A phase I/II study of pemetrexed with sirolimus in advanced, previously treated non-small cell lung cancer
Introduction
Non-small cell lung cancer (NSCLC) accounts for the highest cancer mortality in the US, killing more than 150,000 people annually (1). Approximately 30–40% of NSCLC patients present with metastatic disease where only systemic therapy has an impact on survival (2). Once patients relapse after initial treatment, they have limited therapeutic options that can modestly improve survival. At the time this study was conducted, approved chemotherapies in this setting included single-agent pemetrexed and docetaxel (3,4).
Discovery of driver oncogenes such as epidermal growth factor receptor (EGFR) led to development of tyrosine kinase inhibitors, benefiting patients with activating mutations or translocations. However, the population with driver mutations for which tyrosine kinase inhibitors are available is small in Western countries (20% in Caucasians), limiting its application to a subset of patients, and development of resistance is an inevitable consequence of treatment (5). More recently, a greater understanding of tumor immunology has resulted in the identification of immune checkpoints that can be therapeutically targeted to enhance anti-tumor immune responses. Antibodies targeting the programed death-1 (PD-1) and its ligand (PD-L1) (e.g., nivolumab, pembrolizumab, and atezolizumab), have been approved for treatment of metastatic NSCLC (6). Although durable benefit is observed in patients responding to treatment, a relatively small number of patients develop an objective response to immune checkpoint inhibition therapy and escape mechanisms ultimately result in development of resistance in most cases. Despite the promise and benefit of targeted therapies and immunotherapies in selected NSCLC populations, newer forms of treatment are needed to expand the efficacy of standard agents such as pemetrexed in patients with advanced NSCLC.
Our group has previously demonstrated that the Akt-mammalian target of rapamycin (mTOR) pathway is frequently activated in NSCLC, and its activation is associated with a worse clinical outcome (7,8). Several agents related to rapamycin targeting mTOR signaling are clinically available. Temsirolimus (Torisel®) and everolimus (Afinitor®) are approved in the first and second-line treatment of renal cell carcinoma, respectively (9,10). Sirolimus (Rapamune®) is indicated for post-transplant immunosuppression, with a well described toxicity profile (11). Preclinical studies from our group showed a synergistic anti-cancer effect between pemetrexed and sirolimus in vitro and in vivo. Sirolimus blocks pemetrexed-induced thymidylate synthase (TS) activation in vivo, which may further enhance activity of pemetrexed (12). The addition of mTOR inhibitors such as sirolimus may therefore synergistically cause anti-tumor effects.
The combination of mTOR inhibitors with pemetrexed has previously been evaluated in small phase I trials and found to be safe and tolerable (13,14). To our knowledge this is the largest trial for the phase II portion reported to date to determine the safety and clinical activity of pemetrexed in combination with an mTOR inhibitor in previously treated NSCLC (13,14).
Methods
Study design and treatment
The study was approved by the National Cancer Institute (NCI) Institutional Review Board (IRB), conducted at the NCI, and registered in a clinical trial registry (ClinicalTrials.gov Identifier: NCT00923273). All patients signed the written informed consent approved by the NCI IRB. The primary objective of the phase I portion of the study was to determine the maximum tolerated dose (MTD) of pemetrexed and sirolimus in combination in patients with NSCLC. The secondary objectives were to analyze the pharmacokinetics (PK) of both agents, and mTOR pathway inhibition in peripheral blood mononuclear cells (PBMC). A standard 3+3 dose escalation design was used for the phase I portion (15). Cohorts of 3 patients were enrolled at each dose level (Table S1), and an additional 3 patients were enrolled if one or more of the first 3 patients developed dose limiting toxicity (DLT). Doses of pemetrexed and sirolimus were based on cohort assignment. Starting doses of both pemetrexed and sirolimus were below FDA-approved doses.

Full table
The MTDs were based on the tolerability observed during the first 4 weeks of treatment, although several patients required dose reduction at later cycles. The MTDs were defined as the highest doses at which less than two out of six patients experienced DLT. The primary objective of phase II component was to determine the activity of the combination at the MTD. The objective response rate (ORR) was evaluated by study investigators using RECIST v1.0 (16).
Prior to the first dose of pemetrexed on cycle 1 day 8 all patients received a loading dose and one-week lead-in course of once daily oral sirolimus. Patients who tolerated the lead-in period then continued the same dose of daily sirolimus and received pemetrexed infusion intravenously every three weeks at the assigned doses. Each treatment cycle consisted of three weeks except cycle 1. Standard premedications including corticosteroids, folic acid and vitamin B12 were administered to all patients. After several patients in the phase II portion with a high steady-state sirolimus level developed significant toxicities, the protocol was amended. Sirolimus dose reduction was mandated if a trough sirolimus level exceeded 15 ng/mL at any subsequent cycles.
Dose reduction to the next lower dose level was also mandated if patients in either phase I or II portion developed DLT during any cycle of the treatment. Patients with tumor response of stable disease (SD) or better as defined by RECIST1.0 continued the treatment until disease progression or intolerable toxicities.
Patient eligibility
Eligible patients were aged 18 or older, and had histologically confirmed NSCLC that had relapsed after at least one standard chemotherapeutic regimen. Patients must have had measurable disease for the phase II portion of the study. Patients must also have had an expected survival time of at least 3 months, an ECOG performance status of 0–2, and adequate organ function as determined by absolute neutrophil count (ANC) ≥1,500/mL; platelets ≥100,000/mL; total bilirubin <1.5× upper limit of institutional normal (ULN); AST (SGOT) <2.5× ULN; ALT (SGPT) <2.5× ULN; serum triglycerides <2.5× ULN; serum cholesterol <300 mg/dL; estimated creatinine clearance as calculated using the MDRD equation11 must be ≥60 mL/min/1.73 m2. Tumor EGFR mutation status was not required for study eligibility because genomic analysis of the tumor was not routine practice at the time of study initiation.
Study assessment
Adverse events (AEs) were evaluated and graded by NCI Common Terminology Criteria for Adverse Events (CTCAE Version 3.0) (17), and were monitored throughout the study and for up to 28 days after the last pemetrexed or sirolimus dose. DLTs were defined as any grade 4 hematologic toxicity (except grade 4 lymphopenia or grade 4 neutropenia with duration of 7 days or less), febrile neutropenia (neutrophil count <1,000 cells/mm3 and temperature ≥38.5 °C), grade 3 or 4 hypercholesterolemia (>400 mg/dL or 10.34 mmol/L) or grade 3 or 4 hypertriglyceridemia (>5× ULN) in spite of treatment with HMG-CoA reductase inhibitors, grade 3 or 4 diarrhea that has not resolved to grade 2 within 24 hours and grade 1 within 48 hours of anti-diarrhea agents, grade 3 or 4 pneumonitis, grade 3 or 4 mucositis, grade 3 nausea or vomiting that did not resolve to grade 2 within 24 hours and grade 1 within 48 hours of anti-emetic agents.
At the dose level identified as the MTD, up to 30 subjects were required for the phase I portion of the trial (5 levels with a maximum of 6 patients per level). If dose level 5 was reached and the MTD had not been established, dose level 5 would be used as the dose for subjects enrolling on the phase II portion of the study. Phase II portion was divided into pemetrexed-naïve and pre-treated groups, and objective response rates were assessed separately. Patients enrolled in the MTD level of phase I portion were added onto phase II portion for both safety and efficacy analysis.
Tumor re-staging by CT imaging was conducted at screening, after completion of cycle 2, and every six weeks thereafter, and radiographic response was evaluated by RECIST1.0. Body PET/CT imaging was performed at baseline and completion of cycle 2. Patients who developed DLTs or completed cycle 1 were considered evaluable for AEs. Tumor responses were assessed on an intent-to-treat basis.
On cycle 1 days 7 and 8, blood samples from phase I patients were obtained as baseline and selected time points after the administration of sirolimus and were analyzed for sirolimus and pemetrexed levels. Blood samples for steady-state sirolimus level were drawn from both phase I and II patients at baseline, weekly during cycle 1 and day 1 of subsequent cycles.
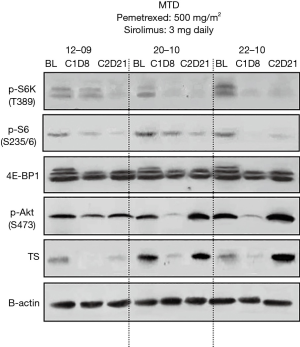
Blood samples were collected at baseline, cycle 1 day 8, cycle 2 day 21 and subsequent every 2 cycles for mTOR pathway analysis. Protein extracted from PBMC was subsequently analyzed for mTOR pathway inhibition (mTOR, P-S6K, 4E-BP1, P-S6, and Akt) by immunoblotting (12). Suppression of TS was also evaluated by western blotting.
Twenty-two patients were accrued on the phase I portion of this trial. Table 1 contains the sirolimus and pemetrexed doses for each dose level. Plasma concentrations of cycle 1 day 8 (C1D8) pemetrexed were measured using a validated HPLC-MS/MS method with a calibration range of 50–20,000 ng/mL. Sirolimus trough measurements were independently analyzed from blood on C1D8.
Full table
A noncompartmental PK analysis of C1D8 pemetrexed was performed on 20 patients using WinNonlin v5 (Pharsight Corp, Mountainview, CA, USA) with patients receiving either 375 mg/m2 (n=6) or the FDA recommended (18) dose of 500 mg/m2 (n=14). The maximum plasma concentration (CMAX) was recorded as observed values and the area under the plasma-concentration time curve extrapolated to time infinity (AUCINF) was calculated using the Linear Trapezoidal rule.
Statistics
Following a phase I cohort with up to 6 patients per dose level, patients were enrolled in two cohorts depending upon whether they were pemetrexed-naïve or had prior pemetrexed. Both cohorts employed a Simon two-stage phase II optimal design to evaluate the responses, with alpha =0.10 and beta =0.10 in each.
In the pemetrexed-naïve cohort, the objective was to rule out a 9% response rate (P0=0.09) in favor of a 34% response rate (P1=0.34). The first stage would enroll 7 patients, and if there were 0 responses, accrual would end. If 1 or more patients responded, accrual would continue until a total of 20 evaluable patients had been accrued. One to 3 responses in 20 patients would have been considered inadequate while 4 or more of 20 with a response would be desirable. The probability of early termination under the null hypothesis was 52%.
In the cohort that received prior pemetrexed, the objective was to rule out a 5% response rate (P0=0.05) in favor of a 20% response rate (P1=0.20). The first stage would enroll 12 patients, and if there were 0 responses, accrual would end. If 1 or more patients responded, accrual would continue until a total of 37 evaluable patients had been accrued. One to 3 responses in 37 patients would have been considered inadequate while 4 or more of 37 with a response would be desirable. The probability of early termination under the null hypothesis was 54%.
For PK analysis, the Wilcoxon rank sum test was used to test the difference between parameters of interest in two groups of patients. The Spearman correlation coefficient (r) was used to determine the correlation between continuous variables, and was interpreted by the following: strong association: |r|>0.7, moderate association: 0.5<|r|<0.7, moderate to weak association: 0.3<|r|<0.5, and weak association: |r|<0.3. Somers D was used to determine the correlation between ordered categorical data and can be interpreted similarly to Spearman’s correlation coefficient. The Jonckheere-Terpstra trend test was used to test for a trend between dose level and C1D8 sirolimus blood trough concentration. All P values are two-tailed and unadjusted for multiple comparisons.
Progression-free survival (PFS) was determined from the on-study date until the date of death, date of progression, or last follow-up; patients who died without progression were also considered to be failures for this analysis. The probability of PFS as a function of time was determined by the Kaplan-Meier method. Fisher’s exact test was used to determine the statistical significance of the difference between two proportions.
Results
Patient characteristics
Between November 2008 and June 2012, 42 patients were enrolled; 22 in phase I and 20 in the phase II portion. Patient characteristics are listed in Table 1. Due to a slow accrual rate, the study was prematurely terminated before the accrual ceiling was reached.
Safety
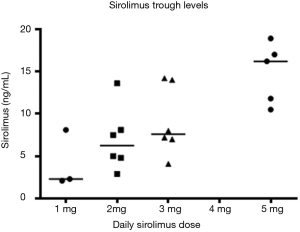
The phase I dose escalation continued up to dose level 5 (pemetrexed 500 mg/m2, sirolimus 15 mg loading/5 mg subsequent), at which one patient developed a DLT (grade 3 fatigue, Table 2), and two patients required dose reduction at cycle 2. Therefore, the decision was made to enroll 4 more patients in dose level 4 where one patient developed a DLT (grade 3 infection). Dose level 4 (pemetrexed 500 mg/m2, sirolimus 10 mg load/3 mg/day) was determined to be the MTD, and all patients enrolled in the phase II portion were initially treated at this dose level. However, four of the first seven phase II patients developed significant AEs. One patient developed grade 5 febrile neutropenia, and three patients required dose reduction of sirolimus for grade 3 or 4 nonhematologic toxicities (two patients with pulmonary toxicities, one with hyponatremia). These four patients had a steady-state sirolimus trough level higher than 15 ng/mL, whereas only one of the remaining three patients who tolerated DL4 had a high sirolimus level. Additional dose reduction of sirolimus was thereafter mandated in the later part of phase II if patients had a sirolimus trough level of 15 ng/mL or higher at any time point.
Full table
Of 27 patients enrolled at the MTD/DL4 (7 in phase I and 20 in phase II), 13 patients received at least 5 cycles of the combination treatment (Table 2: treatment exposure and dose reduction), and 20 patients completed at least first cycle (Table 2). Twelve patients at all dose levels resulted in a dose reduction at subsequent cycles (Table 2) due to AEs (n=8) or high sirolimus trough level (n=4). Nevertheless, none required further dose modification at DL3. The majority (83.3%, 35 of 42) came off study due to disease progression.
All AEs related to the study drugs (possible, probable and definite) at all dose levels/phases are listed in Table 3. The most common hematologic and nonhematologic AEs at grade 3–4 were lymphopenia (31%), neutropenia (14%), anemia (12%), and leukopenia (10%), hypophosphatemia (19%), fatigue (14%), and hyperglycemia (10%). Two patients receiving pemetrexed 500 mg/m2 and sirolimus 3 mg developed grade 5 neutropenia and infection. Both patients developed AEs a few days after the first pemetrexed infusion and had steady-state sirolimus levels higher than 15 ng/mL.
Full table
Clinical activity
A total of 27 patients were enrolled at the MTD/recommended phase II dose (RP2D) and analyzed for efficacy (Table 4, Figure 1A). Six (22.2%) patients achieved a partial response, 12 (44.4%) had SD and 5 (18.5%) had progressive disease. The overall response rate was 22.2%. We observed clinical activity in two patients not expected to respond to pemetrexed as a single-agent, such as a patient who had been treated previously with pemetrexed and progressed on it who achieved a PR as well as in a patient with squamous histology. Tumor responses were more frequent in pemetrexed naïve vs. those with prior pemetrexed [5/19 (26.3%) vs. 1/8 (12.5%); P=0.63]. This indicates that the pemetrexed-naïve group reached at least desirable response as defined in the methods section, whereas efficacy in patients with prior exposure to pemetrexed group was not adequately evaluated due to an underpowered sample size.
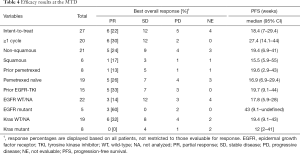
Full table
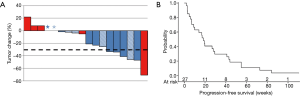
Higher response rates were seen in and non-squamous vs. squamous patients [5/21 (23.8%) vs. 1/6 (16.7%); P=1.00] and EGFR-mutated patients vs. patients with no EGFR mutations or unknown mutation status [3/5 (60%) vs. 3/22 (13.6%); P=0.056]. When another efficacy analysis for all patients (n=42) was performed, these trends were maintained (Table S2). Survival analysis showed a median PFS of 18.4 (95% CI: 7.0–29.4) weeks in patients at the MTD (Figure 1B).
Full table
PK
Twenty of the twenty-two enrolled patients on the phase I portion of this trial had evaluable data for PK analysis. One patient on dose level 1 (taken off study due to brain metastasis) and one patient on dose level 4 (taken off study due to progressive disease) were not evaluable for PK analysis.
Pemetrexed administered at the 500 mg/m2 dose level exhibited a similar exposure and half-life (t1/2) of 144.7 ug×h/mL and 2.79 h, respectively, as a previous report using the same dose, where AUCINF and t1/2 were 158 ug×h/mL and 2.62 h, respectively (19). There were no statistically significant differences in half-life (P=0.97) or clearance (P=0.72) between the two dose levels (Figure S1). However, there was a statistical trend of increasing CMAX (P=0.041, Figure S2A), but not AUCINF (P=0.31, Figure S2B).
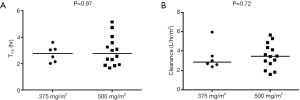
When comparing C1D8 sirolimus trough blood concentrations on each dose level, there is a clear increasing trend with dose (Jonckheere-Terpstra trend test P=0.0013) (Figure S3). To examine whether increasing sirolimus trough levels were associated with pemetrexed PK, sirolimus trough levels were plotted against pemetrexed CMAX and AUCINF (Figure S4). There were only moderate or weak correlations between sirolimus trough levels and pemetrexed CMAX or AUCINF. This suggests that increasing sirolimus levels are not associated with pemetrexed PK.
Pharmacodynamics
Assessment for mTOR in PBMC was performed in a total of 23 available patients. No inhibition on mTOR signaling was observed at DL 1 or 2 (Figure S5A). P-S6K and P-S6 were inhibited in response to sirolimus treatment in patients treated at the MTD (Figures 2 and S5B,C). Feedback activation of P-Akt, which is generally considered as a resistance mechanism to mTOR-targeted therapies such as rapamycin that only target mTORC1 complexes was not observed in the majority of patients. To determine if concurrent administration of sirolimus can influence pemetrexed-induced activation of TS at the MTD, western blotting with protein derived from PBMC was performed in a total of 18 patients who were treated at the MTD. In contrast to our in vitro and in vivo mouse study, where sirolimus blocked activation of TS in cells/tumor tissue (14), TS activation was only temporarily affected by concurrent treatment of sirolimus (Figure 2).
Discussion
In this phase I/II study we demonstrate the tolerability and clinical activity of a combination of pemetrexed with sirolimus in patients with recurrent NSCLC. Pemetrexed is approved for first line therapy of non-squamous NSCLC as part of a platinum doublet and is frequently used for treatment of recurrent non-squamous NSCLC. However, response rates remain low and the survival benefit is modest. Various attempts to combine pemetrexed with other cytotoxic drugs have not shown any benefit over pemetrexed alone (20-23). This group and others have shown that the Akt-mTOR signaling cascade is frequently activated in NSCLC cells, indicating that TORC1 inhibitors such as sirolimus could be potential therapies for NSCLC (7,8). Previously published in vitro and in vivo studies also showed enhanced anti-cancer efficacy of the combination of pemetrexed and sirolimus over either agent alone in NSCLC (12). Based on these preclinical observations, the current study was designed to evaluate the combination of pemetrexed and sirolimus in recurrent NSCLC.
The best overall response for intent-to-treat patients at pemetrexed 500 mg/m2/sirolimus 10 mg load/3 mg/day was 22% which appears to be higher than historical data from single-agent pemetrexed studies in unselected patients in the literature (4). Other regimens using pemetrexed with mTOR inhibitors yielded relatively low response rates of 0–11% (13,14). The response rate was higher in patients with EGFR mutation. Other prior studies also reported higher response rate to single-agent pemetrexed in EGFR-mutated or ALK-rearranged NSCLC (24-27). The reason for better response in theses populations is unclear.
What mechanisms might underlie the combination of pemetrexed and sirolimus? Preclinical and clinical studies indicated that squamous carcinoma has high TS expression which is one of molecular targets of the anti-folate agent pemetrexed (26). However, it has also been shown that a low level of TS expression is associated with high anti-tumor activity of pemetrexed (26-29). This suggests that the clinical responsiveness to pemetrexed in squamous NSCLC might be improved if additional agents were able to decrease TS expression. Our group has been investigating potential mechanism of action for synergistic effect of pemetrexed and sirolimus in preclinical models. Preclinical studies suggest that sirolimus blocks pemetrexed-induced TS activation in tumor tissue (12), which in turn is expected to enhance sensitivity to pemetrexed according to the multiple preclinical studies (27-29). This clinical trial intended to test this hypothesis by correlative studies. It was not feasible to analyze tumor tissue for TS activation; however, contrary to the preclinical in vitro and in vivo study, analysis in PBMC showed that an inhibitory effect of sirolimus on TS activation was observed but only temporary (Figure 2). Sustained suppression of TS level in tumor tissue might be required to further enhance anti-tumor effect of pemetrexed. Although these observations still need to be validated, inhibition of TS activation is a mechanism that may support the observation of activity with the combination in this phase I/II study.
The combination of pemetrexed and sirolimus in this study was relatively well tolerated, and appeared to have no new safety concern when compared with other combinations of pemetrexed with an mTOR inhibitor except as described below (13,14). Although grade 3 and higher neutropenia frequently occurred in other studies using pemetrexed and mTOR inhibitors (37–60%) (13,14), it was observed in 14% of participants in this study. Other hematological and nonhematological grade 3–4 events such as dyspnea (5%) were also less frequently or similarly seen in this study. As described earlier, early deaths possibly attributable to the study drugs occurred in two patients (4.8%), whereas six deaths on study were reported in another study using pemetrexed and everolimus in 43 NSCLC patients (13). The two treatment-related deaths in the first 7 patients in phase II led to the modification of the design to incorporate monitoring of trough sirolimus level. Subsequent 20 patients did tolerate well without grade 5 toxicities. Careful monitoring of myelosuppression is strongly recommended for future studies.
Conclusions
The findings from this study indicate that the combination of pemetrexed and sirolimus is feasible and active in patients with heavily pretreated, advanced NSCLC. Additional clinical trials would be required to optimize dosing schedules to minimize toxicity and maximize benefit and identify the role of this combination in the context of the evolving therapeutic landscape of NSCLC.
Acknowledgments
The authors wish to thank patients and their families and study personnel for their contributions to this study.
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health.
Footnote
Conflicts of Interest: Phillip A. Dennis is employed by Astrazeneca and owns its stocks. Marc S. Ballas is employed by GlaxoSmithKline and receives personal fees from Astrazeneca and Bristol Myers Squibb. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the National Cancer Institute (NCI) Institutional Review Board (IRB) (NCI Clinical Center protocol number: 08-C-0078; ClinicalTrials.gov Identifier: NCT00923273). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Surveillance Epidemiology and End Results. Available online: www.seer.cancer.gov. Accessed November 16, 2018.
- World Health Organization: Cancer, Key Facts. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed November 16, 2018.
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small- cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011;29:2121-7. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Brognard J, Clark AS, Ni Y, et al. Akt/protein kinase b is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 2001;61:3986-97. [PubMed]
- Tsurutani J, Fukuoka J, Tsurutani H, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol 2006;24:306-14. [Crossref] [PubMed]
- FDA Approval for Temsirolimus. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/fda-temsirolimus. Accessed November 16, 2018.
- FDA Approval for Everolimus. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/fda-everolimus. Accessed November 16, 2018.
- Rapamune® (sirolimus) Oral Solution and Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021083s034,021110s045lbl.pdf. Accessed on May 7, 2019.
- Kawabata S, Chiang CT, Tsurutani J, et al. Rapamycin downregulates thymidylate synthase and potentiates the activity of pemetrexed in non-small cell lung cancer. Oncotarget 2014;5:1062-70. [Crossref] [PubMed]
- Vansteenkiste J, Solomon B, Boyer M, et al. Everolimus in combination with pemetrexed in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a phase I study using a novel, adaptive Bayesian dose-escalation model. J Thorac Oncol 2011;6:2120-9. [Crossref] [PubMed]
- Waqar SN, Baggstrom MQ, Morgensztern D, et al. A phase I trial of temsirolimus and pemetrexed in patients with advanced non-small cell lung cancer. Chemotherapy 2016;61:144-7. [Crossref] [PubMed]
- Smith TL, Lee JJ, Kantarjian HM, et al. Design and results of phase I cancer clinical trials: three-year experience at M.D. Anderson Cancer Center. J Clin Oncol 1996;14:287-95. [Crossref] [PubMed]
- RECIST1.0. Response Evaluation Criteria in Solid Tumors (RECIST). Available online: ctep.cancer.gov/protocolDevelopment/docs/quickrcst.doc
- Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed on May 7, 2019.
- ALIMTA® Pemetrexed for Injection, Prescribing Information. Eli Lilly and Company. Available online: http://pi.lilly.com/us/alimta-pi.pdf
- Nakagawa K, Kudoh S, Matsui K, et al. A phase I study of pemetrexed (LY231514) supplemented with folate and vitamin B12 in Japanese patients with solid tumours. Br J Cancer 2006;95:677-82. [Crossref] [PubMed]
- Ardizzoni A, Tiseo M, Boni L, et al. Pemetrexed versus pemetrexed and carboplatin as second-line chemotherapy in advanced non-small-cell lung cancer: results of the GOIRC 02-2006 randomized phase II study and pooled analysis with the NVALT7 trial. J Clin Oncol 2012;30:4501-7. [Crossref] [PubMed]
- Scagliotti GV, Germonpréb P, Bosquéec L, et al. A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer 2010;68:420-6. [Crossref] [PubMed]
- de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non–small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 2011;29:1067-74. [Crossref] [PubMed]
- Chiappori A, Bepler G, Barlesi F, et al. Phase II, double-blinded, randomized study of enzastaurin plus pemetrexed as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 2010;5:369-75. [Crossref] [PubMed]
- Wu SG, Yang CH, Yu CJ, et al. Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer 2011;72:333-9. [Crossref] [PubMed]
- Lee JO, Kim TM, Lee SH, et al. Anaplastic lymphoma kinase translocation. A predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol 2011;6:1474-80. [Crossref] [PubMed]
- Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006;107:1589-96. [Crossref] [PubMed]
- Sigmond J, Backus HH, Wouters D, et al. Induction of resistance to the multi-targeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol 2003;66:431-8. [Crossref] [PubMed]
- Hanauske AR, Eismann U, Oberschmidt O, et al. In vitro chemosensitivity of freshly explanted tumor cells to pemetrexed is correlated with target gene expression. Invest New Drugs 2007;25:417-23. [Crossref] [PubMed]
- Sun JM, Han J, Ahn JS, et al. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. J Thorac Oncol 2011;6:1392-9. [Crossref] [PubMed]