Hunting for transcription factors: STAT3 decoy in non-small cell lung cancer
Non-small cell lung cancer (NSCLC) is by far the most common type of lung cancer and accounts for 84% of all cases. The majority of these patients present at advanced stages that are incurable and have >90% 5-year mortality rate (1). The cellular origin is of unknown etiology and rapidly becomes an incredibly heterogeneous disease containing myriad mutations and genetic alterations (2). For decades, the mainstay of advanced stage treatment has been centered on traditional chemotherapeutics such as first-line cytotoxic platinum-based drugs, and second-line microtubule targeting docetaxel after recurrence (3). With the advent of broad molecular profiling, treatment (4) for NSCLC is dictated by the presence and/or absences of various mutations or constitutively active proteins; namely, EGFR sensitizing mutations or ALK and ROS1 fusion oncogenes (2). Other potential targetable proteins include BRAF, MET, HER2, RET and NTRK fusions (2). EGFR targeted tyrosine kinase inhibitors (TKIs) e.g., erlotinib (first generation), osimertinib (third generation TKI), and ALK inhibitors e.g., crizotinib (first generation), lorlatinib (third generation), have led to improvements in the progression-free survival of those diagnosed with advanced disease and harboring these alterations (5-7). Patients who do not harbor any of these genetic alterations are left with traditional and less efficacious cytotoxic agents. Though immunotherapy via checkpoint inhibition [pembrolizumab, KEYNOTE-024 (8)] is quite effective for NSCLC patients with adequate target expression, only a small subset of patients respond.
Unfortunately, while targeted therapies demonstrate clinical response in a significant proportion of patients, this is almost inevitably followed by progression. This is due either to the development or selection of new mutations (i.e., EGFR T790M and C797S mutations, ROS1 G2031R mutation), or an increase in copy number (i.e., ALK oncogene duplication). Second and third generation agents have greater binding affinity with longer therapeutic effectiveness and have conferred further improvement in overall and progression-free survival (9).
Transcription factors (TFs) are an attractive yet elusive targets due to their location at the bottleneck of many oncogenic signaling pathways (10,11). STAT3 is a TF that has wide-reaching implications in oncogenesis, cancer progression and immunomodulation (12). Increased STAT3 signaling is associated with poor clinical prognosis (13). The oncogenic capabilities ascribed to STAT3 are mediated via a homodimerization and subsequent transcriptional regulation of many cancer-associated genes including those required for survival, proliferation, invasion and epithelial-to-mesenchymal transition (EMT). Elevated and/or constitutively active STAT3 has been demonstrated in a wide array of cancers including NSCLC with various mutational backgrounds (14). Further, its expression is increased upon induction of resistance to chemotherapy—as well as targeted therapies (15). STAT3 is not required for physiologically normal cells to survive, thus making it a valuable cancer-specific target (16).
TFs, including STAT3, have long been thought of as impossible drug targets. Most current inhibitors lack specificity and extremely high concentrations are required for effective inhibition of STAT3. Recently, double-stranded transcription factor decoy (TFD) oligodeoxynucleotides (ODNs) have emerged as novel drug candidates for the efficacious targeting of TFs (10,11). These synthetic decoys competitively inhibit the activity of the TFs by acting as a functional sink, thereby preventing their interaction with a promoter region and subsequent induction of gene transcription. Jennifer Grandis’ group previously developed a decoy out of double-stranded 15-mer oligonucleotides whose sequence was congruent with the STAT3 response element in the c-fos promoter region (17). This cyclic ODN (cODN) decoy prevented the transcription of STAT3 target genes such as Bcl-xL and cyclin D1 in vitro, in preclinical head and neck squamous cell carcinoma (HNSCC) xenograft mouse models, and in human HNSCC tumors via direct intra-tumoral injection (17). The robust decrease in target gene transcription and the lack of adverse reactions highlighted the clinical safety and efficacy of STAT3 inhibition by a cODN decoy.
Now, a new study by Njatcha and colleagues (18) investigates the efficacy of a cODN to inhibit STAT3 and its transcription targets in NSCLC cells (Figure 1). STAT3 15-mer ODNs were linked and cyclized by hexa-ethyleneglycol spacers to cover the 5’ and 3’ free ends, thereby increasing the thermal stability and improving the oligonucleotide half-life compared to the linear TFD. The cyclic STAT3 decoy (CS3D) uptake by NSCLC cells was verified via fluorescence with >90% transfection efficacy, conferring a great amount of translational potential. CS3D administration was determined to inhibit proliferation, increase apoptosis and effectively block the gene products of STAT3 (namely c-Myc, in the setting of stimulation by EGF) in NSCLC cells. The effects of this inhibitor were robust in both wild type EGFR expressing cells (201T) as well as cells expressing EGFR resistance mutation (H1975). Further, the presence of CS3D effectively diminished the nuclear concentration of STAT3 as well as increased the ubiquitination of pSTAT3 in the cytoplasm, leading to enhanced degradation (Figure 1). Importantly, all of the findings associated with the active decoy were compared to an inactive circularized decoy mimetic harboring a single nucleotide mutation that prevented STAT3 binding (CS3M). This control was imperative to prove the STAT3 specificity and effectiveness of the active CS3D.
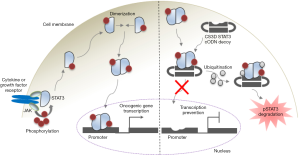
These findings have huge implications for STAT3 decoys to be utilized as a pan-NSCLC treatment method that can transcend the therapeutic constraints of the current targeted therapies. The aforementioned notion that STAT3 activation is increased in NSCLCs that acquire EGFR inhibitor resistance, in concert with CS3D ability to retain functionality in the setting of EGFR resistant disease (inherent or acquired), shows this intervention has great potential as a novel treatment modality for disease resistant to EGFR inhibitors (Figure 1).
The in vivo antitumor effects of CS3D were assessed in 201T and H1975 NSCLC xenograft mouse models. A 97% and 81.7% decrease in tumor size was noted for 201T and H1975 respectively, when comparing CS3D to CS3M treated mice. The authors found a diminished proliferation index (as measured by Ki-67), c-Myc expression, and nuclear pSTAT3 as well as large areas of debris and infiltrating lymphocytes in the CS3D treated group, corroborating with their in vitro results. They further determined the apoptotic induction effects of CS3D were far greater than the control. Of note, no systemic toxicity was observed through the course of the studies.
These in vivo experiments are an important step for the translational implications and eventual clinical usefulness of CS3D. Simply, yet of great practical importance, these results display that the hexa-ethyleneglycol linkers were able to confer the level of stability to CS3D required for efficacious biological activity via systemic treatment, which heretofore had remained unproven. Further, these linkers were not found to hinder the uptake of CS3D from circulation and into the target cells. Given the inaccessibility of NSCLC tumors to direct intratumoral injection, systemic administration is incredibly important, if not a requirement, for any clinically useful therapy. CS3D ability to inhibit the growth of wild type EGFR and EGFR inhibitor resistant mutant NSCLC cells in vivo is incredibly significant. These results offer insight into the possible utility of STAT3 inhibition via CS3D as an alternative treatment strategy for NSCLC.
This study offers many interesting and clinically useful findings, yet contains some limitations. For instance, there is a lack of longitudinal tumor assessment regarding the impact of CS3D on survival and metastasis. 201T tumors were measured for 20 days while H1975 tumors were only observed for 14. The difference between the CS3D and CS3M treated groups was not that large. The xenograft tumor growth was halted but no regression was observed, nor was any assessment conducted on metastasis in the animals. Collection of more robust preclinical survival data would help infer the clinical efficacy of CS3D. The results would also be bolstered by utilizing multiple cell lines as well as head-to-head comparisons with currently used drugs. In conjunction with this, mechanistic studies to determine how and why pSTAT3 is ubiquitinated as well as how it prevented STAT3 from entering the nucleus will facilitate the development of better decoy ODNs. Increased mechanistic understanding will improve targeting and therapeutic functionality of STAT3 drugs. Given the recent recommendations for immunotherapy as first-line treatment for NSCLC and the immunomodulatory effects of STAT3, CS3D impact on the immunogenic milieu within the tumor microenvironment as well as within the immune cells themselves would offer valuable insight into the interaction of the two therapies.
In summary, Njatcha et al. show that cyclic STAT3 decoys are effective in NSCLC in vitro and in vivo models. The inherent genetic heterogeneity along with a striking ability to acquire resistance to various therapies, make NSCLC incredibly difficult to treat. The ability of CS3D to selectively target and kill cancer cells, necessitate the continued development and preclinical assessment of CS3D with the eventual goal of conducting clinical trials.
Acknowledgements
Funding: The study was supported by the UNMC Graduate Student Fellowship (R44CA224619, R41CA213718, PO1 CA217798, UO1 CA200466).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Society AC, editor. Cancer Facts & Figures 2018. Atlanta, 2018:22-3.
- Testa U, Castelli G, Pelosi E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers (Basel) 2018.10. [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. [Crossref] [PubMed]
- Sanford M, Scott LJ. Gefitinib: a review of its use in the treatment of locally advanced/metastatic non-small cell lung cancer. Drugs 2009;69:2303-28. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Hecker M, Wagner AH. Transcription factor decoy technology: A therapeutic update. Biochem Pharmacol 2017;144:29-34. [Crossref] [PubMed]
- Rad SM, Langroudi L, Kouhkan F, et al. Transcription factor decoy: a pre-transcriptional approach for gene downregulation purpose in cancer. Tumour Biol 2015;36:4871-81. [Crossref] [PubMed]
- Darnell JE. Validating Stat3 in cancer therapy. Nat Med 2005;11:595-6. [Crossref] [PubMed]
- Takemoto S, Ushijima K, Kawano K, et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer 2009;101:967-72. [Crossref] [PubMed]
- Huang Z, Lei W, Hu HB, et al. H19 promotes non-small-cell lung cancer (NSCLC) development through STAT3 signaling via sponging miR-17. J Cell Physiol 2018;233:6768-76. [Crossref] [PubMed]
- Wang J, Wang Y, Zheng C, et al. Tyrosine kinase inhibitor-induced IL-6/STAT3 activation decreases sensitivity of EGFR-mutant non-small cell lung cancer to icotinib. Cell Biol Int 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Schlessinger K, Levy DE. Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer Res 2005;65:5828-34. [Crossref] [PubMed]
- Sen M, Thomas SM, Kim S, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov 2012;2:694-705. [Crossref] [PubMed]
- Njatcha C, Farooqui M, Kornberg A, et al. STAT3 Cyclic Decoy Demonstrates Robust Antitumor Effects in Non-Small Cell Lung Cancer. Mol Cancer Ther 2018. [Epub ahead of print]. [Crossref] [PubMed]


