
Optimizing immobilization, margins, and imaging for lung stereotactic body radiation therapy
Introduction
Stereotactic body radiation therapy (SBRT) is defined by the delivery of high doses of radiation over 1–5 treatments to a tumor volume known in 3D coordinates using image-guided conformal treatment plans. This technique was initially adapted from cranial stereotactic radiosurgery (SRS) to the treatment of cT1-2N0 early stage non-small cell lung cancer (ES-NSCLC) in medically inoperable patients (1). The use of SBRT has rapidly gained acceptance in the treatment of medically inoperable patients due to excellent disease control rates (2). More recently, it has also been employed as an alternative to surgical resection in operable patients due to its relative clinical equipoise from randomized trial data (3). It has also been proven an excellently effective treatment for small lesions in patients with oligometastatic disease (4,5).
Advances in the delivery of radiation therapy (RT) has led to the ability to deliver SBRT accurately and safely, and utilization will likely only continue to increase. NSCLC is the leading cause of cancer-related death in the United States (6). While the majority of patients traditionally present with advanced stage disease, the proportion diagnosed with ES-NSCLC is rising due to increases in medical imaging and the early adoption of CT-based screening of high-risk populations that is associated with survival benefits (7). While, historically, these patients have been managed primarily with surgery, a significant proportion are unable to undergo resection due to medical comorbidities. In limited prospective evaluations, SBRT has demonstrated relative equivalency to lobar resection (8), and additional clinical trials are ongoing in surgical candidates with early stage disease. Finally, SBRT is currently being used in the treatment of limited metastatic disease to many areas of the body, including lung metastases (9,10).
The goal of SBRT is to accurately target the tumor and deliver sufficient dose to achieve local control while minimizing the dose to highly sensitive surrounding organs at risk (OARs) including the lungs and airways, heart and great vessels, esophagus, brachial plexus, and spinal cord. As fraction numbers decrease and dose per fraction rises, treatments have necessarily become more conformal to decrease adverse events. More emphasis is necessarily placed for this technique on accurately targeting the tumor and verifying position at the time of delivery. While SBRT for ES-NSCLC was pioneered based on the principles of SRS in the brain, the lack of a truly stereotactic frame and the unique challenge of intrafractional breathing motion of the tumor require particular attention to immobilization and tumor motion management or mitigation.
Increases in the positional uncertainty of the tumor and normal tissues in ES-NSCLC from breathing motion requires incorporation of motion management into the treatment simulation procedure, radiation planning, and treatment delivery to achieve adequate tumor control. This challenge has led to the development of multiple methods to monitor and mitigate motion during simulation. Additionally, accurate verification of tumor location at the time of treatment using onboard image guidance is necessary to ensure accurate target coverage despite the necessary reductions in the planning target volume (PTV) margins to decrease dose to OARs. Broadly defined, image-guided RT (IGRT) is the utilization of various imaging modalities prior to or during radiation treatments to align and verify anatomical agreement between the simulation anatomy, RT treatment plan, and the patient at the time of treatment. Additionally, changes in anatomy between simulation and treatments are common in thoracic malignancies, and alterations can lead to overtreatment of OARs and/or inadequate dose to the target (11-13). As complications from SBRT can potentially be life-threatening (14), adequate verification is essential to the delivery of high-quality treatments.
Motion management strategies
Introduction
The treatment of ES-NSCLC with SBRT requires understanding, modeling, and controlling two different types of motion: (I) inter-fractional motion resulting from setup uncertainties inherent to fractionated radiation treatments based on a prior CT simulation and (II) intra-fractional motion mostly stemming from the respiratory cycle that has proved to have a significant impact on RT planning and delivery (15). Methodologies have been developed to aid in accurately defining, targeting, computing, aligning, and finally delivering a prescribed radiotherapy treatment.
Inter-fraction motion is largely addressed at the time of simulation. While fixed immobilization was initially used in cranial SRS, this strategy is not directly translatable within the thorax. Early efforts led to a number of solutions to enhance fixation of the thoracic cavity and improve reproducibility of patient set up. While a number of solutions including external coordinate systems and whole-body wrapping were developed, some concerns remained about the reproducibility of deep thoracic tumors despite external fixation (12). Additionally, technological advances in image guidance and motion management, as described below, have led to decreased emphasis on extensive external immobilization techniques.
With respect to management of respiratory cycle-induced intra-fraction motion, there are multiple solutions to evaluate motion in a patient-specific manner and then to manage this motion during treatment delivery. Some such measures include 4-dimensional imaging, respiratory-gating, breath-hold, motion mitigation, and tumor tracking. Compared to locally advanced tumors, ES-NSCLC tumors are often highly mobile, especially in the lower lung lobes. When selecting a motion management strategy for an individual patient, several factors are important to consider, including clinical tolerability, target reproducibility, and OAR positioning. These techniques can often be combined for effective and efficient delivery.
Techniques
The most straightforward solution to manage the uncertainty of respiratory motion is to encompass the entire potential range of tumor motion through the breathing cycle in the treatment field. In practice, this method consists of capturing and targeting the full extent of tumor motion as part of the treatment target. Broadly, the three ways to encompass motion are breath-hold CT, slow CT, and four-dimensional CT scanning (4DCT). Breath-hold CT scans are obtained by performing CT scans at a minimum of two discrete points in the respiratory cycle, including end inspiration and end expiration (16-18). Slow CT scanning refers to performing multiple CT scans over several cycles of respiration. These scans are registered to one another, and subsequently the tumor is drawn on each CT scan to generate the internal target volume (ITV), encompassing all areas where the tumor lies throughout the respiratory cycle. While these methods provide some additional snapshots of tumor location during the breathing cycle, they do not always do so systematically or in positions the patient is likely to be during treatment.
4DCT scanning has largely supplanted both slow CT and breath-hold CTs (19,20). 4DCTs provide clinicians with multiple CT scans spanning the breathing cycle, including the tumor path during the interval portions of the breathing cycle. This method is less vulnerable to the distortion of tissue planes that can appear using the slow CT method. Additional reconstructions, including average CT, maximum intensity projection (MIP) images or cine-mode, should be used with caution as they can decrease the benefit of 4DCT for ITV generation (21-23).
As an example, researchers at Thomas Jefferson University evaluated ITV generation based on utilization of only selective phases of previously acquired 4DCTs. They reported that contouring on all phases of the inhalation portion of the breathing cycle led to a good estimate of the ITV from all contoured phases. In contrast, contouring only on the extreme phases generated an ITV that did not account for centripetal motion of the tumor during the breathing cycle (24).
Drawbacks of 4DCT include requirement for additional software and hardware to gather and reconstruct the acquired images, higher patient radiation exposure, and increases in workload for physician target delineation. Additionally, changes in respiration pattern can occur between 4DCT acquisition and treatment and are not accounted for with this method (25). However, a drawback of all techniques is the lack of method to accurately account for changes in breathing patterns between the time of image acquisition and treatment. Only intra-fractional, fluoroscopic or MR-based imaging can monitor motion in real-time to ensure tumoral position within the target throughout the breathing cycle. Finally, and perhaps most importantly, the 4DCT method leads to increasing target volumes and, therefore, greater exposure of normal tissues to potentially unnecessary radiation.
In an effort to address these concerns, respiratory gating has been evaluated, which utilizes a trigger to activate and deactivate treatment. As an example, when the tumor or tumor surrogate is outside the treatment field, the “gate” is closed and treatment is placed on hold until the tumor or surrogate moves back into position, at which point the “gate” is reopened and treatment is reinitiated or continued.
Respiratory gating has been studied at multiple centers and across multiple disease sites (26-29). As the primary motion of concern for SBRT is respiratory in nature, numerous technology systems have been employed using chest wall motion or breath air volume as surrogates for tumor location (30-32). An alternative or complementary strategy includes placement of intra- or peritumoral fiducials or real-time imaging to monitor tumor location (33,34).
While respiratory gating provides an opportunity to decrease irradiation of normal tissues compared to motion inclusion, treatment during only a portion of the respiratory cycle necessarily prolongs treatment delivery time, which can lead to patient comfort/movement concerns (35), as well as operational inefficiencies. Treatment duration is dependent on the gating parameters, and as the parameters become more relaxed, the treatment time difference decreases; however, the benefit of gating will also be somewhat sacrificed. Gating also often relies on a surrogate in SBRT, and decoupling of the surrogate from the tumor can lead to systematic uncertainty (35).
Breath-hold is a similar approach to respiratory gating except that it includes active participation from the patient during simulation and treatment in an effort to decrease target volume. Deep-inspiration breath hold (DIBH) and mid-inspiration breath hold (MIBH) are two techniques preferably performed with some form of verification such as spirometry (36). Visual coaching during simulation and treatment are used to generate a reproducible point at which the patient will hold his/her breath, and the treatment is interrupted when the patient begins to breathe again.
The Active Breathing Control (ABC) system is one such method to achieve breath-hold that can be used for SBRT. A spirometer measures respiratory levels, and glasses worn by the patient provide feedback to the patient, providing a target inspiration level for the patient that is determined at simulation and subsequently utilized for treatment (37).
Another method to decrease tumor motion that does not include active participation from the patient is the use of a motion mitigation apparatus to limit tumor excursion during the breathing cycle. These methods allow the patient to continue breathing during simulation and treatment and may make tumor motion more regular and limit artifacts from irregular breathing cycles. This technique is typically combined with generation of a 4DCT scan.
For example, abdominal compression forces more shallow breathing by the patient and thus less tumoral excursion, and it is a commonly used technique during SBRT treatment courses for both lung and upper abdominal tumors (38-40) (Figure 1). Abdominal compression can be achieved using a belt-like device or a stereotactic body frame which applies a constant pressure to the upper abdomen and reduces potential diaphragmatic excursion. Similar to breath-hold, appropriate patient selection is key given that erratic breathing from distress in some patients can obviate any benefits achieved from compression. Motion mitigation is predominantly useful in patients with pre-mitigation tumor excursions of >8–10 mm. It is our clinic’s practice to recommend attempts to mitigate motion when above this threshold (41).
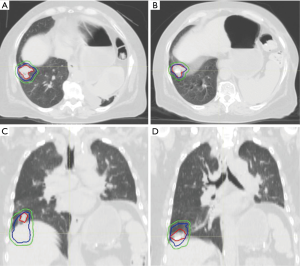
Tumor tracking is the final broadly defined method for motion management with a goal of dynamically tracking the tumor while delivering treatment. This theoretically provides an opportunity to treat the patient more quickly than breath-hold while also minimizing treatment volume and irradiation of normal tissues. Tumor tracking can be accomplished through direct monitoring of the tumor using radiography/fluoroscopy, often coupled with implanted fiducial markers (42) or surrogate chest wall evaluations (43). One particularly compelling approach for tumor tracking of lung cancer involves implantation of electromagnetic radiofrequency (RF) transponders into the tumor. The transponders provide localization to an external electromagnetic array allowing for real-time positional feedback during treatment delivery (44).
Overall, the clinical benefits of SBRT for ES-NSCLC and unique features including significant intra-fraction motion and high-density changes between lung and tumor have led to significant advances in motion management technologies that have been subsequently adapted to multiple disease sites and clinical presentations. Continued research and clinical evaluation are ongoing and have the potential to lead to decreases in toxicities to normal tissues while ensuring accurate, reproducible, and effective tumor treatment.
Image guidance
Introduction
Image guidance at the time of treatment delivery is an important aspect of stereotactic treatment delivery and essential when using the high doses per fraction for treatment of ES-NSCLC. The primary goal of IGRT is to improve treatment accuracy by accurately aligning the patient and his/her tumor prior to delivery. Improved image guidance can lead to smaller PTV margins and, therefore, decreased doses to OARs, while maintaining optimal target coverage. One study demonstrated a systemic uncertainty of 3.4 mm and random uncertainty of 2.7 mm in SBRT with body fixation alone. These values were decreased to 0.6 and 0.9 mm, respectively, with the use of advanced image guidance, highlighting the importance of the complementary nature of these techniques (45).
Accurate alignment is the first goal of IGRT and can be accomplished using orthogonal kilovolt (kV)/megavolt (MV) X-rays or cone-beam (CB) kV/MV CT scans. CBCT provides volumetric imaging and thus the opportunity to assess for changes in tumor size, position relative to critical structures, and tumor position in the PTV. Here, we discuss methods for implementation of IGRT with a specific focus on use in SBRT for ES-NSCLC.
Imaging modalities
Image guidance initially utilized planar film and subsequently electronic portal imaging devices (EPIDs). MV imaging using the treatment beam is still routinely used for treatment field verification. While very useful for soft tissue delineation, MV imaging has lower quality compared to kV imaging for bone. The addition of kV imaging arms to linear accelerators along with EPID data acquisition have been widely adopted due to their lower dose to the patient and superior image quality. Orthogonal kV/EPID arrays can be utilized as a tracking device during treatment (34,46,47) along with their routine use for pre-treatment static imaging. Room-mounted EPIDs are also commercially available for certain stereotactic systems. Limitations include imaging dose, fiducial requirement, lack of volumetric spatial resolution, and directional beam entrance limitations.
Volumetric-based imaging allows for the generation of 3D image sets that can be overlaid onto the simulation CT scan for matching. Initially, this was performed with CT with the patient on a treatment couch; however, this has been largely supplanted by volumetric imaging on-board the linear accelerator that can perform CBCT. CBCT permits patient setup and correction of positional errors based on an assessment of 3D soft tissue anatomy compared to the planning scan (48). Corradetti et al. evaluated kV X-ray matching of bony anatomy to tumor matching using volumetric CBCT datasets. Using data from 87 patients, they demonstrated a difference of 2.2 mm in the anterior-posterior, 1.8 mm in the superior-inferior and 1.6 mm in the right-left directions, suggesting kV matching to bone was inferior to CBCT matching to tumor. They also studied the intra-fractional tumor motion by immediate post-treatment CBCT. Finally, they document significant intra-fraction shifts were required using CBCTs for 27% of treatment fractions (49).
CBCTs significantly increase target accuracy and reduce errors and are routinely utilized, and they are thus recommended for treatment using SBRT in ES-NSCLC (50-52). CBCTs do have some limitations, which include their decreased quality compared to diagnostic scanners due to helical scattering and longer acquisition time. Respiratory correlated 4D CBCT is a novel solution to overcome the acquisition time limitation and uses relative positioning of the diaphragm to prepare a 4D CBCT for evaluation (53).
IGRT in SBRT
Due to the high dose per fraction, interplay between the breathing cycle and tumor location (54), and small PTV margins, evaluation of pretreatment IGRT is critical to the success of SBRT for ES-NSCLC. The close proximity of thoracic SBRT targets to critical, radiosensitive structures necessitates precision and accuracy.
A significant amount of effort has been devoted to determining the optimal alignment paradigm for SBRT. Evidence suggests that the use of stereotactic body frames are not, alone, as accurate as image guidance, and, consequently, motion management and image guidance are essential for the safe delivery of SBRT (11,12). While matching bony landmarks by using X-ray-based kV imaging is widely prevalent even for SBRT treatments in the thorax/abdomen, studies have shown that matching soft tissue/tumor by CBCT/volumetric imaging can be very advantageous (55). Therefore, the use of volumetric imaging is recommended for all thoracic SBRT patients to reduce treatment errors.
Conclusions
Motion management and image guidance are essential to the optimal delivery of SBRT for ES-NSCLC. Continued investigation and technological advances promise to increase accuracy and decrease toxicity with SBRT in the future. As more patients are being recommended to undergo SBRT for ES-NSCLC and for lung metastases—including oligometastatic and oligoprogressive disease—it will be imperative to carefully implement strategies to ensure optimal dose delivery and quality assurance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Simone CB 2nd, Wildt B, Haas AR, et al. Stereotactic body radiation therapy for lung cancer. Chest 2013;143:1784-90. [Crossref] [PubMed]
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: Surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [PubMed]
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28-37. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Williams SB, Shan Y, Jazzar U, et al. Comparing Survival Outcomes and Costs Associated With Radical Cystectomy and Trimodal Therapy for Older Adults With Muscle-Invasive Bladder Cancer. JAMA Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Siva S, Slotman BJ. Stereotactic Ablative Body Radiotherapy for Lung Metastases: Where is the Evidence and What are We Doing With It? Semin Radiat Oncol 2017;27:229-39. [Crossref] [PubMed]
- Baumann BC, Nagda SN, Kolker JD, et al. Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: A potential alternative to resection. J Surg Oncol 2016;114:65-9. [Crossref] [PubMed]
- Worm ES, Hansen AT, Petersen JB, et al. Inter-and intrafractional localisation errors in cone-beam CT guided stereotactic radiation therapy of tumours in the liver and lung. Acta Oncol 2010;49:1177-83. [Crossref] [PubMed]
- Guckenberger M, Meyer J, Wilbert J, et al. Cone-beam CT based image-guidance for extracranial stereotactic radiotherapy of intrapulmonary tumors. Acta Oncol 2006;45:897-906. [Crossref] [PubMed]
- Sonke JJ, Rossi M, Wolthaus J, et al. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance. Int J Radiat Oncol Biol Phys 2009;74:567-74. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM task group 76. Med Phys 2006;33:3874-900. [Crossref] [PubMed]
- Lagerwaard FJ, Van Sornsen de Koste JR, Nijssen-Visser MR, et al. Multiple "slow" CT scans for incorporating lung tumor mobility in radiotherapy planning. Int J Radiat Oncol Biol Phys 2001;51:932-7. [Crossref] [PubMed]
- de Koste JR, Lagerwaard FJ, de Boer HC, et al. Are multiple CT scans required for planning curative radiotherapy in lung tumors of the lower lobe?. Int J Radiat Oncol Biol Phys 2003;55:1394-9. [Crossref] [PubMed]
- van Sörnsen de Koste JR, Lagerwaard FJ, Schuchhard-Schipper RH, et al. Dosimetric consequences of tumor mobility in radiotherapy of stage I non-small cell lung cancer--an analysis of data generated using 'slow' CT scans. Radiother Oncol 2001;61:93-9. [Crossref] [PubMed]
- Vedam SS, Keall P, Kini V, et al. Acquiring a four-dimensional computed tomography dataset using an external respiratory signal. Phys Med Biol 2003;48:45. [Crossref] [PubMed]
- Ford EC, Mageras G, Yorke E, et al. Respiration-correlated spiral CT: A method of measuring respiratory-induced anatomic motion for radiation treatment planning. Med Phys 2003;30:88-97. [Crossref] [PubMed]
- Rietzel E, Liu AK, Chen GT, et al. Maximum-intensity volumes for fast contouring of lung tumors including respiratory motion in 4DCT planning. Int J Radiat Oncol Biol Phys 2008;71:1245-52. [Crossref] [PubMed]
- Weiss E, Wijesooriya K, Ramakrishnan V, et al. Comparison of intensity-modulated radiotherapy planning based on manual and automatically generated contours using deformable image registration in four-dimensional computed tomography of lung cancer patients. Int J Radiat Oncol Biol Phys 2008;70:572-81. [Crossref] [PubMed]
- Park K, Huang L, Gagne H, et al. Do maximum intensity projection images truly capture tumor motion? Int J Radiat Oncol Biol Phys 2009;73:618-25. [Crossref] [PubMed]
- Cao J, Cui Y, Champ CE, et al. Determination of internal target volume using selective phases of a 4-dimensional computed tomography scan. Pract Radiat Oncol 2012;2:186-92. [Crossref] [PubMed]
- Redmond KJ, Song DY, Fox JL, et al. Respiratory motion changes of lung tumors over the course of radiation therapy based on respiration-correlated four-dimensional computed tomography scans. Int J Radiat Oncol Biol Phys 2009;75:1605-12. [Crossref] [PubMed]
- Ohara K, Okumura T, Akisada M, et al. Irradiation synchronized with respiration gate. Int J Radiat Oncol Biol Phys 1989;17:853-7. [Crossref] [PubMed]
- Tada T, Minakuchi K, Fujioka T, et al. Lung cancer: Intermittent irradiation synchronized with respiratory motion--results of a pilot study. Radiology 1998;207:779-83. [Crossref] [PubMed]
- Minohara S, Kanai T, Endo M, et al. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1097-103. [Crossref] [PubMed]
- Hara R, Itami J, Kondo T, et al. Stereotactic single high dose irradiation of lung tumors under respiratory gating. Radiother Oncol 2002;63:159-63. [Crossref] [PubMed]
- Duan J, Shen S, Fiveash JB, et al. Dosimetric effect of respiration-gated beam on IMRT delivery. Med Phys 2003;30:2241-52. [Crossref] [PubMed]
- Ramsey CR, Cordrey IL, Oliver AL. A comparison of beam characteristics for gated and nongated clinical x-ray beams. Med Phys 1999;26:2086-91. [Crossref] [PubMed]
- Nakagawa K, Haga A, Kida S, et al. 4D registration and 4D verification of lung tumor position for stereotactic volumetric modulated arc therapy using respiratory-correlated cone-beam CT. J Radiat Res 2013;54:152-6. [Crossref] [PubMed]
- Shimizu S, Shirato H, Ogura S, et al. Detection of lung tumor movement in real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2001;51:304-10. [Crossref] [PubMed]
- Seppenwoolde Y, Shirato H, Kitamura K, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys 2002;53:822-34. [Crossref] [PubMed]
- Malinowski K, McAvoy TJ, George R, et al. Incidence of changes in respiration-induced tumor motion and its relationship with respiratory surrogates during individual treatment fractions. Int J Radiat Oncol Biol Phys 2012;82:1665-73. [Crossref] [PubMed]
- Duggan DM, Ding GX, Coffey CW II, et al. Deep-inspiration breath-hold kilovoltage cone-beam CT for setup of stereotactic body radiation therapy for lung tumors: Initial experience. Lung Cancer 2007;56:77-88. [Crossref] [PubMed]
- Koto M, Takai Y, Ogawa Y, et al. A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 2007;85:429-34. [Crossref] [PubMed]
- Lax I, Blomgren H, Näslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen: Methodological aspects. Acta Oncol 1994;33:677-83. [Crossref] [PubMed]
- Heinzerling JH, Anderson JF, Papiez L, et al. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys 2008;70:1571-8. [Crossref] [PubMed]
- Lin L, Souris K, Kang M, et al. Evaluation of motion mitigation using abdominal compression in the clinical implementation of pencil beam scanning proton therapy of liver tumors. Med Phys 2017;44:703-12. [Crossref] [PubMed]
- Videtic GM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [Crossref] [PubMed]
- Hughes S, McClelland J, Tarte S, et al. Assessment of two novel ventilatory surrogates for use in the delivery of gated/tracked radiotherapy for non-small cell lung cancer. Radiother Oncol 2009;91:336-41. [Crossref] [PubMed]
- Mayse ML, Smith RL, Park M, et al. Development of a non-migrating electromagnetic transponder system for lung tumor tracking. Int J Radiat Oncol Biol Phys 2008;72:S430. [Crossref]
- Grills IS, Hugo G, Kestin LL, et al. Image-guided radiotherapy via daily online cone-beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1045-56. [Crossref] [PubMed]
- Whyte RI, Crownover R, Murphy MJ, et al. Stereotactic radiosurgery for lung tumors: Preliminary report of a phase I trial. Ann Thorac Surg 2003;75:1097-101. [Crossref] [PubMed]
- Shirato H, Shimizu S, Kitamura K, et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int J Radiat Oncol Biol Phys 2000;48:435-42. [Crossref] [PubMed]
- Higgins J, Bezjak A, Franks K, et al. Comparison of spine, carina, and tumor as registration landmarks for volumetric image-guided lung radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1404-13. [Crossref] [PubMed]
- Corradetti MN, Mitra N, Millar LPB, et al. A moving target: Image guidance for stereotactic body radiation therapy for early-stage non-small cell lung cancer. Pract Radiat Oncol 2013;3:307-15. [Crossref] [PubMed]
- Bissonnette JP, Purdie TG, Higgins JA, et al. Cone-beam computed tomographic image guidance for lung cancer radiation therapy. Int J Radiat Oncol Biol Phys 2009;73:927-34. [Crossref] [PubMed]
- Wang Z, Nelson JW, Yoo S, et al. Refinement of treatment setup and target localization accuracy using three-dimensional cone-beam computed tomography for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:571-7. [Crossref] [PubMed]
- Rosenzweig KE, Amols H, Ling CC. New radiotherapy technologies. Semin Surg Oncol 2003;21:190-5. [Crossref] [PubMed]
- Sonke JJ, Zijp L, Remeijer P, et al. Respiratory correlated cone beam CT. Med Phys 2005;32:1176-86. [Crossref] [PubMed]
- Zou W, Yin L, Shen J, et al. Dynamic simulation of motion effects in IMAT lung SBRT. Radiat Oncol 2014;9:225. [Crossref] [PubMed]
- Hawkins MA, Brock KK, Eccles C, et al. Assessment of residual error in liver position using kV cone-beam computed tomography for liver cancer high-precision radiation therapy. Int J Radiat Oncol Biol Phys 2006;66:610-9. [Crossref] [PubMed]


