
Long-term survival outcomes of video-assisted thoracic surgery lobectomy for stage I-II non-small cell lung cancer are more favorable than thoracotomy: a propensity score-matched analysis from a high-volume center in China
Introduction
It has been more than two decades since the first introduction of video-assisted thoracic surgery (VATS) for the treatment of lung cancer (1,2). Currently, there are mountainous evidences that confirmed the advantages of VATS compared with open thoracotomy for the treatment of non-small cell lung cancer (NSCLC) (3-15). Most of these studies focused on the short-term results of surgery and demonstrated numerous advantages of VATS approach, including shorter chest tube duration (4,5), less pain, better preserved pulmonary and shoulder function (8), fewer postoperative complications (4-6,9,11-13,15), and shorter length of hospital stay (5,9-12,14,15) than open surgery. The oncologic efficacy remains a great concern for thoracic surgeons who performing or planning to perform VATS lung cancer resection (16). Recently, several nationwide database studies showed comparative long-term outcomes of VATS lobectomy for early stage NSCLC when comparing with open approach (10,12,14,15,17). However, few data with long-term outcomes are available in China, even though the VATS approach has been widely adopted for many years.
The main aim of this study was to evaluate the long-term outcomes of VATS versus open thoracotomy lobectomy for NSCLC in a high-volume center of China. The study also analyzed the short-term results of surgery and total in-hospital costs of VATS versus open lobectomy using the data from Western China Lung Cancer Database (WCLCD), which collected the data of lung cancer patients who underwent surgery at West China Hospital, Sichuan University.
Methods
Data source
Lung cancer patients who underwent surgery at the Department of Thoracic Surgery, West China Hospital, Sichuan University were registered in the WCLCD since September 2005. The follow-up data have been updated until June 2018. The study was approved by the institutional review board (IRB) of West China Hospital.
Patient selection
The present study enrolled consecutive patients who underwent VATS or open resection of lung cancer between May 2006 and June 2013 at the Department of Thoracic Surgery, West China Hospital. The inclusion criteria included: VATS or open anatomic lobectomy in combination with systemic lymph node dissection or sampling, pathology report of stage I-II NSCLC according to the 7th edition of the tumor, node and metastasis (TNM) staging system after surgery. The exclusion criteria were as follows: other than VATS or open approaches (video-assisted mini-thoracotomy or other approaches), other than lobectomy (segmentectomy, pneumonectomy, wedge resection or biopsy, lobectomy combined with sub-lobectomy, sleeve resection, chest wall resection or other extended resection, bilateral or combined operations, bilobectomy), preoperative induction therapy, other than NSCLC (small cell lung cancer, lung metastasis), other than stage I-II (stage III and IV, or data missed), and positive surgical margins.
VATS lobectomy was defined as individual transection of lobar vessels and bronchus under the guidance of video screen through three ports in the chest wall without rib spreading. The longest incision should be less than 5 cm. In our center, VATS lobectomy was mainly performed with the single-direction thoracoscopic lobectomy technique as we previously described (18). Open thoracotomy lobectomy was performed through posterolateral thoracotomy with latissimus dorsi and serratus anterior muscle section, as well as rib spreading. The patients were divided into two groups according to the surgical approach, VATS group and open group.
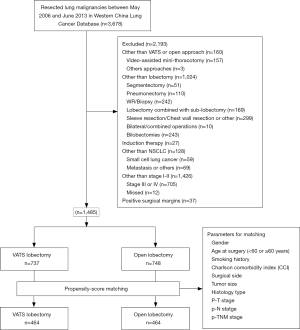
In order to minimize the potential selection bias, propensity score matching (PSM) was performed to balance the confounding factors between the two groups using the nearest matching method with a 1:1 ratio. Patients were matched by the following variables: gender (male, female), age at surgery (<60, ≥60 years), smoking history (never smoker, current or former smoker), Charlson comorbidity index (CCI), surgical side (right, left), tumor size, histological type (adenocarcinoma, squamous carcinoma, adenosquamous carcinoma, and other types of primary NSCLC), p-T status (T1, T2, T3), p-N status (N0, N1), and p-TNM stage (stage I, stage II). The patient inclusion flow chart was shown in Figure 1.
Outcome assessment
Outcomes of different surgical approaches were evaluated using the intent-to-treat analysis. Patients were discharged home 1–2 days after removing all the drainage tubes if no other complications. The primary outcome was overall survival (OS). Patient follow-up was started at the day of surgery according to our daily clinical practice, which included chest computed tomography (CT), brain magnetic resonance imaging (MRI) or CT, upper abdominal CT every 3–6 months for the first 2 years, and every 6 months for the next 3 years, and once annually from then on. In addition, regular telephone follow-up was made for the patients who did not come to the outpatient clinic. If the patient was lost to follow-up, the survival information was achieved from the National Death Registration System as an alternative. Secondary outcomes included the conversion rate of VATS, intraoperative blood loss, postoperative complications, number of lymph nodes and stations removed, chest tube duration, postoperative length of hospital stay and total hospital costs. The recorded postoperative complications included prolonged air leak (more than 5 days), pneumonia, bronchopleural fistula (BPF), empyema, chylothorax, reoperation, pulmonary embolism, arrhythmia, chest re-intubation, and other complications like cerebrovascular accident and gastrointestinal disorders.
Statistical analysis
The variables were summarized using the appropriate descriptive statistics, including absolute and relative frequencies for categorical variables, mean and standard deviation or median and range for quantitative variables. Student’s t-test and Mann-Whitney U test were used to compare quantitative variables between the VATS and open groups wherever applicable. Pearson χ2 test and Fisher’s exact test were applied to compare the categorical variables between the two groups. Kaplan-Meier survival curves were applied to depict the OS of the two groups before and after PSM, while the survival outcomes were compared using log-rank tests. In addition, Cox regression model was carried out to identify the independent prognostic factors for these patients. Statistical tests were performed using the Statistical Package for the Social Sciences (SPSS) for windows, version 23.0 (SPSS IBM Corp; Armonk, NY, USA). For all comparisons, the level of statistical significance was set at 0.05, all two tailed.
Results
A total of 3,678 patients underwent surgery for lung malignancies between May 2006 and June 2013 at the Department of Thoracic Surgery, West China Hospital, Sichuan University. Totally, 2,193 patients were excluded according to the exclusion criteria. The other 1,485 patients were enrolled for the study, including 737 cases of VATS lobectomy and 748 cases of open lobectomy. The number of VATS lobectomies increased annually during the study period. In this group of patients, the proportion of VATS lobectomies increased from 6.6% in 2006 to 66.7% during the first half year of 2013 (Figure 2).

Unmatched population
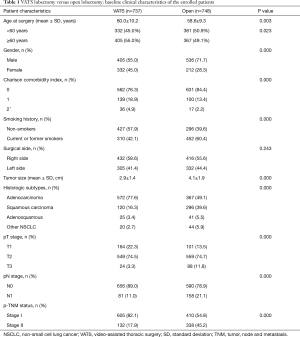
The details on patient and tumor characteristics were listed in Table 1. Patients who underwent VATS lobectomy were more aged than those in the open group (60.0±10.2 vs. 58.6±9.3, P=0.003), and there were fewer patients younger than 60 in the VATS group (45.0% vs. 50.9%, P=0.023). There were more female patients, more patients with comorbidities, but fewer current or former smokers in the VATS group when comparing with the open group. The mean tumor size was smaller in the VATS group than the open group (2.9±1.4 vs. 4.1±1.9 cm, P=0.000). There were more patients with adenocarcinoma in the VATS group than those in the open group (77.6% vs. 49.1%, P=0.000). More patients had p-TNM stage I disease in the VATS group than the open group (82.1% vs. 54.8%, P=0.000).
Full table
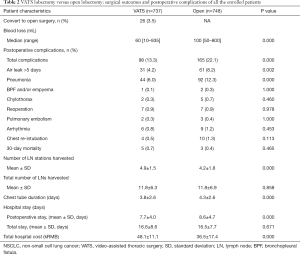
In the VATS group, 26 patients (3.5%) were converted to open surgery (Table 2). The VATS lobectomy was associated with less blood loss than open lobectomy (median: 60 vs. 100 mL, P=0.000). The total incidence of postoperative complications was significantly lower in the VATS group (13.3% vs. 22.1%, P=0.000), which was also associated with fewer prolonged air leak (4.2% vs. 8.2%, P=0.002), and fewer cases of postoperative pneumonia (6.0% vs. 12.3%, P=0.000). More lymph node stations were harvested in the VATS group than the open group (4.9±1.5 vs. 4.2±1.8, P=0.000). However, the total number of resected lymph nodes were nearly the same in VATS and open groups (11.8±6.3 vs. 11.8±6.9, P=0.858). Chest tube drainage was shorter in the VATS group than the open group (3.8±2.6 vs. 4.3±2.6 days, P=0.000), so did the length of postoperative hospital stay (7.7±4.0 vs. 8.6±4.7 days, P=0.000). However, the total hospital costs were higher in the VATS group than the open group (48.1±11.1 vs. 36.5±17.4 kRMB, P=0.000).
Full table
Matched population
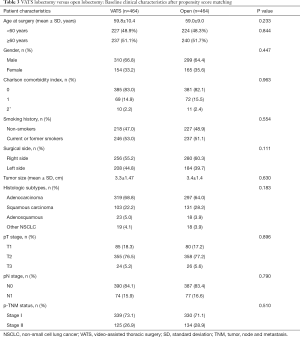
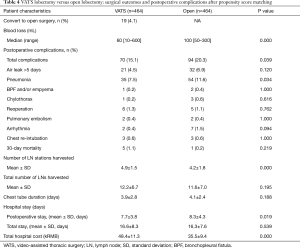
The PSM identified 464 cases of VATS lobectomies and 464 cases of open lobectomies for the study based on patient demographics, CCI, tumor histology and tumor stage. The baseline clinical characteristics of the matched cases were listed in Table 3. The two groups of patients were comparable in age, gender, comorbidities, smoking, tumor size, histology, TNM stage. Surgical outcomes of the two groups were summarized in Table 4. After matching, there was less blood loss in patients underwent VATS lobectomy than open lobectomy (median blood loss: 60 vs. 100 mL, P=0.000). The total incidence of postoperative complications was lower in the VATS group after matching (15.1% vs. 20.3%, P=0.039). VATS lobectomy was also associated with lower incidence of postoperative pneumonia (7.5% vs. 11.6%, P=0.034) in the matched cohort.
Full table
Full table
In the matched cohort, VATS procedures still harvested more lymph node stations (4.9±1.5 vs. 4.2±1.8, P=0.000), but the total number of removed lymph nodes was similar between the two procedures (12.2±6.7 vs. 11.6±7.0, P=0.195). There was no difference in the duration of postoperative chest drainage between the two procedures (3.9±2.8 vs. 4.1±2.4 days, P=0.188). However, patients underwent open lobectomy stayed longer in the hospital after surgery (7.7±3.8 vs. 8.3±4.3 days, P=0.019), but the total in-hospital costs were more expensive in the VATS group than the open group (48.4±11.3 vs. 35.5±9.4 kRMB, P=0.000).
Long-term outcomes
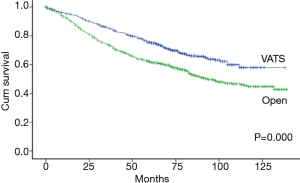
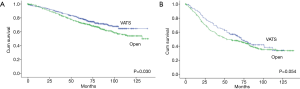
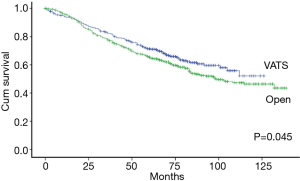
The Kaplan-Meier analysis revealed that the 5-year OS of stage I-II NSCLC patients who underwent VATS lobectomies was significantly better than those who underwent open lobectomies (75.0% vs. 61.9%, P=0.000) (Figure 3). The patients were stratified by pathological stages to compare the long-term outcomes after surgery. The 5-year OS of VATS lobectomy were significantly better for stage I NSCLC patients when comparing with open lobectomy (79.4% vs. 73.4%, P=0.030). In patients with stage II NSCLC, there was also a trend of improved 5-year OS after VATS lobectomy (57.1% vs. 48.4%, P=0.054) (Figure 4). The multivariable adjusted survival analysis revealed that the VATS approach was associated with superior long-term survival (HR =1.336, 95% CI, 1.110–1.607, P=0.002) (Table 5). The other independent predictors of OS included age at surgery, gender, CCI, pT, pN and p-TNM stage (Table 5). After propensity matching, the 5-year OS of VATS lobectomies for stage I-II NSCLC was still better than the open approach (71.1% vs. 65.4%, P=0.045) (Figure 5).

Full table
Discussion
The present study was performed based on the Western China Lung Cancer Database which collecting the clinical information of lung cancer patients underwent surgery at the Department of Thoracic Surgery, West China Hospital since September 2005. We summarized the perioperative and long-term survival outcomes of stage I-II NSCLC patients who underwent VATS or open lobectomies between May 2006 and June 2013. In our center, VATS lobectomy was steadily carried out in 2006 with the procedure named single-direction thoracoscopic lobectomy as we previously described (18). This study enrolled the patients who had more than 5-year of follow-up after surgery in order to compare the long-term outcomes of VATS and open lobectomies for p-stage I-II NSCLC. The patients who underwent surgery during recent years were not included in the study because of two reasons. The first reason was the relative short-term of follow-up after surgery. Secondly, the proportion of VATS lobectomies increased annually during the study period, from 6.6% in 2006 to 66.7% in 2013. The trend of increasing continued during recent years. Most of lung cancer resections were performed through the VATS approach (more than 90% in 2017) and this made the comparative study infeasible in recent case series because open lobectomies were relatively rare in our center.
We found in this cohort that the total conversion rate of VATS was 3.5% which was lower than many reported case series (10,14,19). The total incidence of postoperative complications was lower in the VATS group in both unmatched and matched cohorts. The VATS approach harvested more lymph node stations than the open approach. Even though the postoperative hospital stay was shorter in the VATS group, the total hospital costs were higher. Stage I-II NSCLC patients who underwent VATS lobectomies had superior 5-year OS when compared with those who underwent open lobectomies in both unmatched and matched cohorts.
The VATS approach was found to be associated with fewer postoperative complications in many studies as reported previously (4-6,9,11-13,15), but the incidence of postoperative complications ranged variously in these studies, from less than 10% to as high as 40.8% after VATS lobectomy and 45.1% after open lobectomy. A recent comparative study of the Society of Thoracic Surgeons (STS) and the European Society of Thoracic Surgeons (ESTS) database listed the main complications of lobectomy in these two databases, including air leak >5 days (STS: 10.7%, ESTS: 9.7%), pneumonia (STS: 4.3%, ESTS: 7.8%), atrial arrhythmia (STS: 11%, ESTS: 5.8%), and the 30-day mortality (STS: 1.4%, ESTS: 2.6%) (20). In our cohort, the total incidence of postoperative complications was lower in the VATS group, and lower than the aforementioned reports, so did the main common complications like prolonged air leak. The variation of the incidence of postoperative complications may due to the difference of the studied population, technical aspects in different centers. Another important reason for the variation among different studies was the lack of universal criteria for the definition of postoperative complications like pneumonia.
In this study, we found that the VATS approach was more expensive than the open approach. Several previous reports have demonstrated that the costs of VATS lobectomy were lower than open thoracotomy because of shorter hospital stay (21-27). Most of these studies were from developed countries. However, few data are available on the in-hospital costs for lung cancer patients in China. Even though the patients who underwent VATS lobectomy had shorter postoperative hospital stay and lower incidence of postoperative complications, they cost more money during hospital stay. This could be due to the differences in medical payment between China and the Western counties. When performing VATS lobectomy, more staplers were used to handle the vessels which could be ligated directly.
The most important finding of this study was that VATS lobectomy was associated with improved long-term survival in patients with stage I-II NSCLC, along with other independent predictors of OS like age at surgery (<60 or ≥60 years), gender, T stage, N stage and p-TNM stage. VATS lobectomy was reported with reduced systematic recurrence rate and improved 5-year OS for NSCLC patients (3,5,28). However, a recent national analysis of long-term survival for stage I NSCLC found no difference between the VATS and open approaches (14). When performing VATS lobectomy, the mainstream of surgical procedures in our center was described as single-direction thoracoscopic lobectomy, which transected the pulmonary veins first for every lobe during surgery (18). The open lobectomy was usually performed in a different way which handling the arteries first through the pulmonary fissure. The difference of the two surgical procedures may be an important reason for the improved survival because the sequence of vascular interruption is associated with long-term outcomes. Patients underwent venous-first procedure have improved disease-free survival than those underwent non-venous-first procedure (29). The other potential protective factor was that VATS approach harvested more lymph node stations which may ensure more accurate N-stage.
There are some limitations of the present study. First, this is a retrospective study. The retrospective nature of the study may cause unobserved confounding and selection bias between the two groups even though the multivariable model and PSM could help reduce the bias. Second, this is a single center study from one high-volume center in China. Whether the study could represent the nation-wide real-world practice, is still need further confirmation.
In conclusion, VATS lobectomy for the treatment of stage I-II NSCLC is associated with less intraoperative blood loss, similar postoperative complications, shorter postoperative hospital stay. Despite more hospital cost, VATS lobectomy offer more superior 5-year OS than the open approach. Considering the shorter postoperative hospital stay and the long-term survival benefits, it is reasonable to recommend the VATS approach as the preferred option for the surgical treatment of stage I-II NSCLC.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (NSFC 81602025, to Dr. Mei), the Key Science and Technology Program of Sichuan Province, China (2016FZ0118, to Dr. L Liu).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Use of the data for this study was approved by the institutional review board (IRB) of West China Hospital (NO. 2018-403).
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg 1992;104:1679-85; discussion 85-7.
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Chen FF, Zhang D, Wang YL, et al. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol 2013;39:957-63. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Cai YX, Fu XN, Xu QZ, et al. Thoracoscopic Lobectomy versus Open Lobectomy in Stage I Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS One 2013;8:e82366. [Crossref] [PubMed]
- Pu Q, Ma L, Mei J, et al. Video-assisted thoracoscopic surgery versus posterolateral thoracotomy lobectomy: A more patient-friendly approach on postoperative pain, pulmonary function and shoulder function. Thoracic Cancer 2013;4:84-9. [Crossref] [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [Crossref] [PubMed]
- Yang CF, Sun Z, Speicher PJ, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Pages PB, Delpy JP, Orsini B, et al. Propensity Score Analysis Comparing Videothoracoscopic Lobectomy With Thoracotomy: A French Nationwide Study. Ann Thorac Surg 2016;101:1370-8. [Crossref] [PubMed]
- Laursen LO, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2017. [Epub ahead of print].
- Dziedzic R, Marjanski T, Binczyk F, et al. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: a propensity score-matched analysis. Eur J Cardiothorac Surg 2018;54:547-53. [PubMed]
- Cao C, Tian DH, Wolak K, et al. Cross-sectional Survey on Lobectomy Approach (X-SOLA). Chest 2014;146:292-8. [Crossref] [PubMed]
- Al-Ameri M, Bergman P, Franco-Cereceda A, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy: a Swedish nationwide cohort study. J Thorac Dis 2018;10:3499-506. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Seder CW, Salati M, Kozower BD, et al. Variation in Pulmonary Resection Practices Between The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases. Ann Thorac Surg 2016;101:2077-84. [Crossref] [PubMed]
- Mafe JJ, Planelles B, Asensio S, et al. Cost and effectiveness of lung lobectomy by video-assisted thoracic surgery for lung cancer. J Thorac Dis 2017;9:2534-43. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Stitzenberg KB, Shah PC, Snyder JA, et al. Disparities in access to video-assisted thoracic surgical lobectomy for treatment of early-stage lung cancer. J Laparoendosc Adv Surg Tech A 2012;22:753-7. [Crossref] [PubMed]
- Ramos R, Masuet C, Gossot D. Lobectomy for early-stage lung carcinoma: a cost analysis of full thoracoscopy versus posterolateral thoracotomy. Surg Endosc 2012;26:431-7. [Crossref] [PubMed]
- Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc 2011;25:1054-61. [Crossref] [PubMed]
- Burfeind WR Jr, Jaik NP, Villamizar N, et al. A cost-minimisation analysis of lobectomy: thoracoscopic versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2010;37:827-32. [Crossref] [PubMed]
- Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: can we afford it? Eur J Cardiothorac Surg 2009;35:423-8. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Sumitomo R, Fukui T, Marumo S, et al. Effects of vessel interruption sequence during thoracoscopic lobectomy for non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2018;66:464-70. [Crossref] [PubMed]


