The impact of epidermal growth factor receptor mutations on the prognosis of resected non-small cell lung cancer: a meta-analysis of literatures
Introduction
Primary lung cancer has become the leading cause of cancer-related death in both male and female populations in most part of the world (1). Non-small cell lung cancer (NSCLC) which predominantly consists of adenocarcinoma, squamous cell carcinoma and large cell carcinoma, accounts for 80–85% of all lung cancer cases (2). Complete surgical resection is the gold standard treatment of early-stage NSCLC. However, even patients with completely resected stage IA NSCLC have a 5-year mortality rate of approximately 30% (3). A meta-analysis which was composed of 52 randomized clinical trials and included 9,387 cancer patients concluded that adjuvant chemotherapy only reduced 13% of the risk mortality in the first year and suggested a considerable number of patients do not benefit from adjuvant chemotherapy (4). It is important to identify high-risk patients for poor outcome with adjuvant chemotherapy and find other adjuvant therapies that can improve survival in this population. Previous studies regarding the prognostic factor of lung cancer focus on the clinical features and gene expression (5-7). In recent years, the discovery of oncogenic driver mutations have led to the emergence of a new therapeutic strategy in lung cancer. Furthermore, different oncogenic driver mutations have exhibited a tendency to correlate with different biological behaviors which suggests that some clinical findings need to be reconsidered. Epidermal growth factor receptor (EGFR) is a crucial mutation that the frequency of which ranges from 15% to 44% in East Asian patients with adenosquamous lung carcinoma.
EGFR, a 170-kDa receptor tyrosine kinase (TK), plays a critical role in promoting cell division, migration, angiogenesis and inhibits apoptosis (8). The exon 19 deletion and exon 21 L858R mutations are the most common EGFR mutations which account for 85–90% of all EGFR mutation cases (9,10). EGFR mutation is associated with advantageous clinical outcomes in patients with advanced NSCLC, predominantly as a result of its favorable response to EGFR-tyrosine kinase inhibitors (11,12). In addition to their important role in the planning of treatment strategies for advanced or recurrent NSCLC, EGFR mutations have intrinsic impact on the prognosis (13). However, its impact on the prognosis of resectable NSCLC after complete surgery remains controversial. To elucidate the prognostic value of EGFR mutation status, we attempted to conduct a meta-analysis of all available evidence to assess the correlation between EGFR mutations and prognosis in surgically resected lung cancer.
Methods
Literature search
Relevant studies were retrieved by searching PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library, using the following terms: “EGFR mutation” AND “disease-free survival” AND Resected OR resectable OR Prognosis. The last research time was May 6, 2017. Language was restricted to Chinese and English. In addition, a manual search through reference lists of relevant reviews and included studies were conducted. The search was carried out independently by two authors.
Inclusion criteria and Exclusion criteria
The following criteria was used to select publications: (I) studies assessed the correlation between disease-free survival (DFS) and EGFR status in resectable NSCLC after complete surgery; (II) all patients had never been treated with EGFR-TKI; (III) the available tumor tissue samples instead of circulating free DNA in serum were used to analyze the EGFR mutations; (IV) studies needs to be in English or Chinese in spite of publication time. Studies that fail to meet all above criteria were excluded from analyses.
Data collection
The primary outcome of our study was progression-free survival (PFS). Publication characteristics details such as first author’s name, publication year, tumor type, sample size, EGFR status, stage, evaluation method of EGFR mutation status was extracted by two independent investigators. Any disagreement was discussed amongst investigators to reach consensus. Only univariate analysis results were used when both univariate and multivariate analysis results were supplied in a study as the most of included studies were univariate analysis. We used the data directly when the included studies provided precise HR (95% CI). In the case of the studies only provided Kaplan-Meier survival curves, Engage Digitizer version 2.11 software was used to extract relevant numerical value from survival curves and calculate the HR (95% CI) (14,15).
Statistical analysis
The heterogeneity of the individual HR was calculated using Cochran’s Q-statistic test and I2 test. I2<25% was considered as no heterogeneity, I2=25–50% and I2>50% were considered as moderate heterogeneity and strong heterogeneity respectively (16,17). P value less than 0.05 was considered to be statistical significance. In order to avoid any potential heterogeneity, a random-effects model was used in this meta-analysis. When available, subgroup and sensitivity analysis were stratified for predisposed factors. To assess the strength of the findings, sensitivity analyses were conducted by excluding one study at a time. Egger’s test was used to investigate publication bias. All statistical analyses were performed using STATA 11.0 software.
Results
Eligible studies
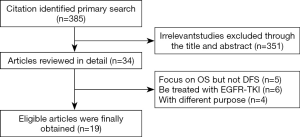
We identified 385 potentially relevant records through the search strategy. And 351 studies were excluded after checking the title and abstract, for it was very clear that their research contents didn’t meet our inclusion criteria. Then the full texts of 34 articles were carefully screened, and a total of 19 studies (18-36) were eligible for the final analysis. Figure 1 summarized the flow chart.
Our meta-analysis was composed of 19 studies to include a total of 4,872 cancer patients with no history of EGFR-TKI as adjuvant or neoadjuvant therapy. The period of included studies ranged from 2007 to 2016. The DFS between EGFR mutated and wild-type patients were compared in 18 studies (18-35); 7 of them reported specific data on stage I patients. DFS of patients with 19 exon deletion (19del) and 21 exon L858R mutation (L858R) were compared in 4 studies. Table 1 summarized the characteristics of all involved studies.
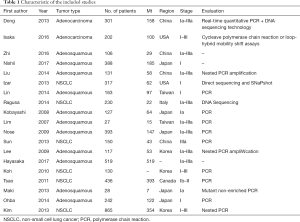
Full table
Meta-analysis
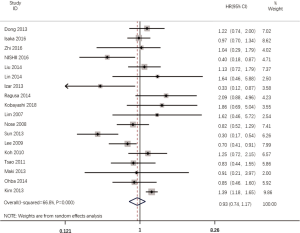
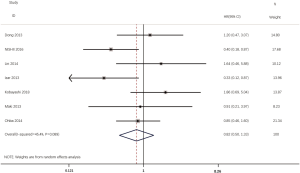
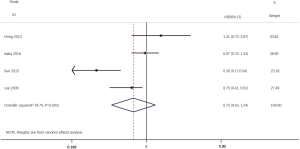
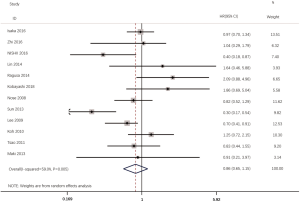
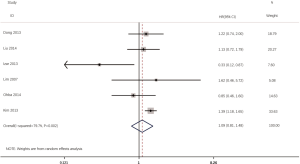
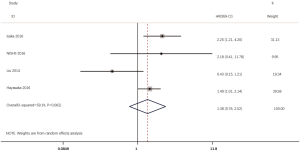
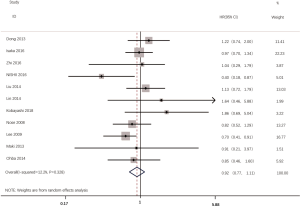
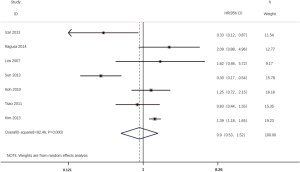
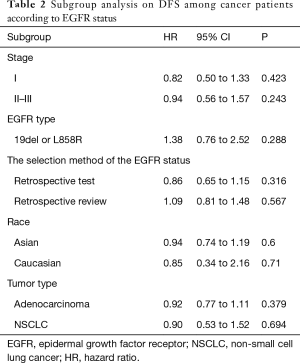
According to all literatures with available data, 18 studies consisting of 4,353 patients reported the results on the DFS between EGFR mutated and wild-type patients. As shown in Figure 2, the DFS of EGFR-mutated patients were similar to wild type patients in overall population (HR 0.93, 95% CI: 0.74 to 1.17; heterogeneity, P=0.000, I2=66.8%). We conducted subgroup analysis based on information provided by 7 studies (18,21-23,26,33,34). We found that EGFR status had no significant effect on DFS in stage I patients, with the HR of 0.82 (95% CI: 0.50 to 1.33), and obvious heterogeneity existed among them (I2=45.4%, P=0.089; Figure 3). There was also no significant difference between patients with EGFR mutation and patients with wide type on DFS in stage II–III patients (HR 0.73, 95% CI: 0.43 to 1.24; P=0.243; heterogeneity, P=0.002, I2=79.7%; Figure 4). To avoid the selection bias of the EGFR status, we performed retrospective test subgroup and retrospective review subgroup. And no significant difference was observed in retrospective test subgroup (HR 0.86, 95% CI: 0.65 to 1.15; P=0.316; heterogeneity, P=0.005, I2=59%; Figure 5) and retrospective review subgroup (HR 1.09, 95% CI: 0.81 to 1.48; P=0.568; heterogeneity, P=0.002, I2=79.7%; Figure 6). Additionally, we pooled the results of 19del patients and L858R patients. Four studies composed of 1,471 patients reported this data (19,21,22,36). However, no differences between 19del and L858R groups were observed (HR 1.38, 95% CI: 0.76 to 2.52; heterogeneity: P=0.062, I2=59.1%; Figure 7). Additional subgroup analysis was performed since significant heterogeneity was observed in the overall analysis. After stratification by tumor type, no significant difference was observed in adenocarcinoma studies (HR 0.92, 95% CI: 0.77 to 1.11; heterogeneity, P=0.328, I2=12.2%; Figure 8) and NSCLC studies (HR 0.90, 95% CI: 0.53 to 1.52; heterogeneity, P=0.000, I2=82.4%; Figure 9). Table 2 summarized the results of all subgroups.
Full table
Publication bias
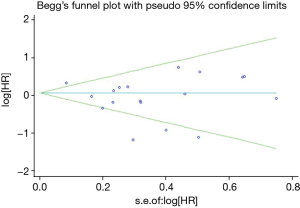
The funnel plot and Egger’s test were performed for the overall comparison. No obvious visual asymmetry was observed in funnel plots (Figure 10) for DFS, and the P values of the Egger’s test were greater than 0.05.
Discussion
Due to the predictive value to EGFR-TKI, EGFR mutation plays a crucial role in precision medicine. Except for its contribution to predict the response to EGFR-TKI, EGFR mutations are anticipated to have inherent prognostic implication. A prior research indicated that EGFR may be a positive prognostic factor for survival in patients with advanced NSCLC (35). In addition, the effect of EGFR mutations on the prognosis of resected NSCLC has been reported by previous studies, but the results were disputed. A meta-analysis is required to integrate all available results to provide further insight on this controversial issue. Combined with the available data provided by the included studies, our results supported the assumption that EGFR mutations have no prognostic value in complete resected NSCLC, but significant heterogeneity was observed.
A previous study has suggested that the status of EGFR mutations was associated with sensitivity to chemotherapy (37), however, it is difficult to determine the prognostic value of EGFR mutations when patients have received adjuvant treatment. Thus, we carried out subgroup analyses in patients with stage I NSLCL who rarely receive adjuvant therapy. Interestingly, our results show that there is no significant different in DFS between the EGFR-mutated patients and those with wild type in stage I subgroup (HR 0.82, 95% CI: 0.5 to 1.33), which is consistent with the overall result. This result implies that EGFR mutation may not an independent prognostic factor for DFS.
Our study found no significant difference in DFS between patients with mutant-type EGFR and those with wild-type which is consistent with a previous meta-analysis (38). It suggested that EGFR mutations may merely play a detrimental role in advanced stage and may not have much impact on early stages of NSCLC. However, the population of this study is mixed with significant heterogeneity (P<0.01, I2=66.8%). After carried out a subgroup analyses by tumor type, we suspect that it was the tumor type resulted in the heterogeneity.
Several studies have showed that patients with advanced NSCLC harboring Ex19 mutations have better OS than those with Ex21 mutations treated with EGFR-TKI (39-41). Moreover, a prior study showed advanced NSCLC patients harboring Ex19 mutations have shown better responses to chemotherapy than those with Ex21 deletions (42). Conversely, a recently published study from Tetsuya Isaka (43) reported that Ex21 adenocarcinoma were low grade with a lepidic growth pattern, whereas wild-type tumors were high grade and contained solid and papillary components with vascular invasion; Ex19 tumors were intermediate grade (44). This finding is consistent with the findings of Yang et al. (44) which showed that Ex21 adenocarcinomas had a higher ratio of ground-glass opacity than Ex19 tumors (43). In the present study, patients with 19del potentially had inferior DFS to those with L858R but the result did not reach statistical significance (HR 1.38, 95% CI: 0.76 to 2.52). As stated above, we surmise that the result may be confused by chemotherapy which is a subsequent treatment after surgery for patients with stage II–III disease.
Our study gives further evidence to support previous assumptions that EGFR mutations had no impact on the prognosis of resected NSCLC. However, there are several limitations. First, this is a retrospective analysis, prospective analysis is needed to further illustrate these issues. Second, since follow-up time of each study was not the same, significant heterogeneity was observed. In addition, we can’t avoid the influence of the adjuvant chemotherapy or postoperative radiotherapy based on the original reports. Further studies are necessary.
Conclusions
In summary, our results demonstrated that EGFR mutations showed no prognostic value in primary resected NSCLC. When deciding treatment strategy for postoperative (especially stage I) patients, there is no evidence support difference between mutated and wild-type patients. However, 19 del might be a negative factor through indirect reason, which may require more strict management. We strongly encourage reporting the specific prognostic impacts of different mutation types.
Acknowledgements
We want to give sincere thanks to all authors and patients of our included studies. We cannot complete this work without your work and participation.
Funding: This study was funded by the grant 2016YFC0905403 and 2016YFC0905403 from the National Key R&D Program of China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Mountain CF. Revisions in the International system for staging lung cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized clinical trials. Non-small cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [Crossref] [PubMed]
- Kuo SW, Chen JS, Huang PM, et al. Prognostic significance of histologic differentiation, carcinoembryonic antigen value, and lymphovascular invasion in stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;148:1200-7.e3. [Crossref] [PubMed]
- Sun Z, Aubry MC, Deschamps C, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg 2006;131:1014-20. [Crossref] [PubMed]
- Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Gazdar AF, Shigematsu H, Herz J, et al. Mutations and addiction to EGFR: the Achilles ‘heal’ of lung cancers? Trends Mol Med 2004;10:481-6. [Crossref] [PubMed]
- Pao W, Miller VA. Epidermal growth factor receptor mutations, small molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 2005;23:2556-68. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Ou SH, Zell JA, Ziogas A, et al. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: apopulation-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007;110:1532-41. [Crossref] [PubMed]
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to event data in meta-analysis. Trials 2007;8:article16.
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring in consistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Geller N, Freedman L, Lee YJ, et al. Conference on meta-analysis in the design and monitoring of clinical trials. Stat Med 1999;18:753-54. [PubMed]
- Dong Y, Li Y, Peng H, et al. Predictive role of EGFR mutation status on postoperative prognosis in patients with resected lung adenocarcinomas. Zhongguo Fei Ai Za Zhi 2013;16:177-83. [PubMed]
- Isaka T, Nakayama H, Yokose T, et al. Epidermal Growth Factor Receptor Mutations and Prognosis in Pathologic N1-N2 Pulmonary Adenocarcinoma. Ann Thorac Surg 2016;102:1821-8. [Crossref] [PubMed]
- Zhi Q, Wang Y, Wang X, et al. Predictive and prognostic value of preoperative serum tumor markers in resectable adenosqamous lungcarcinoma. Oncotarget 2016;7:64798-809. [Crossref] [PubMed]
- Nishii T, Yokose T, Miyagi Y, et al. Prognostic value of EGFR mutations in surgically resected pathological stage I lung adenocarcinoma. Asia Pac J Clin Oncol 2017;13:e204-11. [Crossref] [PubMed]
- Liu WS, Zhao LJ, Pang QS, et al. Prognostic value of epidermal growth factor receptor mutations in resected lung adenocarcinomas. Med Oncol 2014;31:771. [Crossref] [PubMed]
- Izar B, Sequist L, Lee M, et al. The impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancers. Ann Thorac Surg 2013;96:962-8. [Crossref] [PubMed]
- Lin MW, Wu CT, Shih JY, et al. Clinicopathologic characteristics and prognostic significance of EGFR and p53 mutations in surgically resected lung adenocarcinomas ≤2 cm in maximal dimension. J Surg Oncol 2014;110:99-106. [Crossref] [PubMed]
- Ragusa M, Vannucci J, Ludovini V, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcome in resected non-small cell lung cancer patients. Am J Clin Oncol 2014;37:343-9. [Crossref] [PubMed]
- Kobayashi N, Toyooka S, Ichimura K, et al. Non-BAC component but not epidermal growth factor receptor gene mutation is associated with poor outcomes in small adenocarcinoma of the lung. J Thorac Oncol 2008;3:704-10. [Crossref] [PubMed]
- Lim KH, Huang MJ, Liu HC, et al. Lack of prognostic value of EGFR mutations in primary resected non-small cell lung cancer. Med Oncol 2007;24:388-93. [Crossref] [PubMed]
- Nose N, Sugio K, Oyama T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol 2009;27:411-7. [Crossref] [PubMed]
- Sun HB, Ou W, Li Y, et al. Epidermal growth factor receptor mutation status and adjuvant chemotherapy in resected advanced non-small-cell lung cancer. Clin Lung Cancer 2013;14:376-82. [Crossref] [PubMed]
- Lee YJ, Park IK, Park MS, et al. Activating mutations within the EGFR kinase domain: a molecular predictor of disease-free survival in resected pulmonary adenocarcinoma. J Cancer Res Clin Oncol 2009;135:1647-54. [Crossref] [PubMed]
- Koh Y, Jang B, Han SW, et al. Expression of class III beta-tubulin correlates with unfavorable survival outcome in patients with resected non-small cell lung cancer. J Thorac Oncol 2010;5:320-5. [Crossref] [PubMed]
- Tsao MS, Sakurada A, Ding K, et al. Prognostic and predictive value of epidermal growth factor receptor tyrosine kinase domain mutation status and gene copy number for adjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol 2011;6:139-47. [Crossref] [PubMed]
- Maki Y, Soh J, Ichimura K, et al. Impact of GLUT1 and Ki-67 expression on early stage lung adenocarcinoma diagnosed according to a new international multidisciplinary classification. Oncol Rep 2013;29:133-40. [Crossref] [PubMed]
- Ohba T, Toyokawa G, Kometani T, et al. Mutations of the EGFR and K-ras genes in resected stage I lung adenocarcinoma and their clinical significance. Surg Today 2014;44:478-86. [Crossref] [PubMed]
- Kim YT, Seong YW, Jung YJ, et al. The presence of mutations in epidermal growth factor receptor gene is not a prognostic factor for long-term outcome after surgical resection of non-small-cell lung cancer. J Thorac Oncol 2013;8:171-8. [Crossref] [PubMed]
- Hayasaka K, Shiono S, Matsumura Y, et al. Difference of Postoperative Survival Due to the Type of EGFR Gene Mutation in Surgically Resected Lung Adenocarcinomas. J Thorac Oncol 2017;12:S320-1. [Crossref]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhang Z, Wang T, Zhang J, et al. Prognostic value of epidermal growth factor receptor mutations in resected non-small cell lung cancer: a systematic review with meta-analysis. PLoS One 2014;9:e106053. [Crossref] [PubMed]
- Wang Y, Li RQ, Ai YQ, et al. Exon 19 deletion was associated with better survival outcomes in advanced lung adenocarcinoma with mutant EGFR treated with EGFR-TKIs as second line therapy after first-line chemotherapy: a retrospective analysis of 128 patients. Clin Transl Oncol 2015;17:727-36. [Crossref] [PubMed]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839-44. [Crossref] [PubMed]
- Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:3908-14. [Crossref] [PubMed]
- Fang S, Wang Z, Guo J, et al. Correlation between EGFR mutation status and response to first-line platinum-based chemotherapy in patients with advanced non-small cell lung cancer. Onco Targets Ther 2014;7:1185-93. [PubMed]
- Isaka T, Yokose T, Ito H, et al. Correlations between the EGFR mutation status and clinicopathological features of clinical stage I lung adenocarcinoma. Medicine (Baltimore) 2015;94:e1784. [Crossref] [PubMed]
- Yang Y, Yang Y, Zhou X, et al. EGFR L858R mutation is associated with lung adenocarcinoma patients with dominant ground-glass opacity. Lung Cancer 2015;87:272-7. [Crossref] [PubMed]