22 (1). In lung cancer, studies demonstrated elevated IL22 levels in the sera as well as infiltration of IL22 positive cells into the primary tumors of patients with lung cancer (2). IL22 is known to exert its effects through a heterodimeric IL22 receptor (IL22R) which includes IL22R1 chain. High levels of IL22R1, expressed on non-hematopoietic cells, is an independent marker for poor overall survival in patients with non-small cell lung cancer (NSCLC) (3). Lung cancer continues to remain the leading cause of cancer-related deaths in the US. KRAS mutations are the most frequent oncogenic aberrations to occur in NSCLCs and activation of KRAS in lung cancer induces the NF-κB pathway and release of IL22 (4). KRAS mutant cancers also portend poor prognosis and chemoresistance. Unfortunately, targeted therapies for KRAS mutant cancer have not demonstrated clinical benefit. Therefore, understanding how IL22 induces tumor promoting inflammation as well as its effects on remodeling the tumor microenvironment and tumor immunity would potentially offer a therapeutic strategy in which IL22 could be targeted in combination with chemotherapy, checkpoint inhibitors or targeted therapy to improve outcomes in KRAS mutant lung cancer patients.
In the current study, Khosravi et al. first observed that higher IL22R1 mRNA expression was associated with worse recurrence free survival (RFS) in patients with KRAS mutant lung adenocarcinoma however, this association was not statistically significant (P=0.08) In an effort to explore the importance of IL22 in KRAS mutant lung adenocarcinoma, Khosravi et al. preformed experiments in which the authors knocked down IL22 in a mouse model of Kras-induced lung cancer (5). Using a Kras mutant lung cancer mouse model, in which the authors induced expression of mutant Kras in lung epithelial cells by crossing mice harboring the LSL-KrasG12D allele with mice containing Cre recombinase inserted into the Club cell secretory protein (CCSP) locus (CCSPCre mice) to create a CCSPCre/LSL-KrasG12D (CC-LR) mouse, IL22 expression was higher in in CC-LR mice as compared to control mice during tumor progression. Additionally, there were increased numbers of IL22 positive cells in CC-LR mice compared to control mice. These IL22 positive cells were comprised of primarily CD4+ and γδ T cells (14.4% and 59.3% respectively).
To more fully characterize the functional significance of IL22 overexpression in Kras mutant lung cancer, CC-LR mice were crossed with IL22 knock out (KO) mice. CC-LR/IL22KO mice demonstrated reduced visible lung tumors, which was confirmed by histopathology of CC-LR/IL22KO mice lungs showing fewer adenomas. Furthermore, histopathology demonstrated the CC-LR/IL22KO mice tumors displayed lower levels of Ki67 and ERG staining indicating that IL22 may promote tumor proliferation and angiogenesis in Kras mutant lung cancer. Given these findings, the authors were interested in understanding the mechanism behind IL22’s effect on proliferation and angiogenesis. The IL22 receptor complex is known to activate the JAK/STAT pathway which leads to STAT3 and mitogen-activated protein kinase (MAPK) activation (4,6). These pathways are important in the induction of both proliferation and angiogenesis within a tumor. Indeed, CC-LR/IL22KO mice had reduced STAT3 and ERK activation indicating proliferation and angiogenesis in Kras mutant lung cancer may be dependent on IL22’s induction of STAT3 and MAPK pathways.
Given IL22 plays a central role in tumor promoting inflammation and inflammation can in turn affect the tumor microenvironment, the authors next explored IL22’s effect on the immune response within the lung microenvironment. Analysis of bronchoalveolar lavage fluid (BALF) from CC-LR/IL22KO mice demonstrated reduced white blood cells (WBC) and macrophages and marginally reduced infiltrating lymphocytes and neutrophils. The authors further examined the phenotypic changes of the infiltrating leukocytes from CC-LR/IL22KO versus CC-LR mice and found Fizz1, Arg and iNos, markers of immunosuppression, to be reduced in CC-LR/IL22KO mice. Furthermore, Il6 and Il17 mRNA expression, important for Th17 differentiation and infiltration, were reduced in CC-LR/IL22KO mice. Increased expression of Ifng and granzyme B in CC-LR/IL22KO mice suggested that Th1 differentiation and CD8+ cytotoxic T-cell activation were upregulated as well. In addition, Tbet expression was significantly increased in CC-LR/IL22KO mice concordant with Th1 induction. Finally, lungs isolated from CC-LR/IL22KO mice had decreased T-regulatory cells. Taken together, these results suggest that elevated levels of IL22 may induce a more immunosuppressive tumor microenvironment in Kras driven lung cancer.
In addition to IL22’s role as a mediator of inflammation, it is also involved in epithelial barrier integrity and repair. Stem cells play an important role in epithelial renewal and evidence suggests that downstream targets of IL22 such as STAT3 are involved in maintaining “stemness” (7). Therefore, the authors hypothesized that IL22 may be required to maintain or enhance “stemness” in the lung epithelium. Isolation of lung tissue from CC-LR/IL22KO mice demonstrated fewer “stem-like” cells associated with tumors as compared to tumors isolated from CC-LR mice. mRNA expression of stem cell genes, Oct4, Sox2 and Nanog as well as ALDH1 immunostaining expression, was reduced in whole lungs from CC-LR/IL22KO mice compared to CC-LR mice lungs. Taken together this suggests a role for IL22 in the propagation of “stemness” in Kras-mediated lung cancer. Furthermore, the authors discovered that when they crossed CC-LR mice with STAT3 conditional KO mice there was a significant reduction in Sox2 and Nanog suggesting that IL22 induces lung cancer “stemness” through STAT3 activation.
Finally, Khosravi et al. were interested in understanding if overexpression of IL22 was important for tumor initiation in the lung. They employed a model in which they induced COPD-type inflammation in CC-LR mice to produce lung tumors within 8 weeks. The authors induced COPD-type inflammation within the lungs of CC-LR and CC-LR/IL22KO mice with an aerosolized Haemophilus influenza strain 12 (NTHi) and found only a modest decrease in tumor reduction (~31%) in mice lacking IL22 compared to CC-LR mice. This decrease correlated to tumor reduction seen in IL22 deficient mice in the absence of COPD-type airway inflammation (~54%). While there was a reduction in tumor cell proliferation, changes in STAT3 activation were not observed. Interestingly, they did see increased numbers of macrophages and lymphocytes in IL22 deficient mice exposed to COPD-type inflammation suggesting that IL22 may be necessary for a more immunosuppressive tumor microenvironment.
This study had several limitations. First, it is still unclear if IL-22 expression portends a poorer survival in patients with KRAS mutant lung cancer. As the authors suggest they did not see a significant association between IL22R1 mRNA expression and recurrence-free survival in a pool of 150 lung adenocarcinomas. While higher IL22R1 mRNA expression was associated with worse recurrence free survival (RFS) in KRAS mutant lung adenocarcinomas this was not statistically significant. Interestingly, loss of IL22 lead to a reduction of MAPK signaling pathway activation in Kras mutant lung tumors in mice. It is well known that MAP kinases are involved in regulation of the immune response. A more recent study by He et al. demonstrated that expression of MAP3K3, a gene that encodes mitogen protein kinase kinase 3, an upstream regulator of the MAPK pathway, correlated with favorable patient survival in lung cancer (8). While this study did not look specifically at the subgroup of patients with KRAS mutant lung cancer, it suggests that differential regulation of the immune system by the MAPK pathway may have varying effects on cancer outcomes. Perhaps activation of certain MAPKs by IL22 offset the deleterious effects of IL22 expression in KRAS driven human lung cancers.
Second, this study relies solely on a mouse model of Kras mutant lung adenocarcinoma. Mouse models of cancer are not perfect recapitulations of human cancers. The Kras mutant mouse model employed in the current study is a validated research tool for assessing how different genes affect biological outcomes in Kras mutant mouse lung cancer. However, the mechanism of tumor development does not mirror the human as mice expressing the Kras-mutant allele in the lung do not develop adenocarcinomas but rather adenomas (9). The fact that this model does not completely mirror human KRAS-mutant lung cancer could explain the discrepancies the authors see between the association of high IL22 expression in Kras mutant lung cancers in mice with tumor progression and lack of statistically significant association of IL22 expression with RFS in human KRAS mutant lung adenocarcinomas.
Despite these limitations, this study demonstrated a novel role for IL22 in Kras driven lung cancer through mediation of tumor proliferation and remodeling of the tumor microenvironment. The results suggest that IL22 could be a potential therapeutic target. IL22 is uniquely positioned at the interface between the immune system and epithelial cells. IL22 is the only cytokine secreted by immune cells that solely affects non-immune epithelial cells. It is now appreciated that IL22 plays a pivotal role in preservation of mucosal barriers and protection of the host from microbial insults (1,10). IL22 stimulates epithelial proliferation and regeneration which play key roles in IL22’s ability to aid in wound healing and tissue repair (1). In addition, IL22 facilitates pro-inflammatory responses through induction of cytokines and chemokines that leads to the recruitment of leukocytes to the site of epithelial injury at the epithelium (11,12). Therefore, IL22 can be seen as a potent mediator of immune cell-epithelial cell cross talk. Given IL22’s role as a promoter of proliferation and inflammation much research has gone into exploring the role of IL22 in cancer. Studies in which IL22 was overexpressed in the liver and adipose tissue in mice did not demonstrate that IL22 alone could induce spontaneous tumor development (13,14). However, seminal studies in both carcinogen and genetically induced models of mouse colon cancer demonstrated that increased IL22 activity resulted in greater tumor burden and reduced survival (15,16). Furthermore, a study found that in a model of colitis-induced colon cancer, blocking IL22 at an early stage resulted in enhanced tumorigenesis suggesting a role of IL22 as a cancer-repressor. Additionally, blocking IL22 after tumor initiation decreased tumor burden, which suggests a role of IL22 in tumor progression (15). These studies imply that while IL22 may not play a central role in tumor initiation it is important for tumor progression and could be an attractive therapeutic target to use in combination with chemotherapy, immunotherapy or targeted therapy.
Not unlike the gut, the lung lies at the interface between the outside environment and host. IL22 regulates mucosal immunity and epithelial barrier protection in the lung (17,18). As previously discussed, elevated levels of IL22 are found in many primary lung tumors. More recently, a study demonstrated that IL22 is elevated in the bronchoalveolar lavage fluid of patients with lung cancer (2). Further, IL22 exerts anti-apoptotic effects on lung xenografts and induces growth of chemoresistant cancer cells (19). Oncogenic mutations, such as KRAS, lead to tumor promoting inflammation, proliferation and migration through the activation of the NF-kB pathway and release of IL22 (6,20,21). As shown in the current study by Khosravi et al. IL22 also plays an important role in modulating the tumor microenvironment in Kras driven lung cancer. Therefore, IL22 is an attractive therapeutic candidate for the treatment of lung cancers.
Indeed, targeting the IL22 pathway as a way to modulate inflammatory and immune responses has gained momentum, especially in the treatment of autoimmune conditions. In a recent Phase IIa randomized, double blind study, patients with moderate-to-severe atopic dermatitis had improvement in symptoms and disease extent when treated with an IL22 monoclonal antibody, fezakinumab, compared to placebo (22). Further, treatment was well tolerated with the most common adverse event being upper respiratory tract infections (22). Given fezakinumab tolerability, this would be a potential therapy to apply to lung cancer patients. As previously mentioned, IL22 has both tumor promoting and tumor repressing effects. IL22 is involved in the progression of Kras mutant lung cancer and therefore using an IL22 monoclonal antibody such as, fezakinumab, to treat lung cancer would likely be most efficacious in the setting of progressive disease and in combination with other anti-tumor agents. Another way of targeting IL22 therapeutically is to inhibit upstream activators of IL22 secretion. Recently, a study demonstrated lung cancer cells induce IL22 secretion through memory CD4+ T cells mediated release of IL-1β to promote tumor growth (23). Interestingly, anakinra, a clinically approved IL-1 receptor antagonist, abrogated IL22 production and reduced tumor growth in a murine breast cancer model (23). Multiple clinical trials are now underway evaluating anakinra as a potential cancer therapy.
In the era of immune checkpoint blockade, understanding how to improve outcomes and responses to this treatment are of great significance given the potential for durable responses in patients of multiple cancer types. In lung cancer, checkpoint blockade improves overall survival in patients with metastatic disease (24). However, even patients demonstrating greater than 50% expression of programmed death ligand 1 (PD-L1) only respond to checkpoint blockade ~45% of the time (25). Checkpoint inhibition works by disrupting co-inhibitory T-cell signaling allowing for anti-tumor immune responses to occur. This generally occurs through the activation of tumor-reactive CD8 T cells. As mentioned previously, IL22 affects the tumor microenvironment. In the current study, Khosravi et al. demonstrate that IL22 is important for decreasing the amount of tumor infiltrating leukocytes as well as maintaining an immunosuppressive tumor microenvironment in Kras-driven lung cancers. This suggests that IL22 inhibition may augment response to checkpoint blockade in combination. Given IL22’s role as an anti-inflammatory molecule in certain settings it would be imperative to test this combination of checkpoint blockade and IL22 inhibition in murine models of cancer prior to treating cancer patients as IL22 could potentially decrease response to checkpoint blockade.
Understanding how the tumor microenvironment and inflammation affects not only tumorigenesis but response to treatments for cancer will likely provide more efficacious therapies. In the current study, Khosravi et al., for the first time, show that IL22 is important for Kras-mediated lung cancer tumorigenesis. Through IL22 effects on tumor proliferation, remodeling of the tumor microenvironment and maintenance of tumor “stemness”, they offer an attractive therapeutic target for Kras-mutant lung cancers. Given the broad reaching biologic effects of IL22 on tumor progression, targeted IL22 therapy represents a potential strategy in the treatment of cancer in combination with existing therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lanfranca MP, Lin YW, Fang JY, et al. Biological and pathological activities of interleukin-22. J Mol Med (Berl) 2016;94:523-34. [Crossref] [PubMed]
- Tufman A, Huber RM, Volk S, et al. Interleukin-22 is elevated in lavage from patients with lung cancer and other pulmonary diseases. BMC Cancer 2016;16:409. [Crossref] [PubMed]
- Guillon A, Gueugnon F, Mavridis K, et al. Interleukin-22 receptor is overexpressed in nonsmall cell lung cancer and portends a poor prognosis. Eur Respir J 2016;47:1277-80. [Crossref] [PubMed]
- Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 2002;277:33676-82. [Crossref] [PubMed]
- Khosravi N, Caetano MS, Cumpian AM, et al. IL22 Promotes Kras-Mutant Lung Cancer by Induction of a Protumor Immune Response and Protection of Stemness Properties. Cancer Immunol Res 2018;6:788-97. [Crossref] [PubMed]
- Bi Y, Cao J, Jin S, et al. Interleukin-22 promotes lung cancer cell proliferation and migration via the IL-22R1/STAT3 and IL-22R1/AKT signaling pathways. Mol Cell Biochem 2016;415:1-11. [Crossref] [PubMed]
- Kryczek I, Lin Y, Nagarsheth N, et al. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 2014;40:772-84. [Crossref] [PubMed]
- He Y, Wang L, Liu W, et al. MAP3K3 expression in tumor cells and tumor-infiltrating lymphocytes is correlated with favorable patient survival in lung cancer. Sci Rep 2015;5:11471. [Crossref] [PubMed]
- Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 2001;15:3243-8. [Crossref] [PubMed]
- Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006;203:2271-9. [Crossref] [PubMed]
- Sa SM, Valdez PA, Wu J, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 2007;178:2229-40. [Crossref] [PubMed]
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005;174:3695-702. [Crossref] [PubMed]
- Park O, Wang H, Weng H, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology 2011;54:252-61. [Crossref] [PubMed]
- Wang Z, Yang L, Jiang Y, et al. High fat diet induces formation of spontaneous liposarcoma in mouse adipose tissue with overexpression of interleukin 22. PLoS One 2011;6. [Crossref] [PubMed]
- Huber S, Gagliani N, Zenewicz LA, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012;491:259-63. [Crossref] [PubMed]
- Kirchberger S, Royston DJ, Boulard O, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 2013;210:917-31. [Crossref] [PubMed]
- Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 2008;14:275-81. [Crossref] [PubMed]
- Paget C, Ivanov S, Fontaine J, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem 2012;287:8816-29. [Crossref] [PubMed]
- Kobold S, Volk S, Clauditz T, et al. Interleukin-22 is frequently expressed in small- and large-cell lung cancer and promotes growth in chemotherapy-resistant cancer cells. J Thorac Oncol 2013;8:1032-42. [Crossref] [PubMed]
- Ji H, Houghton AM, Mariani TJ, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006;25:2105-12. [Crossref] [PubMed]
- Moghaddam SJ, Li H, Cho SN, et al. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol 2009;40:443-53. [Crossref] [PubMed]
- Guttman-Yassky E, Brunner PM, Neumann AU, et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J Am Acad Dermatol 2018;78:872-81.e6. [Crossref] [PubMed]
- Voigt C, May P, Gottschlich A, et al. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proc Natl Acad Sci U S A 2017;114:12994-9. [Crossref] [PubMed]
- Soria JC, Marabelle A, Brahmer JR, et al. Immune checkpoint modulation for non-small cell lung cancer. Clin Cancer Res 2015;21:2256-62. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]


