Treatment of uncommon EGFR mutations in non-small cell lung cancer: new evidence and treatment
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases and has high mortality worldwide (1,2). In China, it is also the most common cancer and leading cause of cancer-related deaths (3). Targeted therapy has led to a new era in the treatment of NSCLC with the development of detection techniques for epidermal growth factor receptor (EGFR) mutations. NSCLC harboring EGFR mutations constitutes about 10–20% of all lung cancer cases in Europe and North America (4,5) and up to 50% of Asian patients with NSCLC (5). Exon 19 deletions and exon 21 L858R substitution are the most common mutations, accounting for approximately 90% of mutations in NSCLC; these are termed classic mutations and lead to high sensitivity to tyrosine kinase inhibitor (TKIs) (6-9). NSCLC patients with exon 19 deletions and exon 21 L858R substitution have longer progression-free survival (PFS) when treated with TKIs compared with traditional chemotherapy (10-12).
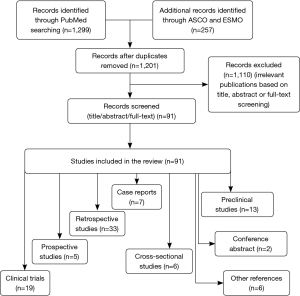
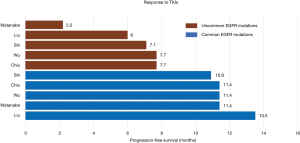
Other EGFR mutations are termed uncommon mutations, and account for 10–20% of all EGFR mutations (Figure 1) (10-13). Uncommon EGFR mutations show variable efficacy to EGFR-targeted drugs depending on the molecular alterations within exons 18–21, which are still not completely understood. The substitution mutations of G719X in exon 18, L861Q in exon 21, S768I in exon 20, and exon 20 insertions are the most frequent mutations among the uncommon mutations (14-16). Patients with these substitution mutations benefit from first-generation EGFR-TKIs such as erlotinib and gefitinib (14-17). The second-generation TKIs afatinib and dacomitinib have also demonstrated improved outcomes as first-line treatment of patients with classical EGFR mutations, which suggest that they may be the optimal therapy for this population (18,19). Increasing evidence has shown improved outcomes for patients with uncommon EGFR mutations such as G719X, L861Q, S768I, and complex mutations upon treatment with second-generation TKIs (17). However, there is no clear consensus on a treatment strategy for this population. The exon 20 mutation is traditionally considered to be insensitive to EGFR-targeted drugs (20-22). Figure 2 compares the clinical outcome of EGFR-TKIs between common and uncommon EGFR mutation-positive patients (15,23-26). Recently, treatment with chemotherapy and immune checkpoint inhibitors (ICIs) has been reported in NSCLC patients with uncommon EGFR mutations (27-29). Controversy over the treatment of uncommon EGFR mutation-positive patients still remains despite completed and ongoing clinical trials. This review provides an overview of the treatment of patients with G719X, S768I, L861Q, exon 20 insertions, and complex mutations based on existing clinical data.
Literature search
We conducted a systemic review in PubMed using the terms “NSCLC,” “EGFR,” “uncommon mutations,” “rare mutations,” “G719X,” “S768I,” “L861Q,” “exon 20,” and references from relevant articles. T790M mutations were excluded from uncommon EGFR mutations. Only articles in English were included and the search had no date limit. Some unpublished studies were searched online in the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology, and results were obtained from conference abstracts. We included diverse study types including clinical trials, retrospective and prospective studies, case reports, preclinical research, and systemic reviews. The details are shown in Figure 3.
Incidence and clinical characteristics
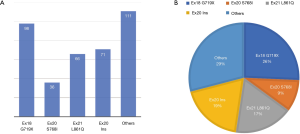
The G719X mutation in EGFR has alanine (A), serine (S), cysteine (C), or aspartic acid (D) in the position of the glycine residue, and is the most frequent point mutation in exon 18, representing 2–4% of all EGFR mutations (30,31). The exon 20 S768I substitution accounts for approximately less than 5% of all EGFR mutations and often exists with other mutations such as G719X and L861Q (31-33). L861Q in exon 21 consists of approximately 2% of EGFR-positive mutations, and is sometimes compounded with other mutations (34,35). G719X, L861Q, and S768I are thought to sensitize EGFR mutations to TKIs, just inferior to the prognosis of classical mutations, exon 19 deletions and exon 21 L858R substitution (17,23,24,36). Exon 20 insertions account for approximately 1–10% of all EGFR mutations (37). A multicenter observational study by Beau-Faller et al. (31) reported that A767V769dupASV was the most frequent variant, accounting for 12% of exon 20 insertions, which differs from a previous study that reported that V769_D770insASV is the most common mutation, constituting 22% of exon 20 insertions. Sasaki’s study (38) demonstrated that exon 20 insertions are more common in non-smokers and females. However, in a study involving 367 EGFR-positive patients, exon 20 insertions represented 9% (33/367) of mutations with more females (67%) and more smokers (52%) affected, consistent with another study (37). However, there was no significant difference in age, sex, ethnic origin, or stage at diagnosis (37,39). A comprehensive view of uncommon EGFR mutations from five studies (14,15,17,40,41) (Figure 4) demonstrated that the exon 18 G719X mutation was the most frequent point mutation, representing approximately 26% of uncommon EGFR mutations, followed by exon 21 L861Q mutation and exon 20 S768I mutation. Exon 20 insertions accounted for 9% of uncommon EGFR mutations (71/382). Complex mutations were not listed alone and were included as part of the other mutations.
Clinical evidence
G719X, S768I, L861Q mutations
It has been reported that G719X in exon 18 is responsive to EGFR-TKIs (35,42). A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6 clinical trials demonstrated that NSCLC patients harboring G719X had an objective response rate (ORR) of 77.8% to afatinib, with a PFS of 13.8 months, which was longer than that with chemotherapy, but a shorter overall survival (OS) than that with chemotherapy (17). A retrospective study by Chiu et al. (25) demonstrated higher sensitivity to TKIs in patients with complex mutations (G719X+ L861Q and G719X+ S768I) than with a single G719X mutation (PFS: 11.9 vs. 6.5 m, respectively, P=0.010). However, the ORR in Zhang’s study (14) was only 22.7% compared with a retrospective study by Xu (16), which showed an ORR of 42.9% with TKIs. By contrast, the PFS for patients harboring the G719X mutation in Zhang’s study (14) was longer than that in Xu’s study (16) (7.6 vs. 5.98 m, respectively) (Table 1).
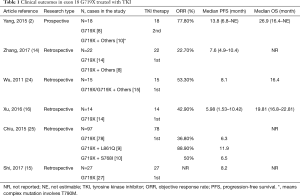
Full table
The S768I in exon 20 is a rare mutation that commonly exists with L858R, G719X, and other mutations (43). Several small case studies have demonstrated the clinical efficacy of EGFR-TKIs in NSCLC patients harboring S768I mutations (44,45). Two small retrospective studies by Shi (15) and Chen (46) reported moderate clinical efficacy inferior to that of other common mutations, but better than that of other exon 20 mutations, with a PFS of 3.4 months and 2.7 months, respectively. Zhang’s study (14) showed a longer PFS of 8.0 months, although the ORR was only 27.3%. Surprisingly, a post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6 clinical trials of NSCLC patients carrying S768I with complex mutations showed the longest PFS of 14.7 months among the uncommon EGFR mutations (17). A female patient with advanced NSCLC harboring a single S768I mutation achieved 6-month survival from afatinib after showing no response to gefitinib (47). These results suggest that afatinib may be a more effective treatment for NSCLC patients carrying the S768I mutation compared to first-generation TKIs (Table 2).
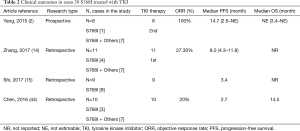
Full table
The exon 21 L861Q mutation is the second most frequent uncommon mutation, representing approximately 2% of all EGFR mutations. Some preclinical trials have demonstrated low efficacy or complete resistance of the L861Q mutation to EGFR-TKIs (48-50), suggesting a poor prognosis for these patients. However, a large retrospective study by Chiu et al. reported a response rate (RR) of 39.6% and a PFS of 8.1 months, demonstrating a moderate response to TKIs, but the OS was not followed (25). Wu and Xu obtained similar data as Chiu, although with smaller cases (24). In the prospective clinical trial by Yang (17), high afatinib activity was observed in patients harboring the EGFR-L861Q mutation, with an ORR of 56.3%, median PFS of 8.2 months, and median OS of 17.1 months (Table 3).
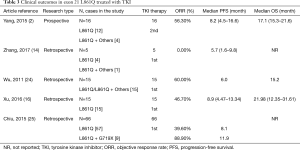
Full table
Exon 20 insertions
Several retrospective studies have reported that EGFR-targeted drugs are ineffective in NSCLC patients with exon 20 insertions (16,24,36). The work by Wu (24) demonstrated that NSCLC patients harboring exon 20 insertions had a much shorter PFS than those with exon 19 deletions and exon 21 L858R substitution (1.4 vs. 8.5 m, P<0.001) (24). In addition, a study involving 12 NSCLC cases harboring exon 20 insertions reported that TKIs might not be the first choice of therapy for these patients (16). In this study, the ORR and PFS were 8.3% and 2 months, respectively. It has been suggested that NSCLC patients who harbor exon 20-ins mutation should be treated with traditional therapy, similar to NSCLC patients with EGFR wild-type. Thus, platinum-based chemotherapy might be the preferred treatment choice rather than TKIs (51). The combined post-hoc analysis of Lux-Lung 2, Lux-Lung 3, and Lux-Lung 6 clinical studies is currently the largest prospective study on the efficacy of EGFR-TKI in patients harboring uncommon EGFR mutations. The study divided patients with rare EGFR mutations into three groups: point mutations or duplications in exons 18–21 (Group 1), de novo Thr790Met mutations in exon 20 alone or in combination with other mutations (Group 2), and exon 20 insertions (Group 3). Patients with exon 20 insertions treated with afatinib had an ORR of less than 10% and a median PFS of 2.7 months, representing the lowest efficacy with afatinib compared to Group 1, Group 2, Group 3, and the chemotherapy-treated group (PFS: 10.7, 2.9, 2.7, and 8.2 months, respectively) (17). Similar results were found in other three studies with a PFS of 2.9, 3.1, and 3.0 months for exon 20ins groups, respectively (Table 4) (36,41,52).
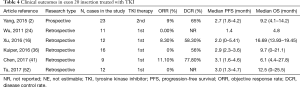
Full table
Complex mutations
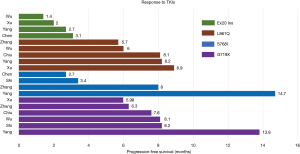
The occurrence and application of new mutational detection techniques s uch as direct sequencing, multiplex PCR systems, and next-generation sequencing have resulted in an increased number of cases with complex mutations. Complex EGFR mutations reportedly account for approximately 3–14% of all EGFR-positive mutations (13,24,53-55). Complex and classical mutations showed similar treatment efficacy toward EGFR-TKIs as that of classical mutations alone in a large retrospective study by Keam (55), demonstrating a RR of 74.8% and 68.8% and a median PFS of 11.9 months and 8.1 months, respectively. Hata et al. also reported similar results on complex mutations, but patients harboring L858R plus G719S mutations did not show a response to gefitinib (56). NSCLC patients harboring uncommon complex mutations without classical mutations (G719X + L861Q and G719X + S768I) had a significantly longer PFS than patients with a single rare mutation (11.9 vs. 6.5 months, respectively) (25). A retrospective multicenter study in 18 Italian institutions by Passaro et al. (57) demonstrated that TKI treatment of patients with complex mutations had superior efficacy than treatment with exon 18 mutation with regard to median PFS (mPFS) and OS (57). Yu et al. (58) reported a worse outcome for compound mutations with exon 20 compared to compound mutations without exon 20 and single common EGFR mutation (mPFS: 6.5, 9.1, and 13 months, respectively, P=0.002). Interestingly, Passaro and colleagues did not observe differences between compound mutation exon 20-positive and exon 20-negative patients with a mPFS of 10 months and 12 months, respectively (P=0.9), in contrast to Yu’s results (57,58). A summary of the efficacy of EGFR-TKIs in patients with each uncommon EGFR mutation is shown in Figure 5. As depicted, the PFS in patients with exon 20 insertions was significantly shorter than that in patients with G719X, S768I, or L861Q mutations. However, inconsistent efficacy to EGFR-TKIs has been shown in different studies of patients with G719X, S768I, or L861Q mutations (2,14-16,24,25,41).
New evidence
G719X, S768I, L861Q, and complex mutations
A retrospective study including 95 NSCLC patients harboring uncommon EGFR mutations conducted by Brindel et al. (27) in France demonstrated an OS of 16.9 months in patients treated with first-line TKIs compared to an OS of 27.7 months in patients treated with first-line chemotherapy. In this study, patients with the exon 21 L861Q mutation had a poor prognosis compared to those with exon 18 and exon 20 mutations (27).
Neratinib, an irreversible pan-ErbB receptor TKI, showed great response in patients harboring a G719X point mutation in exon 18 in a phase II clinical trial, achieving a median PFS of 52.7 weeks (90% confidence interval: 25.6–57.0 weeks) (59). In contrast, low activity was observed in patients with other EGFR mutations. However, another second-generation EGFR-TKI, afatinib, showed high efficacy in the largest prospective study conducted on the efficacy of EGFR-TKI in patients harboring uncommon EGFR mutations, namely a combined post-hoc analysis of the Lux-Lung 2, Lux-Lung 3, and Lux-Lung 6 clinical trials (2,17). More clinical evidence is required to determine whether afatinib should be the first treatment choice for NSCLC patients harboring G719X, S768I, and/or L861Q mutations. A 72-year-old female diagnosed with advanced NSCLC carrying complex exon 18 G719X plus exon 20 S768I mutations reportedly exhibited a good response without progression for 12 months (26). Another patient harboring compound L861Q and L858M mutations experienced tumor regression with afatinib after treatment failure with erlotinib and chemotherapy (60). A retrospective analysis of a Caucasian population in Germany by Kauffmann-Guerrero et al. (61) showed that a patient with G719X mutation achieved a durable partial response (PR) to afatinib that remained after 26 months. In a retrospective analysis, Passaro (57) found good afatinib activity when treating four patients with G719X mutations, all of whom achieved a PR. A large retrospective study (62) demonstrated that patients with uncommon mutations lacking exon 19 deletions or L858R had a significantly longer PFS with afatinib treatment than those who received gefitinib or erlotinib (18.3 vs. 2.8 months, P=0.07). However, compared with gefitinib or erlotinib, patients with complex and classical mutations receiving afatinib exhibited a higher albeit insignificant mPFS (11 vs. 8.2 months, P=0.24). In a small study by Tanaka (63) of patients harboring uncommon EGFR mutations (except exon 19 deletions or exon 21 L858R substitution), afatinib showed superior efficacy over first-generation EGFR-TKIs with an ORR of 75.0% versus 40.0% and PFS of 17.1 versus 5.5 months, respectively (P=0.0481). Therefore, second-generation TKIs might become an optimal choice for patients harboring these mutations.
Osimertinib, a third-generation TKI, has also shown promising results in some cases and studies with small sample sizes. It was confirmed to be effective for treating patients with uncommon EGFR mutations in an open-label, multicenter, phase II single arm trial that included 36 patients (64). A total of 77.8% of patients harboring the L861Q mutation achieved a PR with osimertinib treatment, followed by G719X (52.5%) and S7681 (37.5%). The overall mPFS was 9.5 months and the median duration of response (mDoR) was 7.0 months. Thus, this is a promising drug worth studying.
Exon 20 insertions
Previous studies have demonstrated that NSCLC patients with A763_Y764insFQEA, achieved a PR to treatment with gefitinib and erlotinib (22,65). In another study, two patients carrying N771_P772insNand H773_V774insQ mutations achieved a moderate response to those TKIs (52). The differences in TKI efficacy might be due to the diversity of exon 20 insertions. Comprehensive genomic profiling may allow the detection of a broad spectrum of EGFR exon 20 insertions associated with co-occurring genomic alterations, which will provide insights into effective treatments for improving clinical outcomes in this population (66). In addition, some cases have reported high efficacy to second- and third-generation TKIs (67,68). In a preclinical study, Floc’h and colleagues (69) showed that osimertinib and its metabolite AZ5104 had anti-tumor activity indifferent exon 20 insertion PDX models in vivo, which suggests that its will be efficacious in patients harboring exon 20 insertions. In clinical practice, one NSCLC patient carrying H773L/V774M in exon 20 demonstrated sustained disease control to osimertinib, which suggests that patients with this mutation may clinically benefit from treatment with this TKI (67,68). Nazartinib, a covalent EGFR inhibitor similar to osimertinib, has also exhibited potential benefits for exon 20 insertions in preclinical studies (70). A clinical trial of this drug is ongoing in NSCLC patients with common EGFR mutations, but effects in patients with exon 20 insertions have not been observed. Clinical trials are needed to confirm the role of osimertinib in this patient population.
A female with advanced NSCLC harboring an EGFR exon 20 insertion mutation (A767_S768insSVA tandem duplication) achieved a long survival of 54 months with afatinib after increased pulmonary metastasis with first-generation TKI (67,68). As reported by van Veggela et al. (71) from Netherlands, four progressed patients with NSCLC who had received platinum-based chemotherapy or first-generation TKI with EGFR exon 20 insertion were treated with afatinib 40 mg once daily and cetuximab 250–500 mg/m2 every two weeks. Surprisingly, three patients experienced a radiological response, with significant shrinkage of tumor size. The mPFS for the four patients was 5.4 months, with the longest PFS of 17.6 months. The results of this study provide clinical evidence for the treatment of advanced NSCLC patients harboring EGFR exon 20 insertion with the combination of afatinib plus cetuximab. The efficacy of the combined treatment might be due to the dual blockade of EGFR by the second-generation EGFR-TKI afatinib and the EGFR monoclonal antibody cetuximab, according to previous studies (72,73). This is the same theory underlying the effects of osimertinib plus cetuximab (74). Thus, clinical trials on osimertinib plus monoclonal antibodies should be conducted.
In contrast to classical EGFR-TKIs, new EGFR-TKIs are emerging, and being studied in preclinical studies or clinical trials. Poziotinib is a pan-human epidermal growth factor receptor 2 (HER2) TKI, which was confirmed to be effective in treating advanced or metastatic lung adenocarcinoma that has progressed on erlotinib or gefitinib in a phase II study (75). Robichaux et al. (76) demonstrated that 64% (7/11) of NSCLC patients carrying exon 20 insertion had a confirmed objective partial radiological response based on response evaluation criteria in solid tumors (RECIST) that was due to poziotinib. They also confirmed that poziotinib could overcome changes within the drug-binding pocket induced by insertions in exon 20 by its structural features (76). These results suggest that poziotinib might be a promising TKI for this population both preclinically and clinically, who are considered to be highly resistant to standard therapy. In addition to poziotinib, Ap32788 is a new EGFR or HER2 TKI that has entered into clinical trials in the United States. The latest ASCO meeting reported the preliminary results of safety, pharmacokinetics, and anti-tumor activity of AP32788 in a phase 1/2 clinical trial of NSCLC patients harboring exon 20 insertions (77). This study demonstrated that the ORR was 39% with six of 18 patients achieving a PR and a disease control rate of 94% when the dosage was 80–160 mg. The latest data about the efficacy of AP73288 in treating NSCLC patients with exon 20 insertions was expected to report.
Chemotherapy and uncommon EGFR mutations
Chemotherapy has been demonstrated to have a statistically significant inferior efficacy than EGFR-TKIs in patients with common EGFR mutations (8,11,12,78-81). However, little was concerned about chemotherapy and uncommon EGFR mutations. Brindel et al. (27) found that, compared with first-line TKIs, first-line chemotherapy showed superior efficacy with a longer OS in patients with uncommon EGFR mutations, especially for mutations in exon 18 and exon 20. A real-world study in China had a similar conclusion, showing that chemotherapy led to a better prognosis with an OS of 20.7 months compared to the EGFR-TKI group, which had an OS of 14.3 months (29). Future studies are needed with a larger sample size, and the prognosis and mutation type needs further investigations.
Immunotherapy and uncommon EGFR mutations
The development of ICIs has led to a new era in the treatment of NSCLC. ICIs such as pembrolizumab, nivolumab, and atezolizumab have shown promising results by restoring antitumor activity in clinical trials (82-85). However, it is not recommended that NSCLC patients with EGFR mutations be treated with immunotherapy agents. A meta-analysis reported that ICIs as second-line therapy for EGFR-mutated NSCLC patients did not improve clinical outcome compared to docetaxel (86). The lower expression of PD-L1 and tumor mutation burden in NSCLC patients with EGFR mutations might be associated with a poor prognosis compared to EGFR wild-type patients (82,83,87-89). Akbay et al. (90) found that oncogenic EGFR signaling was related to the change of the tumor microenvironment, which induced immune escape, resulting in a low response to ICIs. However, according to the results of the IMpower150 study, the combination of atezolizumab, bevacizumab, and chemotherapy conferred significant PFS and OS benefits compared to combined treatment without atezolizumab in patients with metastatic nonsquamous NSCLC including patients with EGFR mutations or anaplastic lymphoma kinase (ALK) translocations (91). In subgroup analysis of patients with EGFR mutations or ALK translocations, the PFS was 9.7 months in the group treated with atezolizumab, longer than the PFS of 6.1 months in group of bevacizumab plus chemotherapy. This finding offers a new perspective on the treatment of patients with EGFR mutations using combination therapy including immunotherapy.
Yamada et al. (28) demonstrated that patients with uncommon EGFR mutations, including G719X in exon 18 and insertion in exon 20, showed statistically significant superiority to ICIs compared to those with common EGFR mutations (PFS: 256 vs. 50 days, respectively; P=0.003). These results indicate that immunotherapy might be a potent treatment for NSCLC patients with uncommon EGFR mutations; further studies are needed for confirmation.
Conclusions
G719X, S768I, L861Q, complex mutations, and exon 20 insertions are the most frequent uncommon EGFR mutations. NSCLC patients harboring G719X, S768I, L861Q, and some complex mutations are sensitive to TKIs, but are not sensitive to common mutations (exon 19 deletions and exon 21 L858R substitution). Patients treated with afatinib or osimertinib have a better prognosis than first-generation TKIs, which suggests that second- or third-generation TKIs may be the preferred choice of treatment for this population. More evidence has demonstrated a moderate response to TKIs in NSCLC patients with exon 20 insertions. Novel targeted drugs including poziotinib and Ap32788 have reached late phase clinical trials and achieved good results. Immunotherapy has also achieved promising results for uncommon EGFR mutations. Future research is needed to help determine the treatment choice for NSCLC patients with uncommon EGFR mutations.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant number 81401903, 81572937 and 81572273); China Postdoctoral Science Foundation 64th batch (Postdoctoral number: 45786); Jiangsu Provincial Postdoctoral Science Foundation in 2018; the Natural Science Foundation of Jiangsu province (grant number BK20180139 and BK20161386); Jiangsu Provincial Medical Youth Talent (grant number QNRC2016125), and the Nanjing Medical Science and Technology Development Project (No. ZKX17044), the Jiangsu Provincial Key Research and Development Program (No. BE2016721).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [Crossref] [PubMed]
- D'Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29:2066-70. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Fukuoka M, Wu Y, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Mok TS, Wu Y, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Evans M, O'Sullivan B, Smith M, et al. Large-Scale EGFR Mutation Testing in Clinical Practice: Analysis of a Series of 18,920 Non-Small Cell Lung Cancer Cases. Pathol Oncol Res 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Zhang Y, Wang Z, Hao X, et al. Clinical characteristics and response to tyrosine kinase inhibitors of patients with non-small cell lung cancer harboring uncommon epidermal growth factor receptor mutations. Chin J Cancer Res 2017;29:18-24. [Crossref] [PubMed]
- Shi J, Yang H, Jiang T, et al. Uncommon EGFR mutations in a cohort of Chinese NSCLC patients and outcomes of first-line EGFR-TKIs and platinum-based chemotherapy. Chin J Cancer Res 2017;29:543-52. [Crossref] [PubMed]
- Xu J, Jin B, Chu T, et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer 2016;96:87-92. [Crossref] [PubMed]
- Wu YL, Hirsh V, Sequist LV, et al. Does EGFR Mutation Type Influence Patient-Reported Outcomes in Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer? Analysis of Two Large, Phase III Studies Comparing Afatinib with Chemotherapy (LUX-Lung 3 and LUX-Lung 6). Patient 2018;11:131-41. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Wu JY, Wu S, Yang C, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res 2008;14:4877-82. [Crossref] [PubMed]
- Greulich H, Chen TH, Feng W, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med 2005;2:e313. [Crossref] [PubMed]
- Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013;8:179-84. [Crossref] [PubMed]
- Liu H L, Han G, Peng M, et al. Efficacy of EGFR tyrosine kinase inhibitors in non-small cell lung cancer patients harboring different types of EGFR mutations: A retrospective analysis. J Huazhong Univ Sci Technolog Med Sci 2017;37:864-72. [PubMed]
- Wu JY, Yu C, Chang Y, et al. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 2011;17:3812-21. [Crossref] [PubMed]
- Chiu CH, Yang C, Shih J, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. [Crossref] [PubMed]
- Watanabe M, Oizumi S, Kiuchi S, et al. The Effectiveness of Afatinib in a Patient with Advanced Lung Adenocarcinoma Harboring Rare G719X and S768I Mutations. Intern Med 2018;57:993-6. [Crossref] [PubMed]
- Brindel A, Brevet M, Althakfi W, et al. LBA60Uncommon EGFR mutations in lung adenocarcinomas: Clinical features and response to tyrosine kinase inhibitors. Ann Oncol 2018;29. [Crossref]
- Yamada T, Hirai S, Katayama Y, et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Li H, Wang C, Wang Z, et al. Efficacy and long-term survival of advanced lung adenocarcinoma patients with uncommon EGFR mutations treated with 1st generation EGFR-TKIs compared with chemotherapy as first-line therapy. Lung Cancer 2019;130:42-9. [Crossref] [PubMed]
- Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol 2006;11:190-8. [Crossref] [PubMed]
- Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014;25:126-31. [Crossref] [PubMed]
- Zhu X, Bai Q, Lu Y, et al. Response to Tyrosine Kinase Inhibitors in Lung Adenocarcinoma with the Rare Epidermal Growth Factor Receptor Mutation S768I: a Retrospective Analysis and Literature Review. Target Oncol 2017;12:81-8. [Crossref] [PubMed]
- Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:45-51. [Crossref] [PubMed]
- Murray S, Dahabreh IJ, Linardou H, et al. Somatic mutations of the tyrosine kinase domain of epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical database. J Thorac Oncol 2008;3:832-9. [Crossref] [PubMed]
- Otsuka T, Mori M, Yano Y, et al. Effectiveness of Tyrosine Kinase Inhibitors in Japanese Patients with Non-small Cell Lung Cancer Harboring Minor Epidermal Growth Factor Receptor Mutations: Results from a Multicenter Retrospective Study (HANSHIN Oncology Group 0212). Anticancer Res 2015;35:3885-91. [PubMed]
- Kuiper JL, Hashemi SM, Thunnissen E, et al. Non-classic EGFR mutations in a cohort of Dutch EGFR-mutated NSCLC patients and outcomes following EGFR-TKI treatment. Br J Cancer 2016;115:1504-12. [Crossref] [PubMed]
- Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013;12:220-9. [Crossref] [PubMed]
- Sasaki H, Endo K, Takada M, et al. EGFR exon 20 insertion mutation in Japanese lung cancer. Lung Cancer 2007;58:324-8. [Crossref] [PubMed]
- Lund-Iversen M, Kleinberg L, Fjellbirkeland L, et al. Clinicopathological characteristics of 11 NSCLC patients with EGFR-exon 20 mutations. J Thorac Oncol 2012;7:1471-3. [Crossref] [PubMed]
- Pilotto S, Rossi A, Vavala T, et al. Outcomes of First-Generation EGFR-TKIs Against Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Post Hoc Analysis of the BE-POSITIVE Study. Clin Lung Cancer 2018;19:93-104. [Crossref] [PubMed]
- Chen K, Yu X, Wang H, et al. Uncommon mutation types of epidermal growth factor receptor and response to EGFR tyrosine kinase inhibitors in Chinese non-small cell lung cancer patients. Cancer Chemother Pharmacol 2017;80:1179-87. [Crossref] [PubMed]
- Leduc C, Merlio JP, Besse B, et al. Clinical and molecular characteristics of non-small-cell lung cancer (NSCLC) harboring EGFR mutation: results of the nationwide French Cooperative Thoracic Intergroup (IFCT) program. Ann Oncol 2017;28:2715-24. [Crossref] [PubMed]
- O'Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer 2017;109:137-44. [Crossref] [PubMed]
- Hellmann MD, Reva B, Yu H, et al. Clinical and in vivo evidence that EGFR S768I mutant lung adenocarcinomas are sensitive to erlotinib. J Thorac Oncol 2014;9:e73-4. [Crossref] [PubMed]
- Masago K, Fujita S, Irisa K, et al. Good clinical response to gefitinib in a non-small cell lung cancer patient harboring a rare somatic epidermal growth factor gene point mutation;codon 768 AGC > ATC in exon 20 (S768I). Jpn J Clin Oncol 2010;40:1105-9. [Crossref] [PubMed]
- Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther 2016;9:4181-6. [Crossref] [PubMed]
- Duan H, Peng Y, Cui H, et al. Effectiveness of afatinib after ineffectiveness of gefitinib in an advanced lung adenocarcinoma patient with a single EGFR exon 20 S768I mutation: a case report. Onco Targets Ther 2018;11:2303-9. [Crossref] [PubMed]
- Carey KD, Garton AJ, Romero MS, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res 2006;66:8163-71. [Crossref] [PubMed]
- Furuyama K, Harada T, Iwama E, et al. Sensitivity and kinase activity of epidermal growth factor receptor (EGFR) exon 19 and others to EGFR-tyrosine kinase inhibitors. Cancer Sci 2013;104:584-9. [Crossref] [PubMed]
- Kancha RK, Peschel C, Duyster J. The epidermal growth factor receptor-L861Q mutation increases kinase activity without leading to enhanced sensitivity toward epidermal growth factor receptor kinase inhibitors. J Thorac Oncol 2011;6:387-92. [Crossref] [PubMed]
- Sequist LV, Gettinger S, Senzer NN, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol 2010;28:4953-60. [Crossref] [PubMed]
- Tu HY, Ke E, Yang J, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer 2017;114:96-102. [Crossref] [PubMed]
- Zhang GC, Lin J, Wang Z, et al. Epidermal growth factor receptor double activating mutations involving both exons 19 and 21 exist in Chinese non-small cell lung cancer patients. Clin Oncol (R Coll Radiol) 2007;19:499-506. [Crossref] [PubMed]
- Huang SF, Liu H, Li L, et al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res 2004;10:8195-203. [Crossref] [PubMed]
- Keam B, Kim DW, Park JH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol 2014;19:594-600. [Crossref] [PubMed]
- Hata A, Yoshioka H, Fujita S, et al. Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol 2010;5:1524-8. [Crossref] [PubMed]
- Passaro A, Prelaj A, Bonanno L, et al. Activity of EGFR TKIs in Caucasian Patients With NSCLC Harboring Potentially Sensitive Uncommon EGFR Mutations. Clin Lung Cancer 2019;20:e186-94. [Crossref] [PubMed]
- Yu X, Zhang X, Zhang Z, et al. First-generation EGFR tyrosine kinase inhibitor therapy in 106 patients with compound EGFR-mutated lung cancer: a single institution's clinical practice experience. Cancer Commun (Lond) 2018;38:51. [Crossref] [PubMed]
- Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:3076-83. [Crossref] [PubMed]
- Saxon JA, Sholl LM, Janne PA. EGFR L858M/L861Q cis Mutations Confer Selective Sensitivity to Afatinib. J Thorac Oncol 2017;12:884-9. [Crossref] [PubMed]
- Kauffmann-Guerrero D, Huber RM, Reu S, et al. NSCLC Patients Harbouring Rare or Complex EGFR Mutations Are More Often Smokers and Might Not Benefit from First-Line Tyrosine Kinase Inhibitor Therapy. Respiration 2018;95:169-76. [Crossref] [PubMed]
- Shen YC, Tseng G, Tu C, et al. Comparing the effects of afatinib with gefitinib or Erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer 2017;110:56-62. [Crossref] [PubMed]
- Tanaka I, Morise M, Kodama Y, et al. Potential for afatinib as an optimal treatment for advanced non-small cell lung carcinoma in patients with uncommon EGFR mutations. Lung Cancer 2019;127:169-71. [Crossref] [PubMed]
- Ahn M-J, Cho JH, Sun J-M, et al. An open-label, multicenter, phase II single arm trial of osimertinib in non-small cell lung cancer patients with uncommon EGFR mutation (KCSG-LU15-09). J Clin Oncol 2018;36:9050. [Crossref]
- Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5:216ra177. [Crossref] [PubMed]
- Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J Thorac Oncol 2018;13:1560-8. [Crossref] [PubMed]
- Chan RT. Afatinib for an EGFR exon 20 insertion mutation: A case report of progressive stage IV metastatic lung adenocarcinoma with 54 months' survival. Asia Pac J Clin Oncol 2018;14 Suppl 1:7-9. [Crossref] [PubMed]
- Yang M, Tong X, Xu X, et al. Case Report: Osimertinib achieved remarkable and sustained disease control in an advanced non-small-cell lung cancer harboring EGFR H773L/V774M mutation complex. Lung Cancer 2018;121:1-4. [Crossref] [PubMed]
- Floc'h N, Martin MJ, Riess JW, et al. Antitumor Activity of Osimertinib, an Irreversible Mutant-Selective EGFR Tyrosine Kinase Inhibitor, in NSCLC Harboring EGFR Exon 20 Insertions. Mol Cancer Ther 2018;17:885-96. [Crossref] [PubMed]
- Jia Y, Juarez J, Li J, et al. EGF816 Exerts Anticancer Effects in Non-Small Cell Lung Cancer by Irreversibly and Selectively Targeting Primary and Acquired Activating Mutations in the EGF Receptor. Cancer Res 2016;76:1591-602. [Crossref] [PubMed]
- van Veggel B, de Langen AJ, Hashemi SMS, et al. Afatinib and Cetuximab in Four Patients With EGFR Exon 20 Insertion-Positive Advanced NSCLC. J Thorac Oncol 2018;13:1222-6. [Crossref] [PubMed]
- Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest 2009;119:3000-10. [PubMed]
- Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036-45. [Crossref] [PubMed]
- Della Corte CM, Ciaramella V, Cardone C, et al. Antitumor Efficacy of Dual Blockade of EGFR Signaling by Osimertinib in Combination With Selumetinib or Cetuximab in Activated EGFR Human NCLC Tumor Models. J Thorac Oncol 2018;13:810-20. [Crossref] [PubMed]
- Han JY, Lee KH, Kim SW, et al. A Phase II Study of Poziotinib in Patients with Epidermal Growth Factor Receptor (EGFR)-Mutant Lung Adenocarcinoma Who Have Acquired Resistance to EGFR-Tyrosine Kinase Inhibitors. Cancer Res Treat 2017;49:10-9. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Robert CD, Riely GJ, Spira AI, et al. First report of safety, pharmacokinetics, and preliminary antitumor activity of the oral EGFR/HER2 exon 20 inhibitor TAK-788(AP32788) in non-small cell lung cancer (NSCLC). J Clin Oncol 2018;36:9015. [Crossref]
- Zhou C, Wu Y, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu C, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim D W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Rangachari D, VanderLaan PA, Shea M, et al. Correlation between Classic Driver Oncogene Mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 >/=50% Expression in Lung Adenocarcinoma. J Thorac Oncol 2017;12:878-83. [Crossref] [PubMed]
- Offin M, Rizvi H, Tenet M, et al. Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Lung Cancers. Clin Cancer Res 2019;25:1063-9. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]