
Stereotactic body radiotherapy for centrally located stage I non-small cell lung cancer
Introduction
Stereotactic body radiotherapy (SBRT), also known as stereotactic ablative body radiotherapy (SABR), has become the standard of care over the last decade for treating early stage non-small cell lung cancer (NSCLC) in patients who cannot undergo surgery (1). The technique allows for the delivery of very high doses of radiation with a goal of treating tumors with a biological effective dose (BED10) greater than 100 Gy (2). In order to achieve this safely, the planning process requires precise definition of the target, management of target motion, conformal planning with sharp dose fall off and high quality daily set-up verification.
There are several advantages of SBRT compared to conventionally fractionated radiotherapy (1.8–2 Gy per fraction) in early stage NSCLC. Several phase II studies demonstrate impressive 3-year local control rates ranging from 80–95% (3-5). Compared with historical controls, SBRT appears to provide better overall survival (OS) and progression free survival (PFS) than conventionally fractionated radiotherapy (6,7). Two randomized trials of SBRT versus 60–70 Gy in 2 Gy per fraction demonstrate equivalent or superior OS in early analyses (8,9). In addition, SBRT has shorter treatment times, is more convenient, and is associated with better quality of life for patients (8).
With the increasing adoption of lung SBRT, there have been several challenges in standardizing practice including variability in planning, prescription and delivery of SBRT. One particular challenge has been optimizing the management of central tumors due to the proximity of multiple critical organs at risk (OARs), including the major airways and vessels. This is not a unique problem to radiation alone, as centrally located tumors are surgically more challenging to treat as well, requiring more extensive surgeries such as pneumonectomy or sleeve lobectomy, which are associated with higher morbidity and mortality (10).
The often-quoted study by Timmerman et al. [2006] from Indiana University reported increased toxicities in patients with centrally located tumors treated with 60–66 Gy in 3 fractions; on multivariate analysis, tumor location was the strongest predictor for toxicity (11). Patients with perihilar/central tumors had an 11-fold increased risk of grade 3 or higher toxicity. This led to the first definition of the “no-fly zone,” which was adopted in the RTOG 0236 protocol. This was defined as 2 cm in all directions around the proximal bronchial tree (carina, right and left mainstem bronchi, right and left upper lobe bronchi, bronchus intermedius, right middle lobe bronchus, lingular bronchus, right and left lower lobe bronchi) (12). The ASTRO evidence-based guidelines for SBRT in early-stage NSCLC uses this definition for their recommendations (1). A slightly modified definition was developed in the RTOG 0813 protocol, a phase I/II study investigating the tolerance of SBRT dose/fractionation schemes in centrally located tumors specifically. This definition includes the zone of the proximal bronchial tree from RTOG 0236, with the addition of tumors which are “immediately adjacent to mediastinal or pericardial pleura (PTV touching the pleura)” so as to include tumors that may have had heart or vessels as the main OAR (13). Variations in defining the critical volume that overlaps with the central zone exist in standard practice, with some studies specifying the planning target volume (PTV) as the region of overlap (14,15), and some the gross tumor volume (GTV) for overlap. There are other critical structures that need to be considered, which are not covered by either of the aforementioned definitions, namely the brachial plexus, spinal cord and esophagus (16).
In this article, we will review the existing literature and ongoing trials reporting the outcomes and toxicities of SBRT in central tumors. The importance of treatment dose and fractionation will be the primary focus of discussion, but it is critical to consider other radiation related variables that may vary between the reviewed studies. For example, some centers may prescribe their treatment at the isocenter, and others prescribe it to an isodose covering the PTV. In addition, the PTV may be covered by a range of isodose levels, typically from 60–90%, which in turn greatly changes the maximum dose delivered to the center of the tumor. Other variations in practice include the use of heterogeneity correction, accuracy/variability of volume and OAR delineation, potential under-coverage for critical OARs if they are in the immediate vicinity of the target, and the effect of image guided radiotherapy (IGRT) on the dose actually delivered (as compared to the dose planned, in cases where the target has moved closer to an OAR). Most articles and protocols do not describe these practical aspects in sufficient detail to guide practice (17).
Beyond radiation treatment factors, one must also consider other factors which could contribute to toxicity such as patient comorbidities, which could increase pneumonitis or hemorrhage rates and the potential interaction with chemotherapy or biologics and the timing of such systemic therapies. All of these treatments, patient and other variables can affect both local control and toxicities and need to be taken into consideration when interpreting the literature on lung SBRT.
Literature review
Since the publication of Timmerman et al. first recognizing increased toxicities for hilar and pericentral tumors, there has been a response to reduce these risks while maintaining the impressive local control rates seen with SBRT. The ASTRO Evidence-Based Guideline, published in 2017, makes two recommendations regarding SBRT for central tumors. The first statement states that “SBRT directed toward centrally located lung tumors carries unique and significant risks when compared to treatment directed at peripherally located tumors. The use of 3-fraction regimens should be avoided in this setting.” (Strong recommendation; High quality of evidence) (1). The second statement provides suggestions regarding possible fractionation regimens: “SBRT directed at central lung tumors should be delivered in 4 or 5 fractions. Adherence to volumetric and maximum dose constraints may optimize the safety profile of this treatment. For central tumors for which SBRT is deemed too high risk, hypofractionated radiation therapy utilizing 6 to 15 fractions can be considered.” (Conditional recommendation; Moderate quality of evidence) (1).
Based on these recommendations, the treatment of central tumors is currently commonly delivered in five or eight fractions with effective control and promising survival outcomes, as will be discussed. The five-fraction schedule has been utilized in the North American RTOG 0813 study and the eight fraction regimen has also been adopted by many centers, especially outside the United States, and by the European Organization for Research and Treatment of Cancer (EORTC). This dose/fractionation schedule was derived from a publication of a large retrospective series from the VU Medical Center (VUMC), which demonstrated excellent 5-year survival and local control rates of 50% and 93% respectively (15). The Japanese Radiation Oncology Study Group published the findings of their Phase I dose escalation study from 54 to 68 Gy in 8 fractions. Their recommended dose was 60 Gy in 8 fractions after being unable to meet constraints with the higher doses (18). The sections that follow describe the toxicities and outcomes, specifically survival and local control data, from a series of prospective and retrospective studies.
Toxicities
Most of the attention with SBRT for central lesions has been focused on the observed increase in toxicity due to the proximity of critical structures. To provide context, SBRT to peripheral lesions has been well tolerated when delivered in three fractions or less, with grade 3+ toxicity rates between 10–16% (11,12,19). For example, the previously discussed Indiana University study showed grade 3+ toxicities of 10% (11) with a dose of 60–66 Gy in 3 fractions (54–60 Gy with heterogeneity correction) when treating peripheral lesions. This typically includes toxicities of dyspnea, pneumonitis and pleural effusions. In addition, two RTOG studies using the same dose/fractionation schedule examined toxicities in inoperable (RTOG 0236) and operable (RTOG 0618) peripheral tumors and had grade 3+ toxicities of 16% (12,19). In comparison, patients with central tumors who received a similar dose in three fractions had higher rates of toxicities (Table 1). In the updated Indiana University study, the incidence of grade 3+ toxicities were 27% for central tumors (3), with 5 (7%) grade 5 toxicities in total, including pneumonia, hemoptysis and respiratory failure. In another prospective study with a mixed population of peripheral and central tumors, Bral et al. had a similar trend towards increased grade 3+ toxicities in central compared to peripheral tumors (29% vs. 13%) (20), using a 15 Gy ×4 fraction schedule. Certainly, it would appear that the utilization of higher dose per fraction, 15 Gy and higher, may confer an increased number of serious adverse events for central tumors. This association led to reducing the dose of radiation delivered per fraction, while maintaining an adequate BED10 to provide adequate disease control.
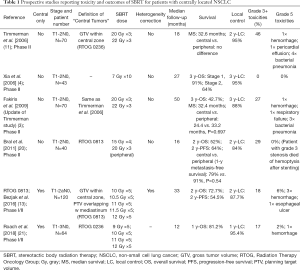
Full table
RTOG 0813 was a multi-institutional phase I/II trial investigating the safety of dose escalation from 10 Gy ×5 fractions to 12 Gy ×5 fractions for its phase I component, using the 11.5 Gy ×5 and 12 Gy ×5 cohorts for a phase II investigation of efficacy and safety. In the reported phase II outcomes of 71 patients, the grade 3+ toxicity rate was 18%, with 13% of patients experiencing toxicities within the first year (13). There were four (6%) reported grade 5 toxicities in the 11.5 and 12 Gy per fraction cohorts, with three bronchopulmonary hemorrhages and one death suspected to be related to an esophageal ulcer (13). In another phase I/II prospective study escalating doses from 9 to 12 Gy ×5 fraction, Roach et al. reported a higher late grade 3+ toxicity rate of 41% (21) with one (2%) grade 5 toxicity due to hemorrhage in a tumor which invaded the pulmonary artery.
Several retrospective series reported the toxicity outcomes of five to eight fraction treatments (Table 2). The group at VUMC reported their outcomes using a 7.5 Gy ×8 fraction schedule in two separate series with grade 3+ toxicities of 6% and 14% (14,15). In Tekatli et al., 6 (8%) incidences of grade 5 toxicities were reported (15). Grade 3+ rates as low as 3% and 1% have been reported in series reviewed by groups at Stanford and MD Anderson, respectively (25,27). The Stanford group used a treatment regimen of 10 Gy ×5 fractions, and the MD Anderson group treated their central tumors with 7 Gy ×10 fractions. These data appear to suggest that reducing the dose per fraction to 10 Gy or less could potentially reduce severe toxicities even further but should be assessed in a prospective setting.
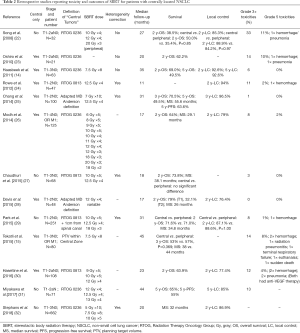
Full table
Hemoptysis is a toxicity of particular concern that is associated with central tumors treated with SBRT and is one of the common grade 5 toxicity reported in both prospective and retrospective studies. Its risk has been associated with endobronchial lesions, squamous cell carcinoma (33) and the use of VEGF inhibitors (34). Another risk factor comes in the form of iatrogenic injuries to the central airways following SBRT. For example, in a case report of central airway necrosis following SBRT for a central lesion treated to 10 Gy ×5, biopsies were taken of a suspected nodal recurrence bronchoscopically. The patient developed hemoptysis several weeks later which originated from the area of necrosis; the patient passed away shortly thereafter. Another example can be found in Bral et al., whereby the only patient who died due to a treatment related toxicity had a stent placed after developing a grade 3 stenosis of the proximal airway (20). With the risk of necrosis and bronchomalacia, it is important for pulmonologists and thoracic surgeons to be particularly cautious with instrumentation in patients who have received SBRT for a central lesion.
Esophageal toxicity is not as well investigated as their respiratory/bronchial counterparts, but certainly represents an important consideration for SBRT of central lesions. The acute toxicities include esophagitis and ulceration, with potential long-term complications of stenosis and fistulization. As previously reviewed, the RTOG 0813 definition of central tumors included tumors with overlapping PTVs with the mediastinal pleura and structures. The protocol mandated a Dmax of 105% of the PTV prescription and a D5cc of 27.5 Gy for the five-fraction regimen (13). In that study, out of 100 patients, 13% had the esophagus as a major organ at risk (13). When evaluating adverse events of dysphagia, esophagitis and esophageal perforation, there was a 4% grade 3+ risk, with one patient (1%) having a grade 4 perforation requiring intervention (13). Nuyttens et al. used a clinical model based on a series of 52 patients to derive the TD50 for grade 2+ esophageal symptoms for D10%, D5cc, D1cc and Dmax for five-fraction SBRT, which were found to be 30.0, 27.4, 32.9 and 43.4 Gy respectively (35). Modh et al. retrospectively reviewed their series on SBRT for central tumors and reported two patients (2%) with grade 3+ esophageal complications of a fistula and hemorrhage (26). Though the overall rate was low, the majority of their patients did not receive a high dose to the esophagus. To address this, they analyzed the rate of esophageal toxicities as a function of the proximity of the esophagus from the PTV. They found that in patients with a PTV overlapping the esophagus, 50% developed grade 2+ toxicities (26). In contrast, patients with PTVs within 2 cm of the esophagus had a 14% rate, and those outside of 2 cm had a 5% risk of esophageal toxicities (26). This would seem to suggest a clear correlation between dose delivered to the esophagus and corresponding toxicities.
Survival and local control
A discussion regarding outcomes of SBRT in central tumors is intimately tied to its toxicity profile as modifications in dose/fractionation were made to accommodate the dose tolerance of critical structures. Decreasing the dose per fraction to minimize toxicities would need to be balanced with the aim of maintaining the significant local control SBRT provided by SBRT with a target BED10 of 100 Gy or higher (2). In an updated report of the series from Timmerman et al., Fakiris et al. demonstrated a median survival of 24.4 months in central tumors compared to 33.2 months in peripheral tumors, which was not statistically significant (P=0.697) (3). Since then, there have been three more prospective studies which provide survival data for central tumors. Tables 1 and 2 provide a list of prospective and retrospective studies, which reported survival and local control data for central tumors treated with SBRT.
In the initial report of RTOG 0813, the 2-year OS was found to be 72.7% and the 2-year PFS was 54.5% (13). The reported 2-year local control was 87.7% in the 12 Gy ×5 arm (14). In another prospective phase I/II study investigating only central tumors, Roach et al. demonstrated a 1-year OS of 82% and a 1-year local control of 95% using an escalating five fraction schedule (9–12 Gy per fraction) (21). Lastly, Bral et al. reported a 2-year OS of 52% in a mix of peripheral and central tumors using a 15 Gy ×4 schedule. They also reported a 1-year metastasis free survival of 79% vs. 91% (P=0.54) in their central and peripheral cohorts (20).
There have been multiple retrospective studies investigating survival and local control outcomes in central tumors alone or in comparison with peripheral tumors. These studies utilized doses ranging from 40–60 Gy in 4–8 fractions. Two-year OS ranged from 50–71%, and two-year local control ranges from 76–94% (14,15,22-32). Three studies have reported long-term outcomes, with 5-year OS of 65% (31) and 50% (14,25). In comparing survival and local control of central and peripheral tumors, there was no difference found in any of these studies for either outcome (15,22,27,29).
In summary, despite reducing the dose per fraction while maintaining a BED10 of 100 Gy, SBRT appears to provide consistent survival and local control outcomes with treated peripheral tumors. Studies comparing outcomes between the two cohorts did not show a significant difference, and the single cohort studies demonstrated survival and local control similar to the known literature.
Practical considerations
Ultracentral tumors
As discussed, there are several definitions of what constitutes a central tumor, but one commonality of these definitions includes tumors within 2 cm of the central airways. Given the arbitrarily defined range of 2 cm, there have been studies investigating the intuitive existence of a gradient of toxicity. Certainly, when the first concept of the “no-fly zone” was developed after publication of Timmerman et al., their two non-pneumonia grade 5 toxicities came from tumors which were treated directly adjacent to the mediastinum and carina (12). Figure 1 shows an ultracentral tumor, before and after it was treated with SBRT.
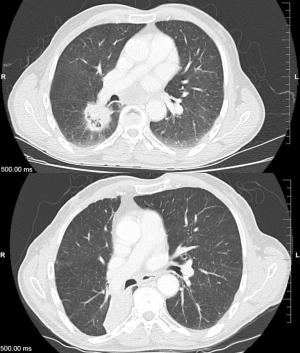
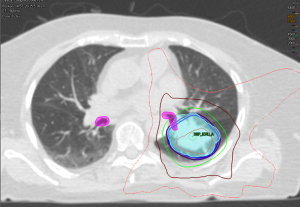
Haseltine et al. retrospectively reviewed central lung tumors receiving SBRT in 5 fractions and stratified tumors located ≤1 cm, defined as “ultracentral”, and >1 cm from the central airways (30). There were no differences noted in local control or survival, but they did note an increase in grade 3+ toxicities in ultracentral tumors (30.7% vs. 7.3%, P=0.01). As with the definition of a central tumor, there are variations in describing ultracentral tumors (Table 3) (27,30,37-39), specifically with regards to the overlap of GTV or PTV with the proximal airways. Daly et al. also performed a retrospective review which showed a similar trend of increased grade 3+ toxicities with ultracentral tumors treated in 5 fractions (22.2% vs. 4.3%, P=0.11) (38). Tekatli et al. reviewed 47 patients with ultracentral tumors treated with 12 fractions of 5 Gy and found a 38% grade 3+ toxicity rate (37). Though there was no comparator arm, this rate is higher than historically reported rates for central tumors. Chaudhuri et al. however showed no difference in toxicity profiles between peripheral, central or ultracentral tumors (27). Put together, there does not appear to be a difference in survival or control rates with ultracentral tumors with SBRT, but greater concerns remain regarding the toxicity profile. Figure 2 demonstrates a treatment plan of an ultracentral tumor, using a definition of PTV overlap with the proximal bronchi.
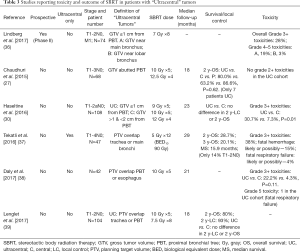
Full table
To better address this question, there are two ongoing prospective studies. Lindberg et al. presented the preliminary data of their Nordic-HILUS trial which looked at tumors within 1 cm of the proximal bronchial tree. They demonstrated overall grade 3+ toxicities of 28%, which is higher than the historical rates of 10–20% seen in central tumors treated with 5 or more fractions (36). They found higher rates of grade 4–5 toxicities associated with tumors in proximity to the main bronchi compared to the lobar bronchi (19% vs. 3%). Another prospective study, the SUNSET trial (40), will investigate the maximally tolerated dose of ultracentral tumors using a time-to-event continual reassessment model starting at 60 Gy in 8 fractions. The study is expected to open in several Canadian centers in 2018. It should be noted that although optimizing the dose schedule may reduce toxicities, the high reported rates may also be a function of the physical effect of the tumor abutting/invading the central airways. Indeed, fatal hemoptysis has been independently associated with central tumor location/endobronchial tumor involvement (33).
Brachial plexus
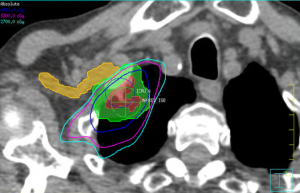
The brachial plexus is not included in either definition of a central structure in RTOG 0236 or RTOG 0813. It is, however, considered a critical structure that should be spared from high doses of radiation to reduce the risk of brachial plexopathy. The brachial plexus is included in an adapted MD Anderson definition, which also includes the proximal bronchial tree, esophagus, heart, major vessels, spinal cord, phrenic nerve and recurrent laryngeal nerve (16). Caution with the brachial plexus is warranted in particular due to the impact of hypofractionation on the low α/β of neuronal tissues, as well as the serial organization of the functional subunits, which places an emphasis on maximum doses for plan evaluation constraints. The number of fractions plays an important role in determining appropriate dose constraints. For lung lesions treated in three fractions, the commonly accepted Dmax is 24 Gy, utilized in the RTOG 0236 and 0618 protocol (12,19). Chang et al. reported a single episode of grade 3 brachial plexopathy when treating with four fractions SBRT (16). In that patient’s case, the brachial plexus had a V40 of 20%. Their published dose constraint recommendation was a Dmax of 40 Gy and D1cc/D10cc of 35 and 30 Gy respectively (16). The RTOG 0915 protocol utilized a more conservative Dmax of 27.2 Gy and D3cc of 23.6 Gy for their four fraction regimen of 48 Gy/4 (41). Finally, for SBRT delivered in five fractions, RTOG 0813 used a Dmax and D3cc of 32 and 30 Gy, respectively (13). In cases where the tumor can be safely treated, clinical judgment must be made to balance sparing the brachial plexus with undercoverage of the target volumes while not compromising tumor control too significantly. Figure 3 illustrates an example of compromising coverage of the PTV to spare the ipsilateral brachial plexus.
Systemic therapies
SBRT for the lung has primarily been utilized to treat early stage lung cancer, which has not historically required adjuvant systemic therapies. Data from stage III NSCLC studies show that concurrent or serial chemotherapy with radical radiotherapy increases acute- and long-term toxicities (42,43). Overall, the effect of chemotherapeutic or other biologic agents on SBRT related toxicities is unknown. The use of VEGF inhibitors may be implicated with an increased risk of pulmonary hemorrhage as reported in a retrospective series by Haseltine et al., where both of the patients who experienced a grade 5 hemorrhage had bevacizumab therapy within a year of SBRT for their ultracentral tumors (30). Bevacizumab has been associated with an increased risk of hemorrhage in cancer patients across a variety of solid tumors with a reported relative risk of 2.3 overall and 5.2 in non-small cell lung cancers (34).
With the increasing prevalence of immunotherapy, multiple ongoing studies are exploring the benefits and toxicity profile of combining the two therapies. Luke et al. reported the results of their phase I study combining pembrolizumab with multisite radiotherapy. Pembrolizumab was administered within 7 days of the final fraction of SBRT. 34 out of 73 received SBRT to the lung, of which 4 (12%) had dose limiting toxicities (DLT), defined as a treatment-related grade 3+ toxicity within three months from the first day of radiotherapy (44). Three of those events were pneumonitis. No radiation dose reduction was necessary, and the authors concluded that SBRT with pembrolizumab was well tolerated with acceptable toxicities (44). Tang et al. reported their experience with CTLA-4 inhibitors, in which lung or liver SBRT was combined with ipilimumab. In the lung SBRT cohort, there were no DLTs, and the overall grade 3+ rates was 30%, though it should be noted that all of the toxicities were extrathoracic (i.e., no pneumonitis, esophagitis, or pulmonary hemorrhage) (45). They also concluded that the combination of therapies was safe with signs of efficacy, with 10% of patients experiencing out of field partial response and 23% experiencing a partial response or stability of disease for ≥6 months (45).
Conclusions
Lung SBRT is the standard of care treatment for inoperable patients with NSCLC and is increasingly being adopted for the treatment of oligometastatic disease. With evidence for the efficacy of low-dose CT screening in NSCLC (46), we expect the role of SBRT to continue expanding. The treatment of central lesions provides a challenge due to an increased risk of injury to the critical structures found in the mediastinum. The primary strategy used to tackle this issue has been reducing the dose delivered per fraction while maintaining a high BED10 of 100 Gy to preserve the excellent local control that SBRT can provide. RTOG 0813 is a multicenter phase I/II study which sought to explore the use of five-fraction treatment, with preliminary data showing a safe toxicity profile at ASTRO 2016 (13). On review of the literature, SBRT for central lesions appears to be safe when treating with five or more fractions with similar efficacy as peripheral lesions. The treatment of ultracentral tumors continues to be associated with increased toxicities, and such lesions may benefit from a more delicate fractionation schedule to minimize serious adverse events.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;66:117-25. [Crossref] [PubMed]
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: Prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thorascopic surgery (VATS): Outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-48. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: A population-based time-trend analysis. J Clin Oncol 2010;28:5153-59. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund JA, et al. SPACE – A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Ball DL, Mai T, Vinod SK, et al. A randomized trial of SABR vs conventional radiotherapy for inoperable stage I non-small cell lung cancer: TROG 09.02 (CHISEL). WCLC 2017;OA 01.01.
- Spiguel L, Ferguson MK. Sleeve lobectomy versus pneumonectomy for lung cancer patients with good pulmonary function. In: Ferguson MK. editor. Difficult decisions in thoracic surgery: an evidence-based approach. 3rd edn. London, UK: Springer, 2007:103-9.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar LE, et al. Efficacy and Toxicity Analysis of NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2016;S96:8. [Crossref]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Otcomes of stereotactic ablastive radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [Crossref] [PubMed]
- Tekatli H, Senan S, Dahele M, et al. Stereotactic ablative radiotherapy (SABR) for central lung tumors: Plan quality and long-term clinical outcomes. Radiother Oncol 2015;117:64-70. [Crossref] [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [Crossref] [PubMed]
- Dahele M, Pearson S, Purdie T, et al. Practical considerations arising from the implementation of lung stereotactic body radiation therapy (SBRT) at a comprehensive cancer center. J Thorac Oncol 2008;3:1332-41. [Crossref] [PubMed]
- Kimura T, Nagata Y, Harada H, et al. Phase I study of stereotactic body radiation therapy for centrally located stage IA non-small cell lung cancer (JROSG10-1). Int J Clin Oncol 2017;22:849-56. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol 2013:31:abstr 7523.
- Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 2011;80:1343-9. [Crossref] [PubMed]
- Roach MC, Robinson CG, DeWees TA, et al. Stereotactic body radiation therapy for central early-stage NSCLC: results of a prospective phase I/II trial. J Thorac Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009;66:89-93. [Crossref] [PubMed]
- Oshiro Y, Aruga T, Tsuboi K, et al. Stereotactic body radiotherapy for lung tumors at the pulmonary hilum. Strahlenther Onkol 2010;186:274-9. [Crossref] [PubMed]
- Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394-9. [Crossref] [PubMed]
- Chang JY, Li QQ, Xu QY, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a "no fly zone". Int J Radiat Oncol Biol Phys 2014;88:1120-8. [Crossref] [PubMed]
- Modh A, Rimner A, Williams E, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;90:1168-76. [Crossref] [PubMed]
- Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015;89:50-6. [Crossref] [PubMed]
- Davis JN, Medbery C, Sharma S, et al. Stereotactic body radiotherapy for early-stage non-small cell lung cancer: clinical outcomes from a National Patient Registry. J Radiat Oncol 2015;4:55-63. [Crossref] [PubMed]
- Park HS, Harder EM, Mancini BR, et al. Central versus Peripheral Tumor Location: Influence on Survival, Local Control, and Toxicity Following Stereotactic Body Radiotherapy for Primary Non-Small-Cell Lung Cancer. J Thorac Oncol 2015;10:832-7. [Crossref] [PubMed]
- Haseltine JM, Rimner A, Gelblum DY, et al. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract Radiat Oncol 2016;6:e27-33. [Crossref] [PubMed]
- Miyakawa A, Shibamoto Y, Baba F, et al. Stereotactic body radiotherapy for stage I non-small-cell lung cancer using higher doses for larger tumors: results of the second study. Radiat Oncol 2017;12:152. [Crossref] [PubMed]
- Stephans KL, Woody NM, Reddy CA, et al. Tumor Control and Toxicity for Common Stereotactic Body Radiation Therapy Dose-Fractionation Regimens in Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;100:462-9. [Crossref] [PubMed]
- Ito M, Niho S, Nihei K, et al. Risk factors associated with fatal pulmonary hemorrhage in locally advanced non-small cell lung cancer treated with chemoradiotherapy. BMC Cancer 2012;12:27. [Crossref] [PubMed]
- Hapani S, Sher A, Chu D, et al. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 2010;79:27-38. [Crossref] [PubMed]
- Nuyttens JJ, Moiseenko V, McLaughlin M, et al. Esophageal dose tolerance in patients treated with stereotactic body radiation therapy. Semin Radiat Oncol 2016;26:120-8. [Crossref] [PubMed]
- Lindberg K, Bergstrom P, Brustugun OT, et al. The Nordic HILUS-Trial – First report of a phase II trial of SBRT of centrally located lung tumors. J Thorac Oncol 2017;12:S340. [Crossref]
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol 2016;11:1081-9. [Crossref] [PubMed]
- Daly M, Novak J, Monjazeb A. Safety of Stereotactic body radiotherapy for central, ultracentral and paramediastinal lung tumors. J Thorac Oncol 2017;12:S1066. [Crossref]
- Lenglet A, Mathieu D, Campeau MP, et al. Risk-adapted lung SBRT for central and ultra-central tumors. Int J Radiat Oncol Biol Phys 2017;99:e473-e474. [Crossref]
- NIH Clinical Trials. “SUNSET: SBRT for Ultra-central NSCLC- a Safety and Efficacy Trial”. Available online: https://clinicaltrials.gov/ct2/show/NCT03306680, accessed Jan 2018.
- Videtic GM, Hu C, Singh AK, et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys 2015;93:757-64. [Crossref] [PubMed]
- Socinski MA, Zhang C, Herndon JE II, et al. Combined modality trials of the Cancer and Leukemia Group B in stage III non-small-cell lung cancer: analysis of factors influencing survival and toxicity. Ann Oncol 2004;15:1033-41. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611-8. [Crossref] [PubMed]
- Tang C, Welsh JW, de Groot P, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23:1388-96. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]


