Hotspots of malignant pleural mesothelioma in Western Europe
Malignant pleural mesothelioma (MPM) has a latency time that ranges from 20 to 50 years but most patients die within 9 to 12 months after diagnosis. The dominant cause of MPM is inhalation of asbestos fibres. Worldwide, there was a peak in asbestos consumption around 1980, with approximately 4.8 million tons of asbestos per year. Hereafter, consumption started to decrease to a stable level around 2 million tons of asbestos per year in 1997 (1). The main reason for this consumption drop was the limitation in asbestos use in Western Europe and North America. Robust ecological correlations have been demonstrated between a country’s incidence of MPM and the per capita amount of imported (or consumed) asbestos in that country, 40 years earlier (2). Asbestos has been used in many industrial applications and in construction. Epidemiological studies have demonstrated links between increased risks of MPM and specific occupations and exposures, such as shipyards and asbestos-cement manufacturing. This has led to a higher prevalence of MPM in certain geographical areas, e.g., close to harbours with shipbuilding or close to plants manufacturing asbestos-cement. An emblematic example of such a hotspot is the city of Casale Monferrato (Italy), where an asbestos-cement company was recently convicted for having caused MPM among its workers and inhabitants.
The epidemiology of asbestos-related diseases in European countries has been intensively studied with regard to its time course. The geographic distribution of MPM has also been studied within countries but, to our knowledge, no studies have investigated the occurrence of mesothelioma hotspots at a European level.
We have compiled the existing evidence of geographical clusters of MPM in Europe, as obtained from recent publications.
We used MEDLINE (PubMed) and Embase to find relevant studies on spatial clustering of MPM in the 21st century in Western Europe (1 January 2000 to 31 December 2015). We focused on publications of MPM, excluding studies dealing only with peritoneal and/or pericardial mesothelioma. Our search included studies for France, Belgium, The Netherlands, Germany, the United Kingdom, Ireland, Luxembourg, Italy, Spain, Portugal, Switzerland, Austria, Denmark, Finland, Sweden, Norway and Poland. The keywords used for our search were (spatial OR clusters OR geographic OR geographic distribution OR mapping OR municipality OR municipal) AND mesothelioma AND the country that was considered.
We created a map describing hotspots of MPM in Europe, as provided in the selected publications. Standardized mortality ratios and relative risks were transformed to incidence rates per 100,000 people by multiplying these values by the age-standardized incidence rates per country (Table S1) (3).

Full table
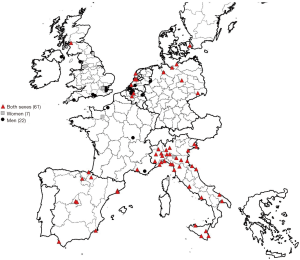
Sixteen different studies published between 2000 and 2015 were selected for an in depth analysis (4-19). We also found one study on spatial MPM clustering for Europe in general (20). All data of these publications were used to visualize MPM hotspots in Western Europe (Figure 1). The symbols of the MPM hotspots in this figure are located at the midpoints of the municipalities or regions, as described in the publications. The specific sources of asbestos exposure for these MPM hotspots were described in most of the publications. In publications where no specific asbestos sources of exposures were described, we did not conduct further searches.
Montanaro et al. used the EUROCIM database to summarize geographical variations for MPM incidence within Europe (20). They observed a high geographical variation in the truncated age-standardized rates (ASR) per 100,000 for mesothelioma between different European countries, with the highest ASR for men occurring in Scotland (8.8/100,000), England (8/100,000) and The Netherlands (7.4/100,000). For other European countries, ASRs of 0.6 to 4.24/100,000 were observed. The ranking was similar for women but with lower ASR values compared to men. The authors also studied regional cancer registries, with highest the ASRs for men being observed in the regions of Trieste (17.2/100,000), Genoa (14.4/100,000) and Rotterdam (13.1/100,000).
We found relevant studies on country-specific spatial distribution of MPM for Italy (n=6), the United Kingdom (n=3), Spain (n=2), Belgium (n=1), the Netherlands (n=1), Germany (n=1), France (n=1), and Denmark (n=1). The hotspots identified in these different publications were visualized in Figure 1.
There is consistency in these epidemiological studies on spatial hotspots of MPM. Most clusters occurred close to shipyards (16 studies) and known asbestos-cement industries (10 studies). It is reasonable to conclude that MPM clusters near the seaside were due to harbours with shipyards (or with petrochemical plants or refineries). It is likely that a high incidence of MPM found among, e.g., dockyard workers or in seafarers of a specific country is also accompanied by a geographical concentration of mesothelioma patients in conurbations close to these harbour regions. However, other MPM clusters were also explained, depending on the study, by the vicinity of railway construction companies (3 studies), asbestos textile manufacturing companies (3 studies), iron and steel industries (4 studies), petrochemical industry (6 studies), asbestos-using industries (4 studies), industrialized areas (2 studies), hazardous dumping sites (1 study), a military defence station (1 study), an electrical power plant (1 study) and furniture industries (1 study). One hotspot attributed to natural asbestos exposure was observed in Biancavilla (Italy) (4 studies) as a result of the presence of a stone quarry contaminated by fluoro-edenite fibres (16).
We did not attempt to pool or meta-analyse data from different sources, mainly because the denominators (expected numbers of deaths) were calculated per country and not for the entire area covered by the studies, and also because the statistical methods (and power) to identify clusters differed between studies. This means that the existence and “magnitude” of clusters depend both on the number of cases of mesothelioma in the area of interest and on the background incidence of malignant mesothelioma in a country (or comparison area). In other words, a high incidence area in one country might not appear as a cluster in another country. Consequently, the present map only shows areas identified as “high incidence” areas within their country and not all clusters should be considered as having the same degree of intensity in quantitative terms.
Also, if no hotspots are shown, this does not necessarily mean that there were no clusters of mesothelioma, but simply that the existence of such clusters was not investigated (or published in accessible journals). The possibility of underestimation of mesothelioma cases must be kept in mind, because not all patients with MPM have been registered in mesothelioma databases. For example, in France, MPM incidence data were only recorded for 26 out of 96 districts by the French National Mesothelioma Surveillance (9). This will lead to an underestimation of mesothelioma incidence in this particular population. Moreover, mesotheliomas are also found outside high incidence areas, because asbestos use has been widespread in industry and buildings throughout Europe, and because previously exposed residents or workers may have moved from hotspots to other areas.
Although there were some limitations for studying the spatial distribution of MPM in Western Europe, there was also much consistency between all these studies. Most MPM clusters occurred near asbestos cement industries and shipyards. We believe that this spatial distribution will continue to be observed in the future. Therefore, even in European countries with persistent environmental asbestos exposure risks (mainly because of not yet completely cleared community asbestos exposures), continuous vigilance for the epidemiological spread of MPM hotspots is required in the next decades.
Acknowledgements
We would like to thank Hilde Vandenhoeck (Dpt Informatics and Communication Technology, KU Leuven), who made the map on spatial distribution of mesothelioma in Europe.
Funding: This research received financial support from the Foundation against Cancer, Belgium (Project 2012-222).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stayner L, Welch LS, Lemen R. The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 2013;34:205-16. [Crossref] [PubMed]
- Lin RT, Takahashi K, Karjalainen A, et al. Ecological association between asbestos-related diseases and historical asbestos consumption: an international analysis. Lancet 2007;369:844-9. [Crossref] [PubMed]
- Bianchi C, Bianchi T. Global mesothelioma epidemic: Trend and features. Indian J Occup Environ Med 2014;18:82-8. [Crossref] [PubMed]
- Van den Borre L, Deboosere P. Asbestos in Belgium: an underestimated health risk. The evolution of mesothelioma mortality rates (1969-2009). Int J Occup Environ Health 2014;20:134-40. [Crossref] [PubMed]
- Burdorf A, Siesling S, Sinninghe Damsté, H. Regionale spreiding van het maligne mesothelioom in Nederland. Deelrapport I. Ministerie van Volkshuisvesting, Ruimtelijke ordening en Milieu (VROM). Opdracht 5040040218. Rotterdam: Ministerie van VROM, 2005.
- McElvenny DM, Darnton AJ, Price MJ, et al. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79-87. [Crossref] [PubMed]
- Health and safety executive. Mesothelioma Mortality in Great Britain: Analyses by Geographical Area and Occupation 2005 [Internet]. [cited 2017 Mar 10]. Available online: http://www.hse.gov.uk/statistics/pdf/mesojune08.pdf
- Mak V, Davies E, Putcha V, et al. The epidemiology and treatment of mesothelioma in South East England 1985-2002. Thorax 2008;63:160-6. [Crossref] [PubMed]
- Schonfeld SJ, McCormack V, Rutherford MJ, et al. Regional variations in German mesothelioma mortality rates: 2000–2010. Cancer Causes Control CCC 2014;25:615-24. [Crossref] [PubMed]
- Goldberg S, Rey G, Luce D, et al. Possible effect of environmental exposure to asbestos on geographical variation in mesothelioma rates. Occup Environ Med 2010;67:417-21. [Crossref] [PubMed]
- López-Abente G, Hernández-Barrera V, Pollán M, et al. Municipal pleural cancer mortality in Spain. Occup Environ Med 2005;62:195-9. [Crossref] [PubMed]
- García-Gómez M, Menéndez-Navarro A, López RC. Asbestos-related occupational cancers compensated under the Spanish National Insurance System, 1978-2011. Int J Occup Environ Health 2015;21:31-9. [Crossref] [PubMed]
- Mastrantonio M, Belli S, Binazzi A, et al. La mortalità per tumore maligno della pleura nei comuni italiani, 1988–1997. Rapp ISTISAN 0212 Ist Super Sanità Roma Italy. 2002.
- Marinaccio A, Binazzi A, Marzio DD, et al. Pleural malignant mesothelioma epidemic: incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int J Cancer 2012;130:2146-54. [Crossref] [PubMed]
- Fazzo L, De Santis M, Minelli G, et al. Pleural mesothelioma mortality and asbestos exposure mapping in Italy. Am J Ind Med 2012;55:11-24. [Crossref] [PubMed]
- Fazzo L, Minelli G, De Santis M, et al. Mesothelioma mortality surveillance and asbestos exposure tracking in Italy. Ann Ist Super Sanita 2012;48:300-10. [Crossref] [PubMed]
- Gatto MP, Tanna D, Tanna GL. Distribution and trends in mesothelioma mortality in Italy from 1974 to 2006. Int Arch Occup Environ Health 2013;86:489-96. [Crossref] [PubMed]
- Corfiati M, Scarselli A, Binazzi A, et al. Epidemiological patterns of asbestos exposure and spatial clusters of incident cases of malignant mesothelioma from the Italian national registry. BMC Cancer 2015;15:286. [Crossref] [PubMed]
- Skammeritz E, Omland Ø, Hansen J, et al. Regional differences in incidence of malignant mesothelioma in Denmark. Dan Med J 2013;60:A4592. [PubMed]
- Montanaro F, Bray F, Gennaro V, et al. Pleural mesothelioma incidence in Europe: evidence of some deceleration in the increasing trends. Cancer Causes Control CCC 2003;14:791-803. [Crossref] [PubMed]


