Focus on treatment complications and optimal management surgery
Introduction
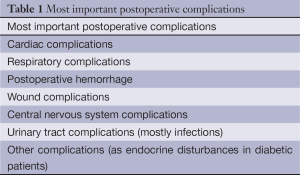
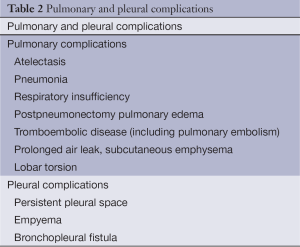
Postoperative complications are a frequent occurrence after thoracic surgery, especially when major procedures are performed as e.g., intrapericardial pneumonectomy, chest wall resections, tracheal surgery and mediastinal tumors invading major anatomical structures. A variety of postoperative complications are encountered and the most important ones are listed in Table 1. Pulmonary and pleural complications occurring after lung resection are listed in Table 2.
Full table
Full table
In a review of more than 3,500 major lung resections, 30-day mortality after lobectomy was 4% and after pneumonectomy was 11.5% (1). Most frequently encountered respiratory complications were pneumonia, unplanned reintubation, failure to wean within 48 hours and pulmonary embolism. These complications occurred as well after pneumonectomy as lobectomy.
Currently, mortality and morbidity have been reduced, mainly due to better anesthetic and intensive care management. Attention should be given to prevention of postoperative complications necessitating a multidisciplinary approach involving not only thoracic surgeons but also anesthesiologists, pulmonary physicians, thoracic oncologists and physiotherapists. Key factors are preoperative smoking cessation, adequate pain control, preferentially by a thoracic epidural catheter, attention to nutritional status and early mobilization.
In this overview complications are addressed which are mainly related to the surgical intervention and of specific interest, not only to thoracic surgeons but also to intensive care and pulmonary physicians taking care of these patients. Short discussions are provided on postpneumonectomy pulmonary edema, thromboembolic disease including pulmonary embolism, prolonged air leak, lobar torsion, persistent pleural space, empyema and bronchopleural fistula.
Pulmonary complications
Postpneumonectomy pulmonary edema
Postpneumonectomy pulmonary edema is a non-cardiogenic, non-infectious pulmonary edema which in fact represents a form of adult respiratory distress syndrome (ARDS) in the remaining lung. Pathophysiologically, this entity is characterized by permeability edema and diffuse alveolar damage. Its incidence after pneumonectomy is approximately 2-5% but mortality remains high, even in centres with a large thoracic surgical experience (2-4).
Risk factors include right pneumonectomy and excessive fluid load as e.g., by overzealous intraoperative transfusion. Diagnosis is one of exclusion and infectious and cardiac complications should be ruled out. Therapy consists of judicious use of diuretics (“keep the patient dry”), inotropic drugs if indicated, restricted fluid intake and in case of respiratory insufficiency, reintubation and ventilation with airway pressures as low as possible (5). Use of NO ventilation may increase oxygen saturation. In severe cases extracorporeal membrane oxygenation (ECMO) may be indicated. The role of steroids remains controversial.
Postpneumonectomy syndrome is caused by extreme mediastinal shifting and although rare, manifests itself by progressive dyspnea due to airway and vascular compression. Several treatment options are available including tissue expanders to reposition the mediastinum towards the midline (6,7).
Thromboembolic disease (including pulmonary embolism)
Pulmonary embolism usually results from deep vein thrombosis and is currently considered to be a single disease entity, called thromboembolic disease. After lung resection, pulmonary embolism is a devastating complication. Its incidence is approximately 1-5% with an elevated mortality after major lung resection. Prevention consists of prophylactic low molecular weight heparin, elastic stockings, early ambulation and in high-risk patients, use of pneumatic compression devices which can also be applied during the intervention. The adagio “the best way to handle a surgical complication is to avoid it” holds certainly true for thromboembolic disease (7). Nowadays, venous duplex examination is the preferred diagnostic test for deep vein thrombosis. Pulmonary emboli can most reliably be detected by high-resolution contrast chest computed tomography (CT). Ventilation-perfusion scanning is an alternative technique, especially in patients with allergy to contrast agents. Treatment consists of low molecular weight heparin in therapeutic doses, adapted to the weight of the patient (8). Thrombolytic therapy or pulmonary embolectomy may be applied in severe cases but the latter is a major procedure which should only be performed in patients with severe hemodynamic compromise.
Prolonged air leak, subcutaneous emphysema
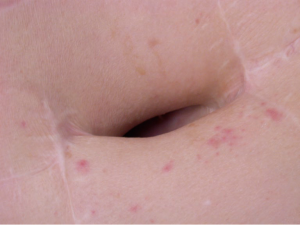
Especially in patients with emphysema, persisting air leakage is often encountered. Its incidence has been reported in up to 50% of patients rendering it the most frequent postoperative complication (9). Air leak is considered abnormal when still present at postoperative day 7, although a limit of 5 days is utilized in some centres (9). Clinically, progressive subcutaneous and mediastinal emphysema may develop, resulting in swollen face and eyes, the so-called “Michelin syndrome” (Figure 1).
Risk factors include bullous emphysema, lung volume reduction surgery and prolonged ventilatory support giving rise to barotrauma. In patients undergoing lung resection, intra-operative control of the bronchial stump and staple lines is necessary to reduce the incidence of this complication. Fibrin glue or other sealants can be applied. In a Cochrane systematic review surgical sealants were found to reduce postoperative air leaks and time to chest tube removal but a reduction in length of hospital stay could not always be demonstrated (10). Initial reinforcement of the staple lines with strips or creation of a pleural tent by detaching the parietal pleura are additional measures for better control of air leakage. In the immediate postoperative period patients should be extubated as soon as possible to reduce the intraalveolar pressure. Whether or not suction should be applied to the chest tubes after pulmonary resection remains a matter of debate. In a systematic review and meta-analysis no differences were found in duration of air leak, incidence of prolonged air leak, chest tube duration and hospital stay when comparing suction drainage versus water seal only (11). Postoperatively, injection of talc, other sclerosing agents or autologous blood may be helpful to close a persisting air leak. In case of adequate lung expansion and persisting air leak, insertion of a pigtail catheter connected to Heimlich valve allows further ambulatory treatment. Intrabronchial valves can also be used to isolate persistent air leaks and close alveolar-pleural fistulas (12).
Subcutaneous emphysema is treated by several small skin incisions with insertion a drain beneath the superficial fascia to drain the air efficiently. In case of pronounced mediastinal emphysema, a mediastinal drain is indicated that is directed caudally underneath the pretracheal fascia from a small suprasternal incision.
Lobar torsion
Lobar torsion is an uncommon event with an incidence of less than 0.5% (4,13). It is induced by rotation of a remaining lobe around its bronchovascular pedicle. This has mainly been described with the right middle lobe but other lobes may be involved as well, also on the left side (14). To prevent lobar torsion fixation of the remaining lobe(s) is indicated in order to avoid twisting around the pedicle of the remaining lung parenchyma.
Clinical signs and symptoms of lobar torsion resemble those of atelectasis and pneumonia. Hemoptysis may be a heralding sign. Progressive opacification of the involved lobe is observed on postoperative chest radiographs. When left untreated, hemorrhagic infarction and necrosis will occur. Bronchoscopy will reveal the precise diagnosis as bronchial rotation occurs at the level of the bronchial stump of the removed lobe (7). Reintervention is indicated and mostly, a lobectomy of the involved lobe will be necessary. When untwisting the involved lobe, emboli may spread through the pulmonary veins. For this reason it is recommended to clamp the pulmonary veins intrapericardially at the start of the reintervention to avoid this dramatic complication (14).
Pleural complications
Persistent pleural space
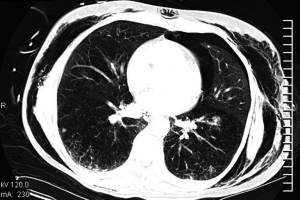
After major lung resection less than a pneumonectomy, there is usually a residual space detected on chest radiograph or CT (Figure 2). This is often described as a “persisting pneumothorax” by thoracic radiologists. However, this is often a wrong term as there is not always a collapse of the remaining lung parenchyma.
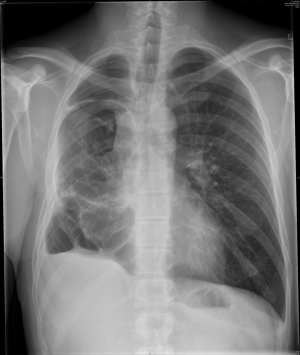
When draining the pleural cavity after lung resection two distinct phases are recognized. Firstly, air and blood have to be evacuated to obtain optimal lung expansion and at a second stage, the air is gradually replaced by fluid as can be observed on postoperative chest radiographs. Overdistension of the remaining lung parenchyma has to be avoided to prevent pleural space complications and lung edema. Due to different compliance characteristics, important differences exist between emphysematous and fibrotic lung parenchyma in development of a residual space.
No standard definition of such a residual space is currently available. Pathophysiologically, four types exist: physiological, those with active air leak or diminished compliance of the lung, and those associated with dense adhesions (trapped lung). Most important factors in the development of space problems are a continuous air leak and decreased compliance. Complicated residual spaces are those with a persisting air leak which may result in subcutaneous and mediastinal emphysema, and those with empyema due to colonization with bacteria or fungi. When no complications occur, a conservative treatment is warranted on the condition that optimal expansion of the remaining lung parenchyma is achieved (15). In case of infection or persistent air leakage drainage of the remaining pleural cavity is indicated. Subsequently, muscle flaps may be used to fill up any residual space and to prevent recurrent infection. When a difficult to control air leak or multibacterial and mycotic superinfections are encountered, a temporary or definitive thoracostomy may be required.
Empyema
By definition empyema implies that the pleural space is infected, usually by bacteria and/or fungi. Its incidence after pneumonectomy varies between 2 and 12%, the majority of these patients also presenting with a bronchopleural fistula. After resections less than a pneumonectomy, it occurs in less than 3% of cases (4). Risk factors include older age, cardiopulmonary impairment, malnutrition, induction therapy (especially chemoradiotherapy), diabetes, steroids, right pneumonectomy, complex procedures as extended resections, postoperative pneumonia and mechanical ventilation giving rise to barotrauma.
Clinical symptoms are mostly age-specific and related to the general condition of the patient. A high index of suspicion is required, especially in patients with systemic toxicity, loss of appetite and general deterioration. Biochemically, a rise in inflammatory parameters is detected. Culture of pleural fluid before starting antibiotics will reveal responsible bacteria and/or fungi. Therapy includes chest tube drainage, antibiotics or antimycotics adapted to the results of the cultures. Irrigation of the pleural space may be indicated to clean the remaining cavity. In patients with severe intoxication thoracostomy may be necessary to control the septic condition (Figure 3). Postlobectomy empyema should be treated as postpneumonic empyema with adequate chest tube drainage (16).
Bronchopleural fistula
In case of a bronchopleural fistula there is a direct communication between the tracheobronchial tree and the pleural space by a variable opening in the bronchial stump. Bronchopleural fistulas may range from small to large, the latter being a nightmare for thoracic surgeons often leading to life-threatening events. Global incidence is highly variable depending on the specific procedures performed (17). This condition is often heralded by progressive sepsis and respiratory distress or insufficiency. Early cases occur within 7 days after the procedure and are usually due to poor technical closure of the bronchial stump. Late cases are observed more than one week after the operation and are mostly related to failure of healing of the bronchial stump, recurrent disease at the bronchial margin or progressive infection.
Risk factors include right or completion pneumonectomy, interventions for infectious or inflammatory diseases, high-dose radiotherapy, prolonged mechanical ventilation, empyema, infected postresection space and residual tumor at the bronchial stump. For early bronchopleural fistulas incomplete closure or devascularisation by extensive mediastinal dissection are the most important causative factors. To prevent a bronchopleural fistula it is advocated to cover every bronchial stump in case one or more risk factors are present (Figure 4).
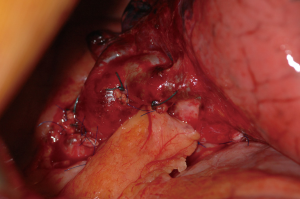
Initially, clinical symptoms are quite similar to those of empyema and equally, a high index of suspicion is necessary. An additional characteristic may be the expectoration of dark brown fluid which may be massive in case of a large bronchopleural fistula.
After pneumonectomy there is a typical decrease in the fluid level on the operated side. Bronchoscopy is indicated to inspect the bronchial stump. Ventilation scanning and injection of methylene blue at the stump may be helpful to detect smaller bronchopleural fistulas.
Therapy is often demanding, difficult and prolonged. Multiple reinterventions may be necessary. Initial treatment is the same as for empyema as both are often encountered together, and consists of closed or open drainage and systemic antibiotics or antimycotics. In case of sudden expectoration the patient should be put in bed with the operated side down and the head elevated.
After control of the septic condition, closure of the bronchopleural fistula should be attempted. Bronchoscopic injection of glue or a sclerosing agent may be successful for smaller bronchopleural fistulas. For larger bronchopleural fistulas, reintervention is necessary with excision of necrotic tissue and reclosure of the bronchial stump, which should be covered by viable tissue as a muscle flap of the chest wall, pericardium or omentum (4). Irrigation of the thoracic cavity is indicated to prevent a recurrent empyema.
Conclusions
A wide variety of postoperative respiratory complications may occur, ranging from minor postoperative problems to life-threatening conditions. Prevention and attention to surgical detail are required to obtain the best postoperative results. Early detection of complications is essential to provide adequate and effective treatment of these complications which will prevent further deterioration of the respiratory and general condition of the patient.
Respiratory physicians and physiotherapists play an important role in prevention and treatment of postoperative morbidity. International registries are required to better define the incidence, diagnosis and treatment of postoperative complications allowing for better management and improved postoperative results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Harpole DH Jr, DeCamp MM Jr, Daley J, et al. Prognostic models of thirty-day mortality and morbidity after major pulmonary resection. J Thorac Cardiovasc Surg 1999;117:969-79. [PubMed]
- Waller DA, Keavey P, Woodfine L, et al. Pulmonary endothelial permeability changes after major lung resection. Ann Thorac Surg 1996;61:1435-40. [PubMed]
- Slinger PD. Perioperative fluid management for thoracic surgery: the puzzle of postpneumonectomy pulmonary edema. J Cardiothorac Vasc Anesth 1995;9:442-51. [PubMed]
- Crabtree TD, Denlinger CE. Complications of surgery for lung cancer. In: Pass HI, Carbone DP, Johnson DH, et al. eds. Principles and practice of lung cancer. Philadelphia: Lippincott Williams & Wilkins, 2010:531-46.
- Parquin F, Marchal M, Mehiri S, et al. Post-pneumonectomy pulmonary edema: analysis and risk factors. Eur J Cardiothorac Surg 1996;10:929-32; discussion 933. [PubMed]
- Villeneuve PJ, Sundaresan S. Complications of pulmonary resection: postpneumonectomy pulmonary edema and postpneumonectomy syndrome. Thorac Surg Clin 2006;16:223-34. [PubMed]
- Haithcock BE, Feins RH. Complications of pulmonary resection. In: Shields TW, LoCicero III J, Reed CE, et al. eds. General Thoracic Surgery, 7th edition. Philadelphia: Lippincott Williams & Wilkins, 2009:551-9.
- Brunelli A. Deep vein thrombosis/pulmonary embolism: prophylaxis, diagnosis, and management. Thorac Surg Clin 2012;22:25-8. [PubMed]
- Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis 2014;6:271-284. [PubMed]
- Serra-Mitjans M, Belda-Sanchis J, Rami-Porta R. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2005;CD003051. [PubMed]
- Coughlin SM, Emmerton-Coughlin HM, Malthaner R. Management of chest tubes after pulmonary resection: a systematic review and meta-analysis. Can J Surg 2012;55:264-70. [PubMed]
- Mahajan AK, Doeing DC, Hogarth DK. Isolation of persistent air leaks and placement of intrabronchial valves. J Thorac Cardiovasc Surg 2013;145:626-30. [PubMed]
- Cable DG, Deschamps C, Allen MS, et al. Lobar torsion after pulmonary resection: presentation and outcome. J Thorac Cardiovasc Surg 2001;122:1091-3. [PubMed]
- Hendriks J, Van Schil P, De Backer W, et al. Massive cerebral infarction after completion pneumonectomy for pulmonary torsion. Thorax 1994;49:1274-5. [PubMed]
- Murthy SC. Air leak and pleural space management. Thorac Surg Clin 2006;16:261-5. [PubMed]
- Gharagozloo F, Margolis M, Facktor M, et al. Postpneumonectomy and postlobectomy empyema. Thorac Surg Clin 2006;16:215-22. [PubMed]
- Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456-64. [PubMed]