|
Cite this article as: Hilbe W, Manegold C, Pircher A. Targeting angiogenesis in lung cancer - Pitfalls in drug development. Transl Lung Cancer Res 2012;1(2):122-128. DOI: 10.3978/j.issn.2218-6751.2012.01.01
Review Article
Targeting angiogenesis in lung cancer-Pitfalls in drug development
Wolfgang Hilbe1, Christian Manegold2, Andreas Pircher,1
1Department of Hematology and Oncology, Medical University Innsbruck, Austria; 2Department of Surgery, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Germany
Wolfgang Hilbe, MD. Medical University Innsbruck, Department of Hematology and Oncology, Anichstrasse 35, A-6020 Innsbruck, Austria. Tel: 0043-512-504-81151; Fax: 0043-512-504-26299. Email: wolfgang.hilbe@i-med.ac.at.
|
|
Abstract
In non-small-cell lung cancer, anti-angiogenic strategies like bevacizumab have developed into standard treatment options. New anti-angiogenic drugs like tyrosine kinase inhibitors generated optimistic results in phase II trials, but failed to translate into positive results in phase III trials. In this overview some critical aspects of the biology of tumor angiogenesis and potential pitfalls of anti-angiogenic drug development are discussed. These include the design of clinical trials, dosage of investigational drugs or the choice of combinational drugs, the lack of validated biomarkers and the complexity of the patho-biology of tumor angiogenesis. Future trials should also direct attention to the role of cigarette smoke and the stage of the disease, which is investigated.
Key words VEGF; VEGFR; anti-antiangiogenic therapy; tyrosine kinase inhibitor (TKI); bevacizumab
Transl Lung Cancer Res Jan 04, 2012. DOI: 10.3978/j.issn.2218-6751.2012.01.01
|
|
Introduction
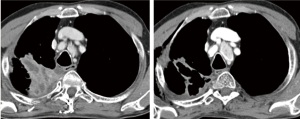
In 1971, Judah Folkman hypothesized that tumor growth is dependent on angiogenesis and suggested that disrupting tumor angiogenesis would inhibit tumor growth, thus providing a method of controlling tumors ( 1). Thereby, tumor hypoxia is the key trigger to induce tumor angiogenesis and, in a simplified model, hypoxia and factors like epidermal growth factor (EGF), insulin-like growth factor (IGF) or platelet-derived growth factor (PDGF) lead to vascular-endothelial growth factor (VEGF)-secretion. Subsequently, VEGF activates VEGF receptors (VEGFR) which by means of dimerization induces the downstream signaling cascade of endothelial cells. Finally, proliferation, migration and permeability of endothelial cells are induced facilitating tumor growth and metastasis. During the following decades tumor angiogenesis has been the subject of extensive research and it is well known that angiogenesis is involved in early as well as in late carcinogenic processes and finally contributes to metastases ( 2). Based on theoretical considerations, anti-angiogenic therapies could target either the VEGF itself by neutralization (Bevacizumab, VEGF-Trap ( 3)) or inhibition of the external epitope of the VEGF receptor with monoclonal antibodies. Further VEGF signaling can be blocked by VEGFR tyrosine kinase inhibitors (TKI) like sunitinib, sorafenib, pazopanib, cediranib, axitinib, motesanib and so on ( 4). In contrast to monoclonal antibodies, these TKIs are not specific for VEGFR 1-3 but also inhibit a plethora of tyrosine kinases and signaling pathways ( 5, 6). The anti-VEGF monoclonal antibody bevacizumab was the first successfully applied antiangiogenic drug in humans. By testing bevacizumab in advanced non-small-cell lung cancer (NSCLC), the well known Sandler trial proved efficacy in all evaluated end-points including overall survival (OS), progression-free survival (PFS) and doubling of response rates (RR) ( 7). This study was the basis for the approval of bevacizumab in NSCLC, a new "standard of care". Impressive effects seen in daily routine supported the enthusiasm for this new anticancer strategy ( Figure 1). Nevertheless, the challenges, experienced during the clinical development in lung cancer, have to be kept in mind. When bevacizumab was evaluated in a randomized phase II trial in an unselected NSCLC cohort, 4 out of 66 treated patients experienced fatal bleedings ( 8). By defining a clear risk profile and by excluding "high-risk" patients, bevacizumab application proved to be tolerable in subsequent phase III trials ( 7, 9). Furthermore, first reports evaluating orally available kinase inhibitors also proved to be effective ( 10). These first positive results initiated a great engagement of the pharmaceutical industry to develop a number of similar products targeting
VEGFR1, 2, 3 or other angiogenic cascades ( 11) (see Table 1). However, these optimistic results generated in phase II
trials did not translate into positive results in phase III trials.
In the following, some critical aspects of the biology of tumor
angiogenesis and potential pitfalls of anti-angiogenic drug
development are discussed.
| Table 1. Relevant phase III trials introducing anti-angiogenic agents in NSCLC. |
| Drug |
Class |
Target |
Clinical Phase |
Combination |
PFS |
OS |
Ref (or clin.trials gov.) |
| Bevacizumab |
MoAb |
VEGF |
III
III |
Carboplatin/PXL
Cisplatin/Gemcitabine |
positive
positive |
positive
negative |
Sandler et al. (7)
Reck et al. (9) |
| Aflibercept |
Soluble decoy receptor |
VEGF |
III |
DXL |
positive |
negative |
Novello et al. (42) |
| ASA4040 |
VDA |
unknown |
III |
Carboplatin/PXL |
negative |
negative |
Lara et al. (15) |
| BIBF1120 |
TKI |
VEGFR-1, 2, 3, FGFR, PDGFR |
III
III |
DXL
Pemetrexed |
pending
pending |
pending
pending |
NCT00805194
NCT00806819 |
| Cediranib |
TKI |
VEGFR-1, 2, 3,
c-kit, Flt-3 |
III
II/III |
Carboplatin/PXL
Carboplatin/PXL |
pending
negative |
pending
negative |
NCT00795340
Goss et al. (43) |
| Motesanib |
TKI |
VEGFR-1, 2, 3,
PDGFR, RET, kit |
III |
Carboplatin/PXL |
positive |
negative |
Scagliotti et al. (44) |
| Sorafenib |
TKI |
Raf, Kit, Flt-3,
VEGFR-2 & 3, PDGFR-β |
III
III
III |
Carboplatin/PXL
Cisplatin/Gemcitabine
Monotherapy |
negative
negative
pending |
negative
negative
pending |
Scagliotti et al. (45)
Gatzemeier et al. (46)
NCT00863746 |
| Sunitinib |
TKI |
VEGFR-1, 2, 3,
PDGFR-α, PDGFR-β, Flt-3, c-kit |
III |
Monotherapy |
pending |
pending |
NCT00693992 |
| Vandetanib |
TKI |
VEGFR-2 & 3, RET, EGFR |
III
III
III |
Docetaxel
Pemetrexed
Monotherapy |
positive
negative
positive |
negative
negative
negative |
Herbst et al. (47)
De Boer et al. (48)
Lee et al. (49) |
| MoAb: monoclonal antibody; VDA: vascular disrupting agent; DXl: docetaxel; PXL: paclitaxel; TKI: tyrosine kinase inhibitor. |
|
|
Tumor response but no prolongation of overall survival
Throughout the last years, most of the phase III studies
evaluating anti-angiogenic drugs failed to prove a survival benefit
despite improvement of PFS. A heavily discussed study, for
example, is the AVAIL trial testing chemotherapy plus minus
bevacizumab ( 9). By addition of bevacizumab in two different
dosages, PFS improved significantly from 6.1 to 6.5 or 6.7
months, the median OS, however, failed to prove a beneficial
effect of bevacizumab (13.1 vs. 13.6 or 13.4 months). Afterwards, a series of phase III trials, evaluating kinase
inhibitors, vascular disrupting agents or new molecules like the
VEGF-trap, failed to improve overall survival despite optimistic
results as far as PFS was concerned ( 12). Why does a drug,
which proves efficacy in terms of tumor response or prolongation
of PFS, not change patient’s outcome? Possible answers for that
question are manifold. Firstly, the design of clinical trials could be an answer.
Subsequent lines of treatment, including cross-over to similar
products in later lines, might hide the absolute benefit of a drug ( 13). In that context the establishment of adequate criteria for
response is warranted, since standard RECIST criteria measure
tumor reduction in diameter but not the development of
necrosis ( 14). Secondly, the dosage of the investigational drug and the
choice of combinational drugs might be other reasons. At the
WCLC meeting in 2011, the presenting author of the ATTRACT
study ( 15) therefore suggested that a suboptimal dosage of
the investigational drug could be responsible for the negative
outcome of the trial. Thirdly, due to the absence of valuable biomarkers for
antiangiogenic drugs ( 16), a proper selection of patients was
impossible. In the fourth place, another explanation supported by
prominent preclinical data suggested that anti-angiogenic drugs
might influence the biology of the disease. This was pointed out
by Ebos et Kerbel ( 12) stating that "Antiangiogenic therapies
might initiate an array of stromal and microenvironmental
defense mechanisms that contribute to eventual drug inefficacy
and, more provocatively, may lead to a more aggressive and
invasive tumor phenotype-one with an increased ability to
metastasize". Their considerations were based on previous
critical publications. In 2009, Ebos et al. ( 17) showed that
sunitinib/SU11248 can accelerate metastatic tumor growth and
decrease OS in mice receiving short-term therapy in various
metastasis assays. Interestingly, mice, receiving sunitinib prior
to intravenous implantation of tumor cells, also experienced
an acceleration of metastases, suggesting possible "metastatic
conditioning" by VEGFR inhibitors in various organs. At the
same time, Paez-Ribes et al. ( 18) observed increased numbers of metastases in distant organs after VEGF-pathway inhibition in a
mouse model. Despite an initial benefit, this mechanism could
lead to limited OS benefits.
|
|
Complexity of the biology of tumor angiogenesis
Angiogenesis is dependent on a complex network of different
cell compartments regulated by a balance of angiogenic and
anti-angiogenic factors, which is much more complicated
than described in the early days of the development of
anti-angiogenic therapies ( 6). In pathologic angiogenesis
the tumor cells themselves produce VEGF and other
angiogenic factors such as beta-fibroblast growth factor
(bFGF), angiopoietins, interleukin-8 and placental-derived
growth factor (PlGF), which leads to an overweight of proangiogenic
factors promoting the angiogenic switch. These
factors stimulate resident endothelial cells to proliferate,
loose cell interactions and migrate. An additional source
of angiogenic factors is the adjacent tumor stroma ,
which is a heterogeneous compartment, comprising of
fibroblastic, inflammator y and immune cells. Tumorassociated
fibroblasts produce chemokines such as stromal cell-derived factor-1 (SDF-1), which may recruit bonemarrow-
derived angiogenic cells (BMC) ( 19). Tumor cells
may also release stromal cell-recruitment factors, such as
PDGF-A, PDGF-C or transforming growth factor (TGF).
A well established function of tumor-associated fibroblasts
is the production of growth factors such as EGFR ligands,
hepatocyte growth factors and heregulin. Endothelial cells
produce PDGF-B, which promotes recruitment of pericytes
in the microvasculature after activation of PDGFR ( 20).
A crucial paper, discussing resistance to a VEGF inhibitor,
has been recently published and analyzes the influence
of the tumor stroma in the development of resistance
against anti-angiogenic therapies ( 21). They showed, that
in a bevacizumab resistant mouse model, multiple genes
(components of the EGFR and FGFR pathways) were upregulated,
and most of them occurred predominantly in
stromal and not in tumor cells. Similarly, Solinas et al. ( 22)
found that alterations of the endothelial microenvironment
(e.g. by chemotherapy or radiation) leads to an induction of
inflammatory mechanisms which increases the metastatic
potential. Others stressed the key role of mast cells ( 23)
which are involved in angiogenic switch, production of pro-angiogenic compounds and the induction of neovascularization.
These data support the special role of the
stromal tissue not only in promoting tumor angiogenesis but
also in the development of evasive resistance mechanisms
against therapies. From the clinical point it is well known
that tumors exposed to antiangiogenic therapies will mostly
become resistant thus leading to a re-growth of the tumor
(for review see Jubb AM et al. ( 24) and Bergers G et al.
( 25)). Various mechanisms are being discussed. On the one
hand, other angiogenic factors like bFGF or PDGF could
be up-regulated; on the other hand tumor vessels could be
protected by an increased coverage of pericytes. A third
option would be that tumor cells increase their invasiveness
by an accumulation of mutations ( 26). Finally, endothelial
progenitor cells, attracted from the bone marrow, could play a
role in inducing resistance ( 27). The most important trigger of the production of proangiogenic
factors is the induction of tumor hypoxia. The role
of hypoxia has already been elucidated by Carmeliet et al. ( 2).
Hypoxic tumor cells switch to a pro-angiogenic phenotype.
One key mediator in that regulation is the hypoxia-inducible
factor 1 (HIF-1), which is a hetero-dimeric protein that
activates the transcription of many genes that code for
proteins involved in angiogenesis, glucose metabolism,
cell proliferation/survival and invasion/metastasis ( 2, 28).
HIFs increase transcription of several angiogenic genes
(for example, genes encoding VEGF, PDGF and nitricoxide
species). HIFs also affect cellular survival/apoptosis
pathways. In that particular setting, the role of anti-angiogenic
therapies was investigated by M. Franco, showing that they
increase the hypoxic tumor fraction ( 29). After a threeweek
treatment period using DC101 (VEGFR2 monoclonal
antibody) the authors found a reduction in micro-vascular
density, blood flow and perfusion, but also an increase in
the hypoxic tumor fraction (measured with pimonidazole)
and an elevation in HIF-1A expression. Tumors can cope
with hypoxia by selection of hypoxia-tolerant clones and
more malignant metastatic cells, which are less sensitive
to antiangiogenic therapies ( 30, 31). Furthermore, tumor
cells might undergo an epithelial-mesenchymal transition to
escape hypoxic conditions ( 31).
|
|
Reconsider the clinical development of anti-angiogenic drugs
Facing the complexity of tumor-angiogenesis together with the
failure of phase III drug combination studies the traditional
pharmaceutical development strategies have to be reconsidered.
Planning of clinical trials evaluating anti-angiogenic drugs should
consider the following points.
-Choice and dose of combinational drugs matter ( 26, 32-34). Evidently, a combination of a platinum plus one of the
third generation cytostatics with one of the anti-angiogenic
kinase inhibitors does not seem to add any benefit. However,
monotherapy with for example sorafenib revealed efficacy in
single cases ( 10). We also learned that chemotherapies such as
cyclophosphamide, administered at maximum tolerated doses,
can mobilize circulating endothelial progenitor cells, which
could contribute to re-growth of the tumor ( 26, 27, 32). On
the other side metronomic therapy (closely spaced, less toxic
doses of chemotherapy) can prevent mobilization of circulating
endothelial progenitor cells ( 33, 34). -As a second point, the stage of the disease, in which the
clinical trial is performed, could be an essential question. It is
known that cancer can develop due to mechanisms evolved by
tumors to escape from surveillance of immune cells ( 35) and
that the immune defense mechanism are altered in late stage
diseases ( 36, 37). Still, the majority of preclinical studies with
anti-VEGF inhibitors were performed in early tumor stages
whereas the majority of clinical phase III trials were done
in advanced metastatic disease. Preclinical evaluations are
dominated by mouse models analyzing early tumor stages with
tumor response or progression as primary endpoints. In the
clinical setting, patients are treated in an advanced stage of the
disease and the primary end-point has to be overall survival ( 38).
These discrepant stages of disease evaluating different end-points
could be responsible for misleading interpretations of results.
Therefore, there is an absolute need to develop appropriate
cancer models for the development of anti-angiogenic drugs. -Thirdly, every trial using anti-angiogenic drugs should
include some kind of biomarker program. Since adequate in
vivo models are missing, the biological role of these substances
in humans has to be closely monitored ( 14, 16, 39). For
example, the MD Anderson group around John V. Heymach
( 39) performed an extensive hypothesis generating biomarker
program in 123 patients who were treated in a randomized
phase II trial evaluating vandetanib ( 40). A large number of
cytokines and angiogenic factors were evaluated at different days
of the treatment and were correlated with progression risk. For
example, plasma levels of VEGF increased and soluble VEGFR-2
decreased by day 43. Increase of VEGF was correlated with
an increased risk of progression. However, validation of such
biomarkers is warranted. -Finally, cigarette smoke induces oxidative/nitrosative
stress, which increases the nitration of tyrosine residues on
VEGFR2, rendering it inactive for downstream signaling. Active
smoking could be responsible for an endothelial dysfunction
( 41). Therefore, a stratification of smoking behavior should be
included. In conclusion anti-angiogenic therapies are already used
successfully in daily clinical practice. But there are still many questions to be answered about mode of action and optimal use
of anti-angiogenic drugs. Further scientific efforts are necessary
to analyze signal pathways and regulatory mechanisms which
could possibly help to identify new targets and biomarkers.
Special attention should be directed to the following points:
Pivotal role of hypoxia, modes of resistance/microenvironment,
need for optimal (mouse) models, role of cigarette smoke, choice
of chemotherapy combination and the stage of the disease,
which is evaluated.
|
|
Acknowledgement
This work was supported by the "Association of Cancer Research
-Innsbruck (Verein für Tumorforschung, Innsbruck)".
|
|
References
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med
1971;285:1182-6.[LinkOut]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature
2000;407:249-57.[LinkOut]
- Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous
vascular endothelial growth factor trap, aflibercept, in patients with
advanced solid tumors. J Clin Oncol 2010;28:207-14.[LinkOut]
- Scagliotti G, Govindan R. Targeting angiogenesis with multitargeted
tyrosine kinase inhibitors in the treatment of non-small cell lung cancer.
Oncologist 2010;15:436-46.[LinkOut]
- Auberger J, Loeffler-Ragg J, Wurzer W, et al. Targeted therapies in nonsmall
cell lung cancer: proven concepts and unfulfilled promises. Curr
Cancer Drug Targets 2006;6:271-94.[LinkOut]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature
2005;438:967-74.[LinkOut]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with
bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-
50.[LinkOut]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase
II trial comparing bevacizumab plus carboplatin and paclitaxel with
carboplatin and paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91.[LinkOut]
- Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatingemcitabine
and bevacizumab or placebo as first-line therapy for
nonsquamous non-small-cell lung cancer: results from a randomised phase
III trial (AVAiL). Ann Oncol 2010;21:1804-9.[LinkOut]
- Blumenschein GR Jr, Gatzemeier U, Fossella F, et al. Phase II, multicenter,
uncontrolled trial of single-agent sorafenib in patients with relapsed
or refractory, advanced non-small-cell lung cancer. J Clin Oncol
2009;27:4274-80.[LinkOut]
- Ulahannan SV, Brahmer JR. Antiangiogenic agents in combination with
chemotherapy in patients with advanced non-small cell lung cancer. Cancer
Invest 2011;29:325-37.[LinkOut]
- Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 2011;8:210-21.[LinkOut]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel
in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.[LinkOut]
- Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance
to antiangiogenic therapy. Nat Rev Clin Oncol 2009;6:327-38.[LinkOut]
- Lara PN Jr, Douillard JY, Nakagawa K, et al. Randomized phase III placebocontrolled
trial of carboplatin and paclitaxel with or without the vascular
disrupting agent vadimezan (ASA404) in advanced non-small-cell lung
cancer. J Clin Oncol 2011;29:2965-71.[LinkOut]
- Pircher A, Hilbe W, Heidegger I, et al. Biomarkers in tumor angiogenesis
and anti-angiogenic therapy. Int J Mol Sci 2011;12:7077-99.[LinkOut]
- Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after shortterm
treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell
2009;15:232-9.[LinkOut]
- Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits
malignant progression of tumors to increased local invasion and distant
metastasis. Cancer Cell 2009;15:220-31.[LinkOut]
- Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive
human breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell 2005;121:335-48.[LinkOut]
- Gaengel K, Genové G, Armulik A, et al. Endothelial-mural cell signaling
in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol
2009;29:630-8.[LinkOut]
- Cascone T, Herynk MH, Xu L, et al. Upregulated stromal EGFR and
vascular remodeling in mouse xenograft models of angiogenesis inhibitorresistant
human lung adenocarcinoma. J Clin Invest 2011;121:1313-28.[LinkOut]
- Solinas G, Marchesi F, Garlanda C, et al. Inflammation-mediated
promotion of invasion and metastasis. Cancer Metastasis Rev 2010;29:243-
8.[LinkOut]
- Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth:
angiogenesis, tissue remodelling and immune-modulation. Biochim
Biophys Acta 2009;1796:19-26.
- Jubb AM, Oates AJ, Holden S, et al. Predicting benefit from anti-angiogenic
agents in malignancy. Nat Rev Cancer 2006;6:626-35.[LinkOut]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat
Rev Cancer 2008;8:592-603.[LinkOut]
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039-49.[LinkOut]
- Resch T, Pircher A, Kähler CM, et al. Endothelial Progenitor Cells: Current
Issues on Characterization and Challenging Clinical Applications. Stem
Cell Rev 2011. [Epub ahead of print]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of
angiogenesis. Cell 2011;146:873-87.[LinkOut]
- Franco M, Man S, Chen L, et al. Targeted anti-vascular endothelial growth
factor receptor-2 therapy leads to short-term and long-term impairment
of vascular function and increase in tumor hypoxia. Cancer Res
2006;66:3639-48.[LinkOut]
- Yu JL, Rak JW, Coomber BL, et al. Effect of p53 status on tumor response
to antiangiogenic therapy. Science 2002;295:1526-8.[LinkOut]
- Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J Mol Med
(Berl) 2007;85:1301-7.[LinkOut]
- Bertolini F, Paul S, Mancuso P, et al. Maximum tolerable dose and low dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 2003;63:4342-6.
- Shaked Y, Emmenegger U, Man S, et al. Optimal biologic dose of
metronomic chemotherapy regimens is associated with maximum
antiangiogenic activity. Blood 2005;106:3058-61.[LinkOut]
- Belotti D, Vergani V, Drudis T, et al. The microtubule-affecting drug
paclitaxel has antiangiogenic activity. Clin Cancer Res 1996;2:1843-9.
- Griffioen AW. Anti-angiogenesis: making the tumor vulnerable to the
immune system. Cancer Immunol Immunother 2008;57:1553-8.[LinkOut]
- Fridman WH, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in
human cancer: prognostic significance and disease control. Curr Top
Microbiol Immunol 2011;344:1-24.[LinkOut]
- Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer
metastasis: historical perspective. Cancer Res 2010;70:5649-69.[LinkOut]
- Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal
benefits: are we making too much of too little or are we achieving too little
by giving too much? Clin Cancer Res 2010;16:5972-80.[LinkOut]
- Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and
angiogenic factor modulation and markers of benefit for vandetanib and/
or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol
2010;28:193-201.[LinkOut]
- Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of
vandetanib alone or with paclitaxel and carboplatin as first-line treatment
for advanced non-small-cell lung cancer. J Clin Oncol 2008 ;26:5407-15.[LinkOut]
- Edirisinghe I, Rahman I. Cigarette smoke-mediated oxidative stress, shear
stress, and endothelial dysfunction: role of VEGFR2. Ann N Y Acad Sci
2010;1203:66-72.[LinkOut]
- Novello S, Ramlau R, Gorbunova VA, et al. Aflibercept in combination with
docetaxel for second-line treatment of locally advanced or metastatic nonsmall-
cell lung cancer (NSCLC): Final results of a multinational placebocontrolled phase III trial (EFC10261-VITAL). 14th World Conference of
Lung Cancer 2011, O43.06. Available online: http://abstracts.webges.com[LinkOut]
- Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial
of carboplatin and paclitaxel with either daily oral cediranib or placebo
in advanced non-small-cell lung cancer: NCIC clinical trials group BR24
study. J Clin Oncol 2010;28:49-55.[LinkOut]
- Scagliotti G, Vynnychenko I, Ichinose Y, et al. An international,
randomized, placebo-controlled, double-blind phase III study (MONET1)
of motesanib plus carboplatin/paclitaxel (C/P) in patients with advanced
nonsquamous non-small cell lung cancer (NSCLC) [abstract]. J Clin
Oncol 2011;29:s7512.
- Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and
paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J
Clin Oncol 2010;28:1835-42.[LinkOut]
- Gatzemeier U, Eisen T, Santoro A., et al. Sorafenib (S)/ Gemcitabine/
Cisplatin (GC) vs GC alone in the first-line treatment of advanced nonsmall
cell lung cancer (NSCLC): phase III NSCLC research experience
utilizing Sorafenib (NEXUS) trial. Ann Oncol 2010;21:viii7(LBA 16).
- Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus
docetaxel as second-line treatment for patients with advanced non-smallcell
lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial.
Lancet Oncol 2010;11:619-26.[LinkOut]
- De Boer R, Arrieta Ó, Gottfried M, et al. Vandetanib plus pemetrexed
versus pemetrexed as second-line therapy in patients with advanced nonsmall
cell lung cancer (NSCLC): A randomized, double-blind phase III
trial (ZEAL) [abstract]. J Clin Oncol 2009;27:s8010.
- Lee J, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with
advanced non-small cell lung cancer (NSCLC) after prior therapy with an
EGFR tyrosine kinase inhibitor (TKI): A randomized, double-blind phase
III trial (ZEPHYR) [abstract]. J Clin Oncol 2010;28:s7525.
|