Multi-targeted tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: an era of individualized therapy
Introduction
In recent years, the application of targeted drugs has dramatically changed the conventional treatment modes for non-small cell lung cancer (NSCLC). The major milestone events include: in 1962, Cohen et al. discovered epidermal growth factor (EGF); in 2008, the iPASS study confirmed the role of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) for advanced EGFR-mutated NSCLC; and in 2010, results from the OPTIMAL trial, for the first time, declared that the median progression-free survival (PFS) of advanced NSCLC patients was prolonged by one year or more after the administration of multi-targeted drug(s) (1,2).
Despite of the scientific carnivals in the past years, however, the efficacies of these drugs for NSCLC were not satisfactory in many clinical trials. For NSCLC patients, these drugs are between a rock and a hard place, although they are theoretically perfect. In this article, we review the up-to-dated research advances on the application of multi-targeted tyrosine kinase inhibitors (TKIs) for NSCLC treatment, with an attempt to summarize evidences for future development.
Anti-tumor mechanisms of multi-targeted TKIs
Multi-targeted TKIs have been regarded as promising for NSCLC due to their unique theoretical bases and anti-tumor mechanisms. First, single-targeted drugs usually have poor efficacies for most NSCLC patients, although they may be highly effective in certain patients. This can be theoretically explained by the fact that NSCLC is a highly heterogeneous malignancy, with its signal pathways exhibit a complex array of cross connections. As a result, when blocking the key signal transduction pathways, the single-targeted drugs can also activate the tumor escape mechanisms; ultimately, the proliferation of tumor cells may be re-activated via other pathways (3). Obviously, when safety and low-toxicity are guaranteed, drugs should be optimized to inhibit as many as possible of the tumor signal pathways. Second, as shown in Table 1, compared with the single-targeted drugs that are targeted at tumor cells only, most multi-targeted drugs can suppress the micro-environment that supports the tumor growth by inhibiting the vascular endothelial growth factor receptor (VEGFR) signal pathway, and thus exert dual anti-angiogenesis and anti-proliferation effects.
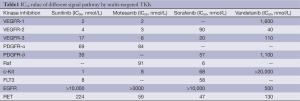
Full table
Some pre-clinical studies have documented that multi-targeted TKIs can exert their anti-angiogenesis effect by inhibiting the activity of VEGF-dependent vascular endothelial cells. Furthermore, by inhibiting the VEGFR signal pathways, multi-targeted TKIs (e.g., sunitinib) not only degrade tumor microvessels but also inhibit the production of new tumor blood vessels and reduce the effusions of tumor vessels, and ultimately block the blood supply of tumor cells and cause the liquefactive necrosis of tumor cells (4-11).
On the other hand, the direct effects of multi-targeted TKIs on the targets at tumor cell signal pathways can also exert certain anti-proliferative activity against NSCLC. For example, vandetanib can inhibit EGFR signal pathway, sunitinib can inhibit platelet-derived growth factor receptor α (PDGFRα) signal pathway, and sorafenib can inhibit RAS/RAF signal pathway.
Therefore, the multi-targeted drugs with dual actions are superior to single-targeted drugs in terms of action mechanism. Then, how about the real-world clinical data?
Up-to-dated advances in clinical research on multi-targeted TKIs
Sunitinib (Sutent, SU11248)
Sunitinib, chemically known as N-[2-(diemylanino)emyl]-5-[(Z)-(5-fluoro-l, 2-dihydro-2-oxo-3H-indol-3-yUdine)memyl]-2, 4-dimemyl-lH-pyrrole-3-carbox ami, is a multi-targeted receptor tyrosine kinase (RTK) inhibitor. Its anti-tumor activities against renal cell carcinoma, gastrointestinal stromal tumors, soft tissue sarcoma, and thyroid cancer have been documented. Since sunitinib has shown good anti-tumor activity in solid tumor cell lines including H226NSCLC (12), many studies have explored its role in fighting against NSCLS. In a phase II trial, Socinski et al. (13) demonstrated the anti-tumor activity of sunitinib monotherapy in treating NSCLC patients for whom chemotherapy had failed. In their study, a total of 63 NSCLC patients for whom conventional chemotherapy had failed were enrolled in this study and received 50 mg/d of sunitinib for 4 weeks followed by 2 weeks of no treatment in 6-week treatment cycles. Compared with the baseline level, tumor size shrank by >30% in 15 patients. According to the Response Evaluation Criteria In Solid Tumors (RECIST), seven patients had confirmed partial responses (PR), an additional 18 patients (28.6%) experienced stable disease (SD) of at least 8 weeks in duration. The median duration of sunitinib treatment among patients whose diseases were controlled was 22.1 weeks. Median PFS was 12.0 weeks, and median overall survival (OS) was 23.4 weeks. The most commonly reported AEs include grade 1-2 and 3-4 fatigue/asthenia (41%/27%), pain/myalgia (43%/17%), and nausea/vomiting (40%/10%). Most AEs were mild to moderate in severity and could be well tolerated. The hematologic toxicities included grade 3 and 4 neutropenia (5%) and thrombocytopenia (5%). For sunitinib, another important issue is the dosing schedule. Therefore, a concurrent phase II trial applied the continuous dosing with 37.5 mg of sunitinib among the same population, and the results are shown in Table 2.

Full table
Some phase III trials on sunitinib have been initiated based on the results of phase II studies. In 2010, European Society for Medical Oncology (ESMO) formerly released the final results of its randomized double-blind phase III trial (SUN1087). SUN1087 were mainly conducted among NSCLC patients who had failed first-line chemotherapy and sought second-line treatment; by comparing the efficacy of sunitinib plus erlotinib vs sunitinib monotherapy, the study tried to alter the second-line treatment mode for NSCLC through the combined application of targeted drugs. For the general populations, once the first- or second-line platinum-based chemotherapy fails, compared with the sunitinib monotherapy, a combination therapy with sunitinib and erlotinib could significantly prolong the PFS (3.6 months vs. 2.0 months, P=0.0023) and increased the objective response rate (P=0.0471) (14). However, due to factors such as subsequent treatment, the OS showed no significant difference (9.0 months vs. 8.5 months). From the perspective of safety, the adverse effects of the combination therapy were predictable, controllable, and manageable.
Notably, the 2011 World Conference on Lung Cancer released the results of subset analysis of East Asian patients participating in a phase III trial (15). Of 103 Asian patients, sunitinib plus erlotinib remarkably improved the OS of the advanced NSCLC patients: the median OS was unreached (95%CI: 13.4 months-unreached) in the combination therapy group, which was significantly higher than that in sunitinib monotherapy group (9.4 months; 95%CI: 7.5-15.4 months); the hazard ratio (HR) reached 0.532 (P=0.0101), and the risk of death decreased by 46.8%. The median PFS in the combination therapy group was 31.2 weeks, versus 15.2 weeks in the monotherapy group (P=0.0889). Furthermore, the objective response rate (ORR) in the combination group was almost three times of that in the monotherapy group (38.5% vs. 13.7%). This study had some limitations: it was conducted at the early stage and the drugs were applied as the second-and third-line therapies; meanwhile, it lacked data on the mutations of EGFR gene. Nevertheless, to identify the optimal population through the subgroup analysis in large-scale trials has gradually become a new research direction of targeted drugs. The efficacy of sunitinib in Asian populations deserves further investigation.
Vandetanib (Zactima, ZD6474)
Slightly different from sunitinib, the chemical structure of vandetanib contains anilinoquinazoline, which is currently the most active and selective inhibitor of tyrosine kinase. The main targets of vandetanib include EGFR, VEGFR, and RET. Theoretically, the concurrent inhibition of EGFR and VEGFR, two most important signal pathways for NSCLC, can achieve better clinical efficacy. Interestingly, an earlier phase II trial seemed to prove this theory: in Natale et al's study, the efficacy and safety of vandetanib was compared with that of gefitinib as the second-line treatment for NSCLC (16). The median PFS was 11.0 weeks for vandetanib and 8.1 weeks for gefitinib (HR=0.69, P=0.025). OS was not significantly different between patients initially randomized to either vandetanib or gefitinib (median 6.1 and 7.4 months, respectively).
Encouraged by the above phase II trial, three large-scale phase III trial on the efficacy of vandetanib on NSCLC were concurrently conducted. However, the released results from these trials were striking. The ZODIAC study (17) enrolled 1400 advanced NSCLC patients to compare the efficacy of vandetanib plus docetaxel with that of docetaxel monotherapy in the second-line treatment of the disease. Although vandetanib prolonged the PFS (median: 4.0 vs. 3.2 months; HR=0.79, P<0.001), OS was not significantly improved (HR=0.91). Furthermore, the results from one study on vandetanib plus pemetrexed (ZEAL) (18) and one study on vandetanib plus erlotinib (19) were even more disappointing: compared with pemetrexed monotherapy or erlotinib monotherapy, either PFS or OS showed no statistically significant improvement although ORR increased to a certain degree. Equally regretfully, the above phase III trials did not carry out stratified analysis on the EGFR gene status and therefore were not able to further identify the potential populations that may benefit from vandetanib. With an IC50 of 500 nmol/L against EGFR (20), vandetanib has a low activity in inhibiting EGFR signal pathway, which may also explain why vandetanib failed in the above trials.
Sorafenib (Nexevar, BAY439006)
Sorafenib has highly active aryl urea structure, which has shown high inhibitory effect on RAS/RAF kinase (21). In fact, sorafenib was initially regarded as a serine/threonine kinase inhibitor; further research has found that it can also inhibit the tyrosine kinase in the VEGFR, PDGFRβ, and c-Kit signal pathways.
Since bevacizumab had achieved some success in the first-line treatment of NSCLC, two phase III trials were conducted: (I) ESCAPE: sorafenib plus taxol/carboplatin; and (II) NEXUS: sorafenib plus gemcitabine/cisplatin (22,23). Regretfully, similar to trials on vandetanib, the results of these two trials were unsatisfactory. Furthermore, the mortality of squamous cell carcinoma was remarkably higher in the sorafenib-based combination therapy than in the single chemotherapy group.
In 2010, ESMO released the final results of NEXUS: after 132 patients with squamous cell carcinoma were ruled out, sorafenib combined with gemcitabine/cisplatin still did not improve the patients' ORR and OS (the primary endpoints), which were 28% vs. 26% (P=0.27) and 379 vs. 376 d, respectively. Enrolment stopped in an ongoing MISSION trial (24), which applied sorfenib as the third-and fourth-line therapy for NSCL with an attempt to explore the role of multi-targeted drugs in the third-and fourth-line treatment of NSCLC. Its final results may be interesting.
Other multi-targeted TKIs
Axitinib (AG013736), with VEGFR, PDGFRβ, and c-KIT as its main targets, is currently the most potent TKI in inhibiting VEGFR signal pathways (25). Its good anti-tumor activity has been demonstrated in many solid tumors. According to the results of a phase III trial released by ASCO this year, axitinib has become a new standard for the second-line treatment of renal cancer (26). In a phase II study examining the efficacy and safety of axitinib monotherapy in patients with advanced NSCLC, three (3/32, 9.4%) responses were reported, with PFS and OS of 4.9 and 14.8 months, receptively, showing promising anti-tumor activity and safety among NSCLC patients (27,28). Currently, three ongoing phase II studies are exploring the effectiveness and safety of axibinib-based combination therapies (i.e. AGILE1030: axibinib combined with taxol/carboplatin; AGILE1039: axibinib combined with pemetrexed/cisplatin; and AGILE1038: axibinib combined with gemcitabine/cisplatin) in treating non-squamous NSCLC.
Motesanib mainly inhibits targets including VEGFR, PDGFR, KIT, and RET. An early phase Ib trial showed that motesanib plus carboplatin/paclitaxel and/or panitumumab showed good safety for advanced NSCLC. Three dose-limiting toxicities occurred among the 55 patients who had received the combined therapy: grade 4 pulmonary embolism (n=1; 50 mg once daily) and grade 3 deep vein thrombosis (n=2; 125 mg once daily). The other adverse effects included fatigue, diarrhea, hypertension, and anorexia. In an ongoing phase III trial, the incidence of hemoptysis increased among squamous NSCLC patients who had been treated with motesanib combined with taxol/carboplatin; as a result, only non-squamous NSCLC patients were included in that study, and the final results require further verification.
Multi-targeted TKIs for NSCLC: Where is the way?
Although multi-targeted TKIs have made certain advances in treating NSCLC, the outcomes remain unsatisfactory if they were applied non-selectively among NSCLC patients. As shown in Table 3, among the non-selective populations, TKI monotherapies showed no significant differences when compared with mono-targeted agent monotherapies in treating NSCLC in terms of ORR, PFS, and OS. Therefore, it is extremely important to identify populations that are suitable for TKIs.
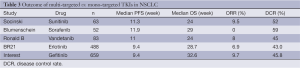
Full table
First, although lung cancer has some tumor-driven genes, only EGFR mutations and EML4-ALK fusion gene are clinically informative (29), and the role of many other tumor driver genes remain unclear. In patients whose disease is resistant to EGFR-TKIs, particularly, the core oncogenic signal pathways that drive cancer have changed and the tumor is highly dependent on the overlapping signal pathways; as a result, the oncogenic signals exert their oncogenic effects through multiple pathways. Therefore, when the mechanisms governing drug resistance remain unclear, TKIs have certain advantages when applied as salvage treatment after EGFR-TKIs fail. It seems a shortcut for improving the treatment effectiveness by maximizing tumor signal pathway inhibition in the premise of ensuring safety.
The results of the identification of driver mutations in lung cancer specimens, as reported in ASCO and World Conference on Lung Cancer (WCLC) in 2011, also explains the "real world" demand for this choice. The cancer driver genes could not be identified among 46% of adenocarcinoma patients and 37% of squamous cancer patients, even after high-throughput screening technology was applied. For these patients, TKIs may play a more active role. Second, compared with the single-targeted agents, TKIs usually can inhibit more signal pathways; when the key inhibitory signal pathways have not been identified, it is difficult to decide the targets both practically and theoretically. The targets for anti-angiogenic therapy for NSCLC remain controversial; on the contrary, TKIs usually have dual anti-angiogenic and anti-proliferative effects. Among NSCLC patients showed partial response to the therapy, the dual mode of inhibition often exists; as a result, a hybrid forecasting model with multiple (rather than single) factors can be produced. This issue has to be faced when exploring for the optimal populations of a specific multi-targeted drug. In addition, according to the basic theories of the anti-tumor effects of multi-targeted drugs, for multiple targeted signal pathways, a single drug can not achieve the optimal inhibitory concentrations for all the targets. In other words, when the blood drug levels of a certain drug differ, its inhibitory effects on different targets can also be varied. Such effect also interferes with the homogeneity of the efficacy of a multi-targeted drug among NSCLC patients.
In this regards, the results of SUN1058 trial, which was released in the 2010 annual meeting of American Association for Cancer Research (AACR), may bring some inspiration (30-32). The SUN1058 trial conducted a biomarker analysis in a phase II trial that compared the efficacy of sunitinib plus erlotinib versus erlotinib monotherapy for the second-and third-line treatment of NSCLC. This study basically covered the expressions of all the sunitinib-related targets as well as the mutations of EGFR and KRAS. In this study, the efficacy of sunitinib showed no significant association with the mutations of EGFR and KRAS. In terms of histological indicators, patients with low PDGFRαmRNA expression showed superior response to the treatment than those with high PDGFRαmRNA expression (HR=0.386, P<0.05). Their study also investigated the role of serum protein molecular markers, and for the first time found the correlation between the changes of sVEGFR-3, VEGFC, and sVEGFR-2 and the efficacy of sunitinib. Actually, it is speculated from the SUN1087 trial that only large-scale target screening might make the multi-targeted drugs become more promising for NSCLC.
Conclusions
The efficacy of multi-targeted drugs for NSCLC has not well recognized. For EGFR-TKIs-resistent patients with unknown driving mechanism, the concept of "cancer driver gene" may help to increase the efficacy of multi-targeted drugs for NSCLC (33-35). In other words, individualized treatment based on molecular markers has become a new direction in the treatment of NSCLC. How to choose the right drugs for the right patients and thus achieve the optimal balance between efficacy and adverse effects has become a new research focus in the field of oncology. The future of multi-targeted drugs is highly depended on the capability of delivering these molecule-targeted therapeutics to patients most likely to benefit.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Efficacy results from the randomised phase III OPTIMAL(CTONG 0802) study comparing first-line er-lotinib versus carboplatin(CBDCA) plus gemcitabine(GEM), in Chi-nese advanced non-small-cell lung cancer (NSCLC) patients (PTS) with EGFR activating mutations. 35th European Society for Medical Oncology, LBA13.
- Gridelli C, Maione P, Del Gaizo F, et al. Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer. Oncologist 2007;12:191-200. [PubMed]
- Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327-37. [PubMed]
- Herbst RS. Imaging in drug development. Clin Adv Hematol Oncol 2004;2:268-9. [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [PubMed]
- Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol 2007;25:884-96. [PubMed]
- Kim DW, Jo YS, Jung HS, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab 2006;91:4070-6. [PubMed]
- Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst 2006;98:326-34. [PubMed]
- Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 2002;62:7284-90. [PubMed]
- Osusky KL, Hallahan DE, Fu A, et al. The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis 2004;7:225-33. [PubMed]
- Potapova O, Laird AD, Nannini MA, et al. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther 2006;5:1280-9. [PubMed]
- Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol 2008;26:650-6. [PubMed]
- Govidan V, Krzakowski M, Szczesna A, et al. Sunitinib in combina- tion with erlotinib for the treatment of advanced/metastatic non- small cell lung cancer (NSCLC): a phase III study. 35th ESMO, LBPL2.
- Thongprasert S, Tung Y, Kim JH, et al. Sunitinib plus erlotinib for the treatment of advanced NSCLC: subset analysis of East Asian patients participating in a phase III trial. 14th WCLC, MO09.02.
- Natale RB, Bodkin D, Govindan R, et al. ZD6474 versus gefitinib in patients with advanced NSCLC: final results from a two-part, double-blind, randomized phase II trial J Clin Oncol 2006;24:S7000.
- Herbst RS, Sun Y, Korfee S, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (NSCLC): a randomized, double-blind phase III trial [Abstract]. J Clin Oncol 2009;27:s abstr CRA8003.
- Vansteenkiste J, Arrieta O, Gottfried M, et al. Vandetanib plus peme-trexed vs pemetrexed as 2nd-line therapy in patients with advanced non-small-cell lung cancer (NSCLC): a randomized, double-blind phase III trial [Abstract]. Program and abstracts of the Joint 15th Congress of the European CanCer Organisation (ECCO) and 34th Congress of the European Society for Medical Oncology (ESMO), 2009;O-9004.
- Goss GD, Thongprasert S, Greco FA, et al. Vandetanib versus erlo- tinib in patients with previously treated advanced non-small-cell lung cancer (NSCLC): a randomized, double-blind phase III trial [Abstract]. Program and abstracts of the Joint 15th Congress of the European CanCer Organisation (ECCO) and 34th Congress of the European Society for Medical Oncology (ESMO), 2009;O-9005.
- Ryan AJ, Wedge SR. ZD6474--a novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br J Cancer 2005;92:S6-13. [PubMed]
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006;5:835-44. [PubMed]
- Scagliotti G, von Pawel J, Reck M, Cupit L, Cihon F, DiMatteo S, O'Leary J, Hanna N. Sorafenib plus carboplatin/paclitaxel in chemonaive patients with stage IIIB-IV non-small cell lung cancer (NSCLC): interim analysis (IA) results from the phase III, randomized, double-blind, placebo-controlled, ESCAPE (Evaluation of Sorafenib, Carboplatin, and Paclitaxel Efficacy in NSCLC) trial. J Thorac Oncol 2008;3:S97-S98.
- Gatzemeier U, Eisen T, Santoro A, et al. Sorafenib (S)+Gemcitabine/ Cisplatin (GC) vs GC alone in the first-line treatment of advanced non-small cell lung cancer (NSCLC): phase III NSCLC research ex- perience utilizing sorafenib (NEXUS) trial. 35th EMSO, LBA16.
- Bayer. A 3rd/4th line placebo-controlled trial of sorafenib in pa- tients with predominantly non squamous non-small cell lung cancer NSCLC (MISSION). NCT00863746.
- Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 2008;14:7272-83. [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC): Results of phase III AXIS trial J Clin Oncol 2011;S4503.
- Schiller JH, Larson T, Ou SH, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol 2009;27:3836-41. [PubMed]
- Blumenschein GR Jr, Reckamp K, Stephenson GJ, et al. Phase 1b study of motesanib, an oral angiogenesis inhibitor, in combination with carboplatin/paclitaxel and/or panitumumab for the treatment of advanced non-small cell lung cancer. Clin Cancer Res 2010;16:279-90. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adeno- carcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC). ASCO 2011: CRA7506.
- Sivachenko A, Hammerman P, Pho N, et al. Genomic characteriza- tion and targeted therapeutics in squamous cell lung cancer. WCLC 2011: PRS1.
- Groen HJ, Harmon CS, Williams JA, et al. Biomarker associations with survival for refractory NSCLC patients receiving erlotinib± sunitinib in a randomized phase 2 Trial. AACR 2010: OA No19.
- Novello S, Scagliotti GV, Rosell R, et al. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer 2009;101:1543-8. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [PubMed]


