
Immunotherapy for non-small cell lung cancer: from clinical trials to real-world practice
Introduction
The introduction of immunotherapy in the treatment of advanced non-small cell lung cancer (NSCLC) has resulted in a new era of treatment options that have substantially improved efficacy and tolerability when compared to chemotherapy. Since 2015, many immunotherapeutic agents have been approved for the in advanced NSCLC. Treatment of nivolumab, pembrolizumab, and atezolizumab are all approved in the second line setting. Pembrolizumab is also approved in the first line setting, both as monotherapy and in combination with chemotherapy. Durvalumab is now approved after concurrent chemoradiation for stage III lung cancer. A large number of ongoing clinical trials are investigating the use of checkpoint inhibitors in combination with other treatments in both NSCLC and small cell lung cancer.
The rapid adoption of immune checkpoint inhibitors into clinical practice has been truly astounding. As these agents move from the highly controlled context of therapeutic clinical trials to real-world oncology practices, a number of key differences are expected. These range from patient characteristics (including age, performance status, concomitant medications, the presence or absence of brain metastases, history of autoimmune disease, and availability of archival tumor tissue), to available guidance for the detection and management of immune-related adverse events, to interpretation of efficacy and decisions on treatment duration. Thus, understanding the experience of immunotherapy in real-life practice provides essential and complementary data to clinical trial findings.
In a recently published analysis of 188 patients treated with immunotherapy for lung cancer in Greece, Areses Manrique et al. have sought to address these questions (1). Before describing the contribution to these considerations rendered by the recent publication, however, it is essential to review the clinical development of immunotherapy and the patient populations in which it has occurred (Table 1).
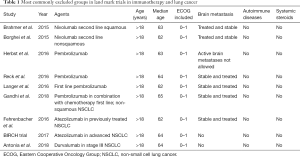
Full table
Clinical trials that studied immunotherapy in lung cancer
Several landmark immunotherapy clinical trials have been conducted in patients with advanced NSCLC, with many more ongoing. In 2015, some of these trials resulted in U.S. Food and Drug Administration (FDA) approval of two immune check point inhibitors for second-line treatment of advanced NSCLC. Data from checkmate 017 (2), checkmate 057 (3) and Keynote 010 (4) showed significant improvement in overall survival (OS) with immune check point inhibitors compared to docetaxel chemotherapy in squamous and non-squamous cell histologies. These treatments were associated with significantly less grade 3–4 side effects. In 2016, Reck et al. (5) showed that pembrolizumab treatment in the front-line setting showed significant survival improvement when compared to platinum doublet chemotherapy in patient with high proportion of programmed death-ligand 1 (PDL1) expression. Again, this was associated with fewer and less severe treatment-related toxicities. Later, the anti-PD-L1 checkpoint inhibitor atezolizumab showed survival benefit and better tolerability compared with docetaxel in the second-line setting (6,7).
Langer et al. conducted a phase II randomized clinical trial exploring the addition of pembrolizumab to chemotherapy as a first-line agent in patients with advanced non-squamous NSCLC (8). Superior response rate, progression, free survival, and OS were observed in the pembrolizumab plus chemotherapy arm, and these results were confirmed in a subsequent phase 3 trial (9). Atezolizumab has also been studied in the first line setting with or without chemotherapy and resulted in favorable outcome (10-12).
For patients with advanced NSCLC without targetable mutations, using single-agent immunotherapy as first-line therapy is dependent on PD-L1 status. If the PD-L1 expression is ≥50%, then pembrolizumab may be chosen for first-line therapy. Nivolumab and atezolizumab are approved for second-line treatment for stage IV NSCLC regardless of tumor PD-L1 expression and therefore can be used in patients without any detectable expression of PD-L1 or those without sufficient tissue for PD-L1 analysis. Pembrolizumab may be used in the second-line setting if tumor expression of PD-L1 is ≥1%. Most recently, immunotherapy has been incorporated in the treatment of locally advanced NSCLC after concurrent chemo and radiation based on the PACIFIC trial that showed significantly improved PFS with durvalumab compared with placebo (13). Durvalumab is currently the only immune check point inhibitor that is approved as consolidation therapy in patients with unresectable stage III NSCLC with no disease progression after at least two cycles of chemoradiation.
Adoption of immunotherapy in clinical practice
Impressive data from clinical trials show that immune checkpoint inhibitors can induce durable responses in some patients, which has led to accelerated approval for some of these drugs prior to confirmatory phase 3 trials. The excitement about checkpoint inhibitors is often coupled with rapid adoption into clinical practice. Patients with advanced lung cancer may be enthusiastic to try new treatment options, especially if these new treatments have shown promising efficacy and relatively low toxicity. It is now common for novel therapies to become the new standard of care in less than 4 months after the FDA approval (14).
Why is real-world practice sometimes different from clinical trial experience?
The risk in rapid application of immunotherapy into clinical practice is the potential discrepancy in patient characteristics and treatment administration and monitoring in real-world practice versus those in clinical trials. Earlier studies have demonstrated that experience with novel treatments in the clinic may differ considerably from effects reported in clinical trials (15,16).
Only 2% of adult patients with cancer participate in clinical trials (17), representing a population that is motivated, has access to clinical trials, and manages to meet increasingly numerous and restrictive eligibility criteria. These individuals are often younger and healthier than the broader oncology patient population (16,18). They are also more likely to tolerate treatment and derive clinical benefits (19). As immune checkpoint inhibitors enter clinical practice, it can be assumed that patients ineligible for immunotherapy clinical trials are receiving these agents, including those with Eastern Cooperative Oncology Group (ECOG) performance status >1, history of autoimmune disease, chronic steroid requirement, significant organ dysfunction, or symptomatic brain metastases.
Accordingly, it may be challenging to anticipate whether the magnitude of benefit of novel treatments observed in clinical trials is reproducible in clinical practice, as sicker individuals who are not represented in clinical trials form a substantial proportion of cancer patients in clinical practice (20). Data on using these novel therapies in a cohort that represents real-world considerations are critically needed. This information may guide not only the selection of patients who should receive these treatments, but also inform their clinical monitoring and expectations.
Real-life challenges in giving immunotherapy to lung cancer patients: central nervous system (CNS) metastasis
Lung cancer cases account for 40% to 50% of brain metastases (21). Patients with active or untreated CNS metastasis are widely excluded in clinical trials (2,3,7), and there are no data to show whether immunotherapy is safe and effective in this situation. Historically, the median OS of patients with metastatic brain tumors has been very poor, typically in the range of 2–4 months (22). However, outcomes are changing rapidly due to the recent introduction of new and more effective therapeutic strategies. Small prospective and retrospective studies have examined the administration of immunotherapy in NSCLC with active brain metastases. While Kanai et al. reported exacerbation of neurologic symptoms in most patients with untreated brain metastasis who received nivolumab (23), another study reported objective response in 33% of NSCLC with active brain metastases treated with pembrolizumab (24). Complete responses in the brain with immunotherapy were also reported in other studies (25). Overall, these results provide evidence that anti-PD-1 monotherapy can induce intracranial ORs in patients with NSCLC and brain metastases, particularly in cases of asymptomatic disease. Nevertheless, because inflammation may exacerbate complications of brain metastases, it remains unclear whether these agents should be used in cases of symptomatic brain metastases or high intracranial disease burden (1).
Patients with autoimmune diseases
It is estimated that 20 to 50 million individuals have autoimmune disease in the United States (26). These patients are universally excluded from clinical trials investigating immunotherapies in lung cancer. This is typically done to lower the risk for developing immune related adverse events, which may potentially be severe and permanent. Interestingly and even though this subgroup of patient is underrepresented in clinical trials, patients with autoimmune disorders are diagnosed with cancer at least as frequently as the general population. In fact, there are some reports that suggest increased risk in this population (27).
About 13% of patients with lung cancer have preexisting autoimmune disease (28). Although these patients are often excluded from immunotherapy clinical trials, small series have reported that administering immune checkpoint inhibitors to patients with autoimmune disease increased the risk for exacerbation but is feasible with careful monitoring (29). Clinicians treating patients outside clinical trials have generally been reluctant to offer cancer immunotherapy to this patient group. It will remain unclear to how to best approach these patients as long as these patients are excluded from clinical trials. Due to the paucity of relevant data, many times the decision is based upon anecdotal experience or small case series or case reports. Therefore, additional studies to clarify whether cancer immunotherapy is safe and effective in patients with preexisting autoimmune diseases are highly warranted.
Patients with poor performance status
While individuals with advanced age, ECOG performance status ≥2, multiple co-morbidities, and/or brain metastases comprise 30–50% of the real-world lung cancer population (30), they are universally underrepresented in the randomized clinical trials (31). These patients are especially prone to toxic treatment effects. It is unclear whether systemic immunotherapies in this category of patients provides more benefit than risk (32). For this population, observational and registry-based data becomes the only available source to guide treatment decisions. More recently, some immunotherapy trials have included these groups. In the CheckMate 153 trial, patients with ECOG PS2 and age older than 70 were included (33). Nivolumab was effective and well tolerated in PS2 patients. Preliminary data from CheckMate 171 found that the efficacy and safety of nivolumab in patients age ≥70 years and PS2 were similar to other groups (34). However, in contrast to these trials, other studies have reported inferior survival in ECOG PS ≥2 patients as compared to patients with ECOG PS 0/1 (1,25). Whether anti-PD-1/anti-PD-L1 agents can provide a clinically meaningful benefit to patients with ECOG 2–4, for whom therapeutic options are limited, remains an important question which warrants further evaluation in a prospective randomized controlled trial.
Patients receiving steroids and other immunosuppressive agents
Currently, a large fraction of immunotherapy clinical trials exclude patients receiving systemic corticosteroids or other immunosuppressive agents. This practice reflects the hypothesis that systemic steroids would antagonize the anti-cancer effects of immunotherapy. Corticosteroids are commonly prescribed to cancer patients as antiemetic or anti-inflammatory agents, particularly in patients with brain metastases who undergo brain radiation. Although the use of corticosteroids to manage immune-related adverse events does not appear to compromise anti-cancer efficacy (35), whether chronic immunosuppression from steroids or other agents can be administered concurrently with checkpoint inhibitors remains unanswered.
The current publication
How does the report by Areses Manrique and colleagues add to our understanding of immunotherapy in real-world practice? Immunotherapy agents in lung cancer are commonly used in patients not represented in the landmark trials. It is currently unclear whether advanced age, poor performance status and brain metastasis have adverse effects on the outcome of lung cancer patients treated with immunotherapy. Areses Manrique et al. studied real world patients (1). In this trial There were no statistically significant differences in OS regarding age. This suggests that elderly with good performance status derive the same benefit from nivolumab as their younger counterparts. These findings are in accordance with the literature (3,7,36) demonstrating similar benefit across all age subgroups. Inferior outcome was reported for patients with ECOG PS2 and for patients with brain metastasis, which is also consistent with prior literature (1,22,25). Unfortunately, similar to other clinical trials, patients with severe autoimmune diseases and patients on systemic corticosteroids were excluded. More investigation is needed, with specific prospective clinical trials for these subgroups of patients.
Conclusions
Real-world experience with immunotherapy in NSCLC differs from clinical trials. Little data exists with regards to safety and efficacy of anti-PD-1/anti-PD-L1 agents in patients with poor ECOG PS, elderly, patients with untreated asymptomatic brain metastases, patients with autoimmune diseases, and patients on chronic steroids. Broader, more inclusive eligibility criteria with large phase III clinical trials, along with retrospective studies examining drug efficacy and tolerability in real-world patient populations, are needed to fill the data gap between real world practice and clinical trials.
Acknowledgments
Funding: This study was supported in part by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24 CA201543-01, to DE Gerber).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Areses Manrique MC, Mosquera Martinez J, Garcia Gonzalez J, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res 2018;7:404-15. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Perez-Gracia JL, et al. P2.41 (also presented as PD1.06): Pembrolizumab vs Docetaxel for Previously Treated NSCLC (KEYNOTE-010): Archival vs New Tumor Samples for PD-L1 Assessment: Track: Immunotherapy. J Thorac Oncol 2016;11:S242-3. [Crossref]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Wakelee H, Patel JD, Heist R, et al. ORAL01.04: Phase II Trial of Atezolizumab for Patients with PD-L1-Selected Advanced NSCLC (BIRCH): Updated Efficacy and Exploratory Biomarker Results: Topic: Medical Oncology. J Thorac Oncol 2016;11:S251-2. [Crossref]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Antonia SJ, Ozguroglu M. Durvalumab in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:869-70. [PubMed]
- O'Connor JM, Fessele KL, Steiner J, et al. Speed of Adoption of Immune Checkpoint Inhibitors of Programmed Cell Death 1 Protein and Comparison of Patient Ages in Clinical Practice vs Pivotal Clinical Trials. JAMA Oncol 2018;4:e180798. [Crossref] [PubMed]
- Elting LS, Cooksley C, Bekele BN, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer 2006;106:2452-8. [Crossref] [PubMed]
- Sorbye H, Pfeiffer P, Cavalli-Bjorkman N, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 2009;115:4679-87. [Crossref] [PubMed]
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291:2720-6. [Crossref] [PubMed]
- Mengis C, Aebi S, Tobler A, et al. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. J Clin Oncol 2003;21:3933-9. [Crossref] [PubMed]
- Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol 2012;30:2036-8. [Crossref] [PubMed]
- Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst 2014;106:dju002. [Crossref] [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Steinmann D, Paelecke-Habermann Y, Geinitz H, et al. Prospective evaluation of quality of life effects in patients undergoing palliative radiotherapy for brain metastases. BMC Cancer 2012;12:283. [Crossref] [PubMed]
- Kanai O, Fujita K, Okamura M, et al. Severe exacerbation or manifestation of primary disease related to nivolumab in non-small-cell lung cancer patients with poor performance status or brain metastases. Ann Oncol 2016;27:1354-6. [Crossref] [PubMed]
- Goldberg KH, Yin AC, Mupparapu A, et al. Components in aqueous Hibiscus rosa-sinensis flower extract inhibit in vitro melanoma cell growth. J Tradit Complement Med 2016;7:45-9. [Crossref] [PubMed]
- Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer 2016;98:114-7. [Crossref] [PubMed]
- American Autoimmune Related Diseases Association, Inc. Autoimmune Disease Statistics. Available online: https://www.aardaorg/news-information/statistics/
- Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012;32:1119-36. [PubMed]
- Khan SA, Pruitt SL, Xuan L, et al. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol 2016;2:1507-8. [Crossref] [PubMed]
- Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol 2016;2:234-40. [Crossref] [PubMed]
- Lilenbaum RC, Cashy J, Hensing TA, et al. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol 2008;3:125-9. [Crossref] [PubMed]
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. [Crossref] [PubMed]
- Langer C, Li S, Schiller J, et al. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol 2007;25:418-23. [Crossref] [PubMed]
- Waterhouse D, Horn L, Reynolds C, et al. Safety profile of nivolumab administered as 30-min infusion: analysis of data from CheckMate 153. Cancer Chemother Pharmacol 2018;81:679-86. [Crossref] [PubMed]
- Popat S, Ardizzoni A, Ciuleanu T, et al. Nivolumab in previously treated patients with metastatic squamous NSCLC: Results of a European single-arm, phase 2 trial (CheckMate 171) including patients aged ≥70 years and with poor performance status. Ann Oncol 2017.28.
- Garant A, Guilbault C, Ekmekjian T, et al. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: A systematic review. Crit Rev Oncol Hematol 2017;120:86-92. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]


