
Clear cell adenocarcinoma of the lung: a SEER analysis
Introduction
Clear cell adenocarcinoma (CCA) is a well-known major histologic type in renal cell carcinoma (1). It is occasionally seen in non-renal primary sites such as ovary (2) and lung (3). CCA derives its name from the clear appearance of the cytoplasm and such changes in neoplastic cells are generally caused by fat, mucin or glycogen accumulation in the cytoplasm. Both CCA and signet ring cell carcinoma (SRC) of lung had been considered as distinct pathologic entities until International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATC)/European Respiratory Society (ERS) committee in 2011 removed both features from subtypes of lung adenocarcinoma (4). This decision was attributed to the unclear clinical significance for both CAA and SRC. World Health Organization (WHO) classification of lung cancer in 2015 also discontinued the subtype of CCA and recognized it as a cytologic feature (5). However, a population study demonstrated that patients with SRC pathologic feature had worse prognosis as compared to lung adenocarcinoma not otherwise specified (LANOS) (6). A small study of CCA of the lung in 1980 reported comparable prognosis (7), whereas a recent retrospective study from China reported relatively poor survival in patients with CCA, suggesting its role as a prognostic predictor (8). Because of the scarcity of the disease and the lack of definitive studies, the clinical role of CCA subtype has not yet been adequately determined.
Retrospective case series have inherent limitations. Cases with rare disease such as CAA cannot be adequately collected by individual researchers. However, publicly available population datasets provide investigators with a large sample size and make such studies feasible. The US National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) database is one such resource for researchers conducting outcomes research (9).
In this SEER database study, we found that patients with CCA had a worse prognosis than those with LANOS in the subgroups of local/regional stages. We also conducted analyses for clinical characteristics and prognosis in subgroups.
Materials and methods
Patient population
This population study used SEER-18 Dataset consisting of 18 cancer registries across United States. Patients with CCA and LANOS diagnosed between 1973 and 2013 were included in this study. Data were extracted using the SEER Stat version 8.3.4 of NCI (10). Histologically diagnosed cases were identified by the specific codes (8310/3, 8140/3) of the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) for CCA and LANOS, respectively. Due to multiple revisions in TNM staging system, use of identical TNM staging versions throughout the study period was not possible. Instead, SEER historic stage A system was used in the analysis (11). It continued to assign cases into local, regional, or distant disease throughout the study period, allowing us to compare cases over the decades. Other clinical characteristics abstracted in the study were gender (male, female), age (0–69, 70+), year of diagnosis (1973–2000, 2001–2013), surgery (yes, no), and radiation therapy (yes, no). Every case was assigned to one of the two groups for each factor for multivariate analysis.
Association between histology and the other characteristics were examined. Cancer-specific survival was studied by Kaplan-Meier method and analyzed for each subgroup. Multivariate analyses excluded cases that did not contain any of the above mentioned clinical factors (gender, age, year of diagnosis, surgery, radiation).
Statistical analysis
Chi-square or Fisher’s exact test was used for comparisons of histologic and demographic characteristics as appropriate. Univariate survival analyses were conducted using the log-rank test. Cox proportional hazards model was used for multivariate survival analysis. All statistical analyses were performed using JMP software version 13 (SAS Institute, Inc., Cary, NC, USA). A two-tailed P value less than 0.05 was considered as statistically significant.
Ethics
This is a population study that involves no identifiable information for individuals throughout the analyses. Institutional Review Board at Tulane University gave exemption because the study was deemed not to constitute human subject research.
Results
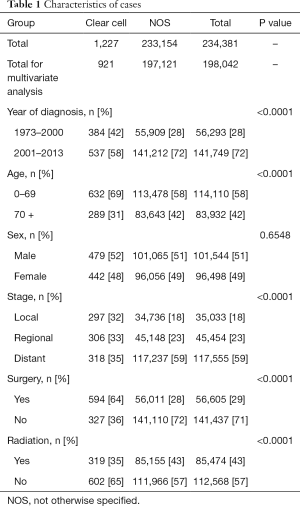
A total of 1,227 CCA and 233,154 LANOS cases were selected and analyzed. Clinical characteristics described above were available for 921 and 197,121 cases, respectively (Table 1). Overall, cases commonly had characteristics with recent year (2001–2013) of diagnosis (72%), age 0–69 (58%), male gender (51%), distant stage (59%), lack of surgery (72%), and lack of radiation (57%).
Full table
As compared to LANOS, CCA histology was significantly associated with early year (1973–2000) of diagnosis, younger age (0–69), early disease stage, presence of surgery, and lack of radiation (all P<0.0001, Table 1).
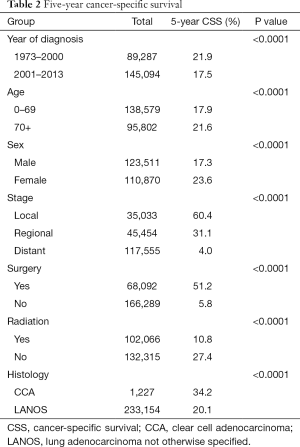
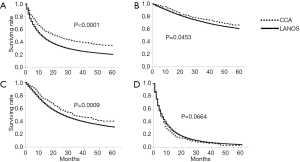
Survival analyses demonstrated that patients with age 0–69, year of diagnosis 2001–2013, female gender, localized disease, undergoing surgery, and lack of radiation had significantly better cancer-specific survival (all P<0.0001, Log-Rank, Table 2). Regarding histology, the five-year cancer-specific survival rates were 34.2% and 20.1% for CCA (n=1,227) and LANOS (n=233,154) histology, respectively, and the difference was statistically significant with P<0.0001 (Table 2, Figure 1A). Subset analysis by SEER historic stage A system showed that the difference in the five-year cancer-specific survival was significant in localized and regional but no distant stage (P=0.0453, 0.0009, 0.0664, Log-Rank, respectively, Figure 1B,C,D).
Full table
Multivariate cox proportional hazard model showed that all the above factors excluding histology were independent predictors of survival (Table 3).

Full table
Discussion
Recent histologic classification discouraged clinicians from considering CCA as a distinct subtype and rather recommend to report it as an associated cytologic change based on lack of its clinical significance (4,5). Although our study does not demonstrate the role of CCA histology as an independent prognostic indicator; univariate analysis in our study is contradictory to a recent retrospective cohort study in China, where patients with lung adenocarcinoma with clear cell component had relatively worse overall survival without confirmation of multivariate analysis (8).
Our study found that CCA histology was associated with more advanced disease stage, whereas the study by Gu et al. reported that it was linked with early stage (8). The discrepancy between the two studies might be related to differences in ethnicity as well as the definition of CCA, where clear cell component was only required in at least 5% of the tumor for their study.
Interestingly, the WHO and IASLC classifications acknowledge that the other discontinued pathologic feature known as signet-ring cell are associated with presence of echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) gene rearrangement (EML4-ALK) (4,5). Little is known about biologic significance of CCA histology in the development of lung cancer. Gu et al. reported that 11 (29%) and 9 (24%) of 38 patients with CCA histology in China had EGFR and Kras mutations, respectively (8). Similarly, a higher proportion of Kras mutations were noted in a small study in US (12). Although somatic alteration in EGFR kinase domain is more common in East Asian nations than in the US, the relative high incidence in their study might suggest the link between EGFR signaling and CCA phenotype. ALK gene rearrangement was not detected in their cohort, although it was reported in the literature (12). No other commonly observed somatic changes such as RET, or ROS1 fusions were identified. Sensitivity of CCA with EGFR/ALK alteration to their kinase inhibitors is unknown.
Subtypes of non-small cell lung cancer (NSCLC) other than adenocarcinoma may also contain component of clear cells. They include benign pulmonary tumor, PEComa, squamous cell carcinoma, metastasis from renal cell carcinoma. Differential diagnosis in patients with a complicated disease history could be a challenge, though immunostaining is useful in determining the diagnosis. Further clinical and preclinical research to help interpret the significance of CCA is required.
We acknowledge limitations in the current study such as lack of information about systemic treatment and TNM stage. Smoking history or molecular information is not included because they are not available in SEER database. However, the current analysis still suggests NSCLC patients with CCA histology have favorable prognosis over LANOS, especially in local/regional stages. Further investigation is needed.
Acknowledgements
The authors thank Dr. Cindy Leissinger and Ms. Kathleen Brumfield for their administrative support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional Review Board at Tulane University gave exemption because the study was deemed not to constitute human subject research.
References
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005;353:2477-90. [Crossref] [PubMed]
- Shu CA, Zhou Q, Jotwani AR, et al. Ovarian clear cell carcinoma, outcomes by stage: the MSK experience. Gynecol Oncol 2015;139:236-41. [Crossref] [PubMed]
- Yamamato T, Yazawa T, Ogata T, et al. Clear cell carcinoma of the lung: a case report and review of the literature. Lung Cancer 1993;10:101-6. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors. Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Ou SH, Ziogas A, Zell JA. Primary Signet-Ring Carcinoma (SRC) of the Lung. A Population-Based Epidemiologic Study of 262 Cases with Comparison to Adenocarcinoma of the Lung. J Thorac Oncol 2010;5:420-7. [Crossref] [PubMed]
- Katzenstein AL, Prioleau PG, Askin FB. The histologic spectrum and significance of clear-cell change in lung carcinoma. Cancer 1980;45:943-7. [Crossref] [PubMed]
- Gu C, Pan X, Wang R, et al. Analysis of mutational and clinicopathologic characteristics of lung adenocarcinoma with clear cell component. Oncotarget 2016;7:24596-603. [Crossref] [PubMed]
- National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER). Available online: https://seer.cancer.gov/, accessed on May 4, 2018.
- SEER* stat software. National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER). Available online: https://seer.cancer.gov/seerstat/, accessed on May 4, 2018.
- Localized/Regional/Distant Stage Adjustments. National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER). Available online: https://seer.cancer.gov/seerstat/variables/seer/lrd-stage/, accessed on May 4, 2018.
- Yousem SA. Immunohistochemical and molecular characterization of clear cell carcinoma of the lung. Hum Pathol 2013;44:2467-74. [Crossref] [PubMed]


