
A propensity score matching study of non-grasping en bloc mediastinal lymph node dissection versus traditional grasping mediastinal lymph node dissection for non-small cell lung cancer by video-assisted thoracic surgery
Introduction
Lung cancer is one of the most common causes of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC) accounts for 80–85% of lung cancer patients (2). Surgery is thus far the most effective treatment for resectable NSCLCC including stage I-II and some stage III patients. With the advent of the video-assisted thoracic surgery (VATS) technique, the use of VATS has now become a viable surgical procedure for resectable NSCLC with superior short-term surgical outcomes and equivalent long-term survival rates when compared with thoracotomy (2-5). Lymph node (LN) metastasis is extremely important for the prognosis of NSCLC, and its correlation with prognosis even exceeds that of the extent of local invasion of tumour (6). This being the case, it is very important to remove the metastatic mediastinal lymph nodes (MLNs) thoroughly during the operation. Systemic mediastinal lymph node dissection (MLND) as an important part of standard NSCLC surgery is also conducive to accurate staging after surgery, providing a basis for postoperative adjuvant chemotherapy (7). The method of systemic MLND under VATS usually follows the traditional technique of thoracotomy, which needs to grasp the target LNs.
We have previously reported the technique of MLND named “non-grasping en bloc mediastinal lymph node dissection” (NE-MLND); this technique resects the MLN with the combined utilization and mutual cooperation of metal endoscopic suction and energy devices (electrocautery hook or ultrasonic scalpel) without grasping the LNs (8). Compared with the traditional “grasping” MLND, the non-grasping strategy can avoid damage to LNs and ensure the integrity of LNs, which is more in meeting with the principles of surgical oncology. Moreover, en bloc dissection of the bounded LNs and fat tissue block can remove LNs as best possible and make sure that there is no LN missed. However, it is still unclear whether NE-MLND can increase the risk of surgery and improve long-term survival. The aim of this study is to compare the efficacy of MLND, short-term surgical outcomes and long-term outcomes between NE-MLND and traditional grasping MLND (G-MLND).
Methods
Patients
Consecutive patients who had been treated with VATS for primary NSCLC between January 2009 and December 2013 were identified from the Western China Lung Cancer database at the West China Hospital, Sichuan University. To determine the preoperative staging, patients received enhanced computed tomography (CT) scanning of the chest and upper abdomen, enhanced brain CT scanning or magnetic resonance imaging (MRI), and whole-body bone scintigraphy. The positron emission tomography-computed tomography (PET-CT) was performed for patients with suspicious LN involvement appearing on CT scans. The seventh edition of the TNM staging system of lung cancer was used for staging (9). The International Association for the Study of Lung Cancer LN map was used to assess LN involvement (10).
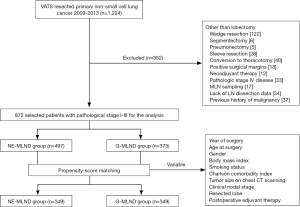
Our exclusion criteria included the following: lung resection other than lobectomy, sleeve resection, conversion to thoracotomy, positive surgical margins (R1 or R2), neoadjuvant therapy, pathologic stage IV disease, merely performed MLN sampling, lack of LN dissection data, and previous history of malignancy (Figure 1). This retrospective study was approved by the institutional review board at the West China Hospital, Sichuan University (No. 2018-404). Patients were grouped into the NE-MLND group or G-MLND group according to the procedure of MLND that was recorded in surgical records.
Surgical technique
A three-portal procedure was applied to each patient after general anaesthesia using double-lumen endotracheal intubation. Lobectomy was performed following the “single-direction” strategy as we previously described elsewhere (11). For patients in the NE-MLND group, we carried out a three-dimensional dissection according to our previous publication (8), which removed the total fat pad located among the anatomic landmarks of each station with the “non-grasping” technique. Meanwhile, for patients in the G-MLND group, the traditional “grasping” method of MLND was carried out, which mainly removed the target LNs.
Data collection
The clinicopathologic variables of these patients were reviewed and included the following items: age at surgery, gender, year of surgery, body mass index (BMI), smoking status, history of comorbidity as defined by the Charlson comorbidity index classification (12), tumor size on chest CT scanning, clinical TNM stage, resected lobe, postoperative adjuvant therapy, length of procedure, intraoperative blood loss, incidence of major postoperative complications (which includes postoperative bleeding, air leak more than 5 days, respiratory failure, pneumonia, chylothorax, arrhythmia, hoarseness, bronchopleural fistula, and pulmonary embolism), perioperative mortality, pleural drainage during the first 3 days after surgery, length of postoperative hospital stay, histologic subtype, pathologic TNM stage, number of LNs removed, nodal upstaging, and follow-up information updated as of June 2018.
Propensity score matching (PSM)
To minimize selection bias between the 2 groups, a PSM using SPSS 23.0 for Windows (SPSS IBM Corp; Armonk, NY, USA) was performed with the ratio of patients of each group being 1:1. The match tolerance was set at 0.02. The following variables were used for the PSM analysis: year of surgery, age at surgery, gender, BMI, smoking status, Charlson comorbidity index, tumour size on chest CT scanning, clinical nodal stage, resected lobe, and postoperative adjuvant therapy.
Statistical analyses
Continuous variables with normal distribution were expressed as the mean ± standard deviation (SD), otherwise they were presented as median and range. The differences between each group were determined using unpaired t-tests or Mann-Whitney U-test. Dichotomous variables were presented as counts and proportions and were analyzed using Pearson’s chi-square test or Fisher exact test. All statistical tests reported in the manuscript were two-sided. The Kaplan-Meier method and the log-rank test were used to analyze the overall survival (OS) and disease-free survival (DFS). The OS was defined as the time from the date of surgery until the last follow-up or death from any cause. The DFS was defined as the time from the date of surgery until any local or distant disease recurrence, or until the last follow-up. Univariable and multivariable analyses for OS were performed using the Cox proportional-hazards regression model in the propensity-matched patients. A P value less than 0.05 was considered to be statistically significant. Statistical analyses were performed using the SPSS 23.0 for Windows. Survival curves were depicted using GraphPad Prism (version 6.01, GraphPad Software Inc, La Jolla, CA, USA).
Results
Clinical characteristics and short-term surgical outcomes
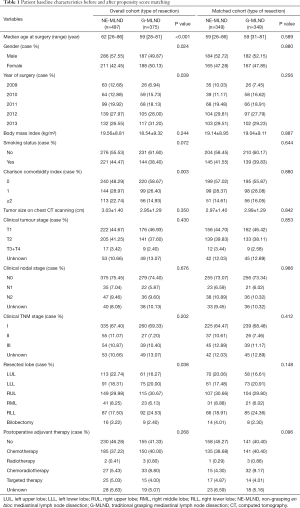
Before PSM, 872 patients who underwent VATS for stage I–III NSCLC met the eligibility criteria, of whom 497 were placed into the NE-MLND group, and 375 were placed into the G-MLND group. Their baseline clinical characteristics before PSM are shown in Table 1. There were significant differences in patient age (P<0.001), gender (P=0.024), year of surgery (P=0.039), Charlson comorbidity index (P=0.003), and resected lobe (P=0.038), between the NE-MLND and G-MLND groups. There were no significant differences in BMI (P=0.244), smoking status (P=0.072), tumour size on chest CT scanning (P=0.350), clinical tumour stage (P=0.430), clinical nodal stage (P=0.676), clinical TNM stage (P=0.202), and postoperative adjuvant therapy (P=0.268) between the 2 groups.
Full table
For short-term surgical outcomes, compared with patients in the G-MLND group, patients in the NE-MLND group had shorter operation time (135.37±49.70 vs. 165.40±70.04 min, P<0.001), more LNs removed (N1: 4.15±3.32 vs. 3.51±2.90, P=0.002; N2: 9.82±4.84 vs. 6.35±4.24, P<0.001; N1+N2: 13.97±6.14 vs. 9.86±5.37, P<0.001), more pleural drainage during the first 3 days after surgery (565.48±344.36 vs. 447.80±332.97 mL, P<0.001), and longer postoperative hospital stay (7.90±4.10 vs. 6.49±2.88 days, P<0.001). However, there were no statistically significant differences between the 2 groups with regard to the major postoperative complications (P=0.839), intraoperative blood loss (P=0.052), perioperative mortality (P=0.657), histologic subtype (P=0.122), pathologic TNM stage (P=0.849), and nodal upstaging (including clinical N0 to pathologic N1, P=0.061 and clinical N0 to pathologic N2, P=0.350) (Tables 2,3 respectively).

Full table

Full table
To reduce potential selection bias, we carried out a PSM analysis. Two matched groups (349 pairs, n=698 patients) were generated, and there were no significant differences in compared baseline clinical characteristics, as shown in Table 1. For short-term surgical outcomes, compared with patients in the G-MLND group, patients in the NE-MLND group had shorter operation time (132.67±50.19 vs. 166.77±70.35, P<0.001), more LNs removed (N1: 4.09±3.41 vs. 3.52±2.92, P=0.019; N2: 9.73±4.63 vs. 6.18±4.01, P<0.001; N1+N2: 13.82±6.00 vs. 9.70±5.23, P<0.001), more pleural drainage during the first 3 days after surgery (557.67±344.00 vs. 439.92±332.42 mL, P<0.001), and longer postoperative hospital stay (7.19±3.14 vs. 6.52±2.91 days, P<0.004). Moreover, in consistent with the results before PSM, there was no difference in other short-term surgical outcomes (Tables 2,3).
Survival
Patient follow-up as a part of our routine clinical practice started from the day of surgery. All patients were asked to review in the clinic every 3–6 months for the first 2 years, every 6 months for years 3, 4 and 5, and annually thereafter. Follow-up investigations for every review included clinical examinations, chest CT, MRI or CT of the brain and upper abdomen. Additionally, whole-body bone scintigraphy was performed once every year. A telephone follow-up would be made if the patient did not come to the clinic, or if patients who initially came from distant geographic locations followed up in their local hospital. Survival data were recorded in the Western China Lung Cancer database at the West China Hospital, Sichuan University. As of the last follow-up, 48 patients (9.66%) in the NE-MLND group and 46 patients (12.27%) in the G-MLND group were lost to follow-up. After matching, there were 35 patients (10.02%) and 38 patients (10.89%) lost to follow-up in the NE-MLND group and G-MLND group, respectively.
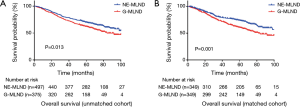
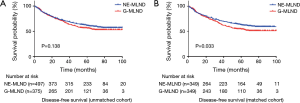
The median follow-up time was 60 months for unmatched patients and 59 months for matched patients. For long-term survival outcomes, NE-MLND showed superior OS in both the unmatched and matched comparisons (P=0.013, P=0.001, respectively) (Figure 2). The unmatched 5-year OS rates were 71.8% in the NE-MLND group and 64.8% in the G-MLND group. The matched 5-year OS rates were 76.4% in the NE-MLND group and 63.5% in the G-MLND group. Although the DFS showed no significant difference between the 2 groups in the unmatched comparison (P=0.138), NE-MLND showed superior DFS in the matched comparison (P=0.033) (Figure 3). The matched 5-year DFS rates were 63.0% in the NE-MLND group and 54.6% in the G-MLND group.
To see whether the application of the MLND technique was an independent prognostic factor, we performed the univariable and multivariable analyses for OS after PSM. On univariable analyses, statistically significant prognostic factors of OS were the application of MLND technique, gender, pathologic tumour stage, and pathologic nodal stage. The final multivariable analyses included these variables. G-MLND, pathologic T2 stage and T3 plus T4 stage (vs. T1 stage), and pathologic N1 and N2 stage (vs. N0 stage) were shown to be independently associated with higher risk of mortality. Table 4 demonstrates the results of univariable and multivariable competing risks regression.
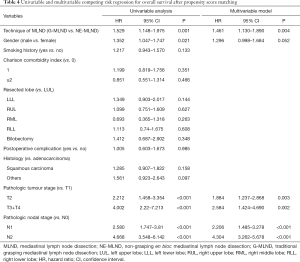
Full table
Discussion
In the development of VATS for lung cancer, MLN resection under VATS is one of the main difficulties hindering its popularization. The biggest controversy about VATS is mainly focused on whether it can completely resect MLN. Due to the complexity of the procedure and the different surgical habits of the surgeons, the methods and procedures of MLN resection are diverse. Although some experts have described their techniques of thoracoscopic MLN resection (13-15), all of their technical processes are relatively cumbersome and use strategies of grasping to resect the target LNs. Ultimately, the standard method of MLN resection has not yet been established. From our experience, we gradually developed a stylized method of MLND named as NE-MLND according to its operating characteristics (8).
In this study, we evaluated the short-term surgical outcomes and long-term survival outcomes of patients with NSCLC who underwent lobectomy plus NE-MLND or G-MLND. For short-term outcomes, our results demonstrated that compared with G-MLND, NE-MLND was associated with a shorter length of procedure, more pleural drainage during the first 3 days after surgery, and longer postoperative hospital stay before and after the PSM process. This might correlate with the en bloc dissection of the bounded fat, LNs and lymphatic vessel block, which can lead to a larger surgical wound and more adjacent lymphatic injury. Because there was no difference in intraoperative blood loss and perioperative bleeding between the 2 groups, the increase in the pleural drainage during the first 3 days after surgery was mainly caused by lymphatic exudation. This further leads to prolonging the postoperative hospital stay. Moreover, there was no significantly different in the other postoperative complications and perioperative mortality rates. This shows that NE-MLND is a safe and acceptable approach to remove MLN.
Nodal upstaging is known to occur in a significant proportion of surgical patients with NSCLC. There are several studies focusing on the topic of nodal upstaging in the literature. D'Cunha et al. (16) demonstrated that nodal upstaging was seen in 28% of the clinical stage I NSCLC patients in their study (14% of clinical stage II and 13.5% of clinical stage III). In another study, the nodal upstaging was found in 18.6% of the patients with clinical stage I NSCLC (17). Nodal upstaging is related to the accuracy of clinical staging. Nowadays, noninvasive imaging with CT alone or combined with PET-CT are recommended to determine the clinical nodal stage (18). Unfortunately, imaging cannot completely identify the occult nodal metastasis. Thus, the rate of nodal upstaging is dependent on the completeness of surgical LN dissection. Intuitively, the more LNs removed, the lower the false negative rate we should get (19). However, it is still unclear how many removed LNs is enough (19). Our study found that although NE-MLND removed more LNs, there was no significant difference in nodal upstaging status between the two groups.
For long-term survival outcomes, our results demonstrated that the OS and DFS in the NE-MLND group were significantly longer than those in the G-MLND group. Additionally, as shown in Table 4, the technique of MLN resection was shown to be an independent prognostic factor of OS. This may benefit from the certain advantages of NE-MLND over G-MLND. For one, as shown in Table 2, the mean number of removed nodes in the NE-MLND group was greater than the G-MLND group, and adequate LN dissection has previously been shown to be the most important prognostic element (20-23). Additionally, non-grasping can avoid LN damage and can further reduce tumour spread if the node is involved, which is in consistent with the tumour-free principles of surgical oncology. Moreover, en bloc dissection can remove the bounded fat block, LNs and lymphatic vessels in each station, which meets the principles of complete resection for surgical oncology. In contrast, the traditional “grasping” method of MLND mainly removes the target LNs without removing the tissue around the LNs, which may lead to LN fragmentation and miss some LNs that have metastasized.
There are several limitations to this study. First, about 10% of the patients in both groups were lost to follow-up, which may threaten the validity of these research results. Second, although the PSM process can reduce potential biases in retrospective studies, unlike randomized controlled trials, the biases caused by unobserved covariates cannot be eliminated. To our knowledge, this is the first study comparing the short- and long-term outcomes between NE-MLND and G-MLND. Therefore, we hope that there will be more studies, particularly large, randomized prospective studies focusing on this topic in the future to further verify our results.
Conclusions
Although patients who underwent NE-MLND have more pleural drainage during the first 3 days after surgery, and longer postoperative hospital stay than those who underwent G-MLND, NE-MLND is still a safe, acceptable and superior approach to remove MLNs, and has shorter operation time. Furthermore, patients with NSCLC may benefit from NE-MLND, which could lead to better OS and DFS as compared to G-MLND.
Acknowledgements
Funding: This study was supported by the Key Science and Technology Program of Sichuan Province, China (2016FZ0118 to Dr. L Liu).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the institutional review board at the West China Hospital, Sichuan University (No. 2018-404).
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Zhang W, Wei Y, Jiang H, et al. Video-Assisted Thoracoscopic Surgery Versus Thoracotomy Lymph Node Dissection in Clinical Stage I Lung Cancer: A Meta-Analysis and System Review. Ann Thorac Surg 2016;101:2417-24. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Liu C, Li Z, Bai C, et al. Video-assisted thoracoscopic surgery and thoracotomy during lobectomy for clinical stage I non-small-cell lung cancer have equivalent oncological outcomes: A single-center experience of 212 consecutive resections. Oncology letters 2015;9:1364-72. [Crossref] [PubMed]
- Zhang J, Mao T, Gu Z, et al. Comparison of complete and minimal mediastinal lymph node dissection for non-small cell lung cancer: Results of a prospective randomized trial. Thorac Cancer 2013;4:416-21. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53. [Crossref] [PubMed]
- Liu C, Pu Q, Guo C, et al. Non-grasping en bloc mediastinal lymph node dissection for video-assisted thoracoscopic lung cancer surgery. BMC Surg 2015;15:38. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Amer K. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg 2012;24:74-8. [Crossref] [PubMed]
- Lee HS, Jang HJ. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg 2012;24:131-41. [Crossref] [PubMed]
- Watanabe A, Nakazawa J, Miyajima M, et al. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg 2012;24:68-73. [Crossref] [PubMed]
- D'Cunha J, Herndon JE II, Herzan DL, et al. Poor correspondence between clinical and pathologic staging in stage 1 non-small cell lung cancer: results from CALGB 9761, a prospective trial. Lung cancer (Amsterdam, Netherlands) 2005;48:241-6. [Crossref] [PubMed]
- Licht PB, Jorgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9. [Crossref] [PubMed]
- Leong TL, Loveland PM, Gorelik A, et al. Preoperative Staging by EBUS in cN0/N1 Lung Cancer: Systematic Review and Meta-Analysis. J Bronchology Interv Pulmonol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Xu BB, Lu J, Zheng ZF, et al. Comparison of short-term and long-term efficacy of laparoscopic and open gastrectomy in high-risk patients with gastric cancer: a propensity score-matching analysis. Surg Endosc 2019;33:58-70. [Crossref]
- Wu YC, Lin CF, Hsu WH, et al. Long-term results of pathological stage I non-small cell lung cancer: validation of using the number of totally removed lymph nodes as a staging control. Eur J Cardiothorac Surg 2003;24:994-1001. [Crossref] [PubMed]
- Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol 2003;21:1029-34. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [Crossref] [PubMed]


