Lung cancer molecular epidemiology in China: recent trends
Introduction
Lung cancer is a major public health problem worldwide (1). Nevertheless, in contrast to most Western countries, where lung cancer death rates are decreasing, lung cancer incidence rate is still increasing in China (1,2) and lung cancer is still the most common diagnosed cancer and the leading cause of cancer related deaths in China (3) (Table 1).
The opposite trend of lung cancer incidence rate in China compared with that in Western countries might mainly be attributable to tobacco epidemic (4,5). As in Western countries, the tobacco epidemic peaked by the middle of the last century, while in China, the smoking prevalence had reached its peak recently in Chinese men and showed signs of stability in Chinese women (6-8). Other identified risk factors for lung cancer in China include air pollution [environmental or occupational exposure to agents such as asbestos, nickel, chromium, and arsenic (9), indoor air pollution from unventilated coal-fueled stoves and cooking fumes, especially for Chinese women (10-12)], chronic pulmonary disease [such as pulmonary tuberculosis, chronic bronchitis, and emphysema (13-16)], and several genetic variants (17-23).
Non-small-cell lung cancer (NSCLC) comprises the most common form of lung cancer and the majority of these patients are at advanced stage when diagnosed and small cell lung cancer (SCLC) comprises small part of total lung cancer cases (24). For SCLC patients, chemotherapy alone or combined with radiation therapy is the standard treatment. For patients with early stage NSCLC, surgery is the preferable option. However, for the majority lung cancer patients with advanced NSCLC, there was still no satisfactory and effective treatment options to date. Palliative chemotherapy was once to be the only standard treatment but with limited efficacy. Encouragingly, recent advances in targeted therapy for a subgroup of molecularly defined NSCLC (patients harboring ‘driver genes’) provided promising alternative approaches for NSCLC patients (25-28).
Nowadays, cancer related public health concern and the economic burden of cancer are getting serious in China. In this review, we summarized current data about the incidence and mortality of lung cancer to increase public attention, and particularly, the detailed epidemiology of driver genes in NSCLC to help clinicians to better screen certain driver genes in China for treatment decisions.
Epidemiology of lung cancer in China
Data source
Population-based cancer registration data nationwide in China has been established in recent few years. National Central Cancer Registry (NCCR) is responsible for collecting, evaluating and analyzing cancer incidence and mortality data from cancer registries in China. In 2010, there were totally 219 submitted cancer registries. After checking and evaluating based on relevant data quality criterion and “Guideline for Chinese Cancer Registration” by NCCR, there were till qualified 145 cancer registries for cancer statistics in 2010, including 28 provinces, autonomous regions or municipalities, 63 cities and 82 counties covered 158,403,248 persons (11.86% of whole national population) (3).
Incidence
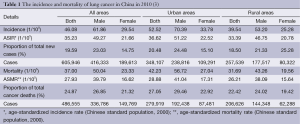
Lung cancer was the most common cancer in males and the second most common cancer in females (breast cancer was the most common cancers in females) in China. Compared with the data in 2008 (29), the number of new diagnosed cases increased 16% (522,050 new cases with 33.5 per 100,000 population in 2008). At the same time, compared with European countries such as Germany and Italy, lung cancer rates of Chinese females were also higher (21.3 cases per 100,000 females vs. 16.4 vs. 11.4, respectively) (1). In 2010, the crude incidence of lung cancer was 46.08 per 100,000 population (61.86 per 100,000 men and 29.54 per 100,000 women). There were 605,946 total new diagnosed lung cancer patients in 2010, including 416,333 males and 189,613 females (3).
Lung cancer was the most frequently diagnosed cancer in both urban and rural and the crude incidences of lung cancer in males and females were higher in urban than that in rural. In urban, the crude incidence of lung cancer was 52.52 per 100,000 population with 348,107 total new cases (70.39 per 100,000 men with 238,816 new men cases and 33.78 per 100,000 women with 109,291 new women cases). In rural, the crude incidence of lung cancer was 39.54 per 100,000 population with 257,839 total new cases (53.20 per 100,000 men with 177,517 new men cases and 25.23 per 100,000 women with 80,322 new women cases) (3).
Mortality
Lung cancer was also the leading cause of cancer deaths in both males and females in China. In 2010, the crude mortality of lung cancer was 37.00 per 100,000 population (50.04 per 100,000 men and 23.33 per 100,000 women). There were total 498,555 estimated deaths from lung cancer in 2010, including 336,786 males and 149,769 females (3).
Lung cancer was the leading cause of cancer death in urban and rural for both males and females and the crude mortality of lung cancer in males and females were higher in urban than that in rural. In urban, the crude mortality of lung cancer was 42.23 per 100,000 population with 279,919 total estimated deaths from lung cancer (56.72 per 100,000 men with 192,438 estimated men deaths from lung cancer and 27.04 per 100,000 women with 87,481 estimated women deaths from lung cancer). In rural, the crude mortality of lung cancer was 31.69 per 100,000 population with 206,636 total estimated deaths from lung cancer (43.36 per 100,000 men with 144,348 estimated men deaths from lung cancer and 19.56 per 100,000 women with 62,288 estimated women deaths from lung cancer) (3).
Based on third National Retrospect Spot-check of Death-Causation, Lung cancer has replaced liver cancer as leading cause of cancer death in China (accounting for 22.7% of total cancer related deaths) (30). With the development of modern medicine, mortality of certain cancer such as esophageal, stomach, and cervical cancers have steadily declined. However, the prognosis of lung cancer is still very poor with a 5-year survival rate lower than 20%. Moreover, the 5-year morbidity rate (an indicator to evaluate therapeutic efficacy) of lung cancer in China was 45.6 per 100,000 population in 2008, which was higher than world average level (34.1) but was significantly lower than the world average level of developed countries (86.4), including other East-Asian countries such as Japan (146.2) and South Korea (59.3) (31). According to population-based analysis, the 5-year morbidity rate of lung cancer was also lower than breast cancer, cervical cancer, colon-rectal cancer, and gastric cancer in China (29).
Clinical characteristics of lung cancer patients in China
Smoking prevalence
Scientific evidence clearly shows that tobacco exposure is significantly associated with the cause of lung cancer and among Chinese lung cancer patients, about 60-70% are ever-smokers. To date, More than 50% of males in many Asian countries are now smokers, about twice the prevalence in many Western countries. Notably, female-smokers are continuously increasing in some Asian countries (32,33). Not surprisingly, the increasing incidence of lung cancer in China is consistent with the increasing prevalence of smoking in China. In 1984, there were approximately 250 million smokers in China, with a prevalence of 61% in men and 7% in women (34). In 2002, the number of smokers in China had increased to approximately 350 million (33). Importantly, the average number of cigarettes smoked per day by men, smoking rates in the young population and females are also increasing rapidly (35). However, the tobacco knowledge among Chinese is relatively poor [e.g., less than one third of the residents of Zhejiang province know that smoking could cause all three diseases (stroke, heart disease, and lung cancer)] (36). It also should be carefully noted that smoking is also popular in Chinese physicians, with an overall prevalence of 23% in all Chinese physicians, 41% for male physicians and 1% for female physicians (37). Furthermore, in spite of the efforts and progress with respect to tobacco control by government (e.g., supply reduction, increased tobacco taxation, decreased second-hand smoking and cessation support) (34), there are still a lot of barriers to tobacco control in China. Thus, more effective advising techniques and education for smoking cessation, including physicians, multiple valid strategies and solutions for tobacco control by government are urgently needed in China.
Age and gender
With the development of modern medicine and the standard of living, the average age of people is increasing rapidly. Generally, both men and women are more likely to get lung cancer with increasing age. According to the data from NCCR (38), during 1989-2008, the average age of lung cancer incidence among male and female dramatically increased from 65.32 and 65.14 to 67.87 and 68.05 years old, respectively. A similar trend of increasing age was found in both urban and rural. In urban, the average age of lung cancer incidence among male and female dramatically increased from 65.51 and 65.34 to 68.19 and 68.34 years old, respectively. Meanwhile, in rural, the average age dramatically increased from 63.07 and 62.63 to 66.40 and 66.34 years old, respectively. In addition, there is a trend of increasing incidence of lung cancer in young patients (younger than 45 years) in Shanghai (39).
Although the incidence of lung cancer of both males and females increased during the past decades, the incidence rate ratios of lung cancer between male and females were significantly decreased from 2.47 to 2.28 during 1989-2008, especially in urban, where incidence rate ratios significantly decreased from 2.45 to 2.21 (38), because the incidence of lung cancer in females increased more rapidly than that in males in China.
Histological type
Adenocarcinoma has replaced squamous cell carcinoma (SQCC) as the most predominant histological subtype of lung cancer in China at present, which is consistent with the change in developed countries (40-42). From 1998 to 2007, the proportion of adenocarcinoma increased from 42.83% to 46.80% while the proportion of SQCC decreased from 30.41% to 24.16% in Beijing (43). A similar trend of the change was observed in Dalian and Shenyang, Liaoning province, China (44,45). A more recently study covering different geographic areas of China further confirmed these findings. In this study, during 2002-2012, the relative frequencies of adenocarcinoma increased from 21.96% to 43.36% and the relative frequencies of SQCC decreased from 39.11% to 32.23% in 15, 427 male lung cancer patients (46).
The changed smoking prevalence in Chinese males and females (7,8), the application of “light” and “low-tar” and filter cigarette (47-49) may partially attribute to the changed histological subtypes and gender proportion in Chinese lung cancer patients. Other identified etiologic factors related to the development of lung cancer in never smokers, of which the most common subtype was adenocarcinoma, including environmental tobacco smoke, exposure to cooking fumes, inherited genetic susceptibility, occupational and environmental exposure (exposure to radon, cooking fumes, asbestos, heavy metals), hormonal factors, and infectious factors (50,51), may also result in the changed histological subtypes.
Molecular epidemiology of Chinese NSCLC patients
More importantly, the changed histological subtypes further accompanied with a process in the development of treatment, in which targeted therapy for patients with adenocarcinoma plays a more and more important role. However, targeted therapy was not like chemotherapy, a “one fits all” option, clinicians had to detect relevant driver genes before the application of targeted drugs. Because of the shortage of tumor tissue in advanced NSCLC and the cost-effectiveness of testing methods in China (not every patient could afford the cost of testing in a developing country), to better understand the molecular epidemiology [such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) gene translocations, ROS1, and RET gene fusions etc.] in Chinese population and ascertain enriched population is of significant clinical value.
Epidermal growth factor receptor (EGFR)
EGFR signaling pathway has emerged as one of the most important molecular aberrations in NSCLC and EGFR tyrosine kinase inhibitors (EGFR-TKIs) have been widely used in patients with positive EGFR mutations. Previous studies have demonstrated patients who were female, never-smoker, adenocarcinoma or East Asian ethnicity were more likely to harbor these mutations (25). Moreover, He et al. recently found that patients with family history of lung cancer were significantly associated higher incidence of positive EGFR mutations in Chinese population (52).
Overall, in unselected NSCLC Chinese population, the mutation rate of EGFR was about 28.4% (147/517) (53). In patients with adenocarcinoma, the EGFR mutations were very popular with the mutation rate ranging from 40.3% to 64.5% (53-57). Notably, in clinical enriched Chinese population (i.e., patients who were never-smoker and adenocarcinoma), approximately 75% of certain patients were found to harbor EGFR mutations (range from 49.8% to 78.8%) (18,53,55,58,59). In smokers with adenocarcinoma, the rate of EGFR mutation was only 22.0% to 43.5% (53,60). Even in Chinese population (East-Asian population), EGFR mutations were rare in SQCC (8.0% for non-smokers with SQCC and 2.1% for smokers with SQCC) (53). Moreover, Pan et al. recently reported that there was no EGFR mutation in 215 NSCLC patients with pure SCC and only 10.5% of patients with SQCC with minor (61). Thus, EGFR mutation analysis should be routinely detected in adenocarcinoma in Chinese population. While, it is not recommended in routine detection in SQCC patients, except for patients who were non-smokers or female, which suggests the tumors of these patients were likely to have adenocarcinoma component.
Gene fusions
ALK rearrangements define another unique molecular subtype of NSCLC and provide a novel molecular target in NSCLC targeted therapy. Among various ALK fusions, the echinoderm microtubule associated protein-like 4 (EML4)-ALK (EML4-ALK) fusion transcript was the most common type (62). Similar to several clinical features that were associated with EGFR mutations, never/light smokers with adenocarcinoma were more likely to harbor EML4-ALK fusion. But patients with ALK fusions were younger, without ethnic specific and mutually exclusive from other known driver genes (63,64). Previous studies have reported a low frequency of ALK fusions that has ranged from 1.5% to 6.7% in unselected populations (65-67). Similarly, Pan et al. reported that, in 1,139 Chinese adenocarcinomas, the incidence rate of ALK fusions was 5.1% (58/1,139) (54). In other relatively small sample studies, the incidence rate of ALK fusions ranged from 5.8% (3/52) to 10% (11/110) (18,53,55,57,68). Notably, in non-smoker patients with adenocarcinoma, approximately 10% of these patients were found to harbor ALK fusions [9.6% (10/104) and 9.3% (8/86)] (53,59]. Furthermore, Shaw et al. reported that in never/light smokers without EGFR mutations, the frequency of EML4-ALK was as high as 33% (63), although the majority patients in this study population were non-Asian ethnicity.
ROS1, which was involved in chromosomal translocations in lung cancer, was recently identified as a novel driver gene in NSCLC (69). Similar to the clinical characteristics observed in ALK-positive NSCLC patients, Shaw et al. found that never smokers with a histologic diagnosis of adenocarcinoma tended to harbor ROS1 fusions (70). However, ROS1 fusions didn’t seem to be exclusive in adenocarcinoma. Notably, Davies et al. identified ROS1 fusions in SQCC (71) and a ROS1 fusion also was discovered in adeno-squamous carcinoma in our previous study (72). Thus, the distinct clinical characteristics of ROS1 fusions-positive patients remain elusive. Generally, the frequency rate of ROS1 fusions was very low in NSCLC, even in Chinese patients, with a prevalence ranging from 1% to 2% (54,58,72,73).
RET gene rearrangement has been demonstrated to be another novel oncogenic driver in a subset of lung adenocarcinomas (73,74). Wang et al. found that patients harboring RET fusions tended to be young never-smokers with early lymph node metastases, poor differentiation, and a solid-predominant subtype (75). Consistently, the identified RET fusions-positive patients in our previous study were all never-smokers (76). Screening results of RET fusions showed that the prevalence of RET fusions was less than 2% in unselected Chinese NSCLC patients, ranging from 1.3% to 1.9% (54,73,75,76). It should be noted that RET fusions didn’t exist exclusively only in adenocarcinoma, but also in adenosquamous (75) and SQCC (76). Furthermore, RET fusions were a little more common in patients with adenocarcinoma than in those with SQCC (1.3%, 1.9%, 1.7%, and 1.73% in adenocarcinoma, respectively vs. 0.84% in SQCC) (54,73,75,76).
The c-MET gene
The c-MET gene amplification was not only a potential novel driver gene in NSCLC but also an important mechanism for both primary and acquired resistance to EGFR-TKIs in NSCLC. The overexpression of c-MET, the only known receptor for hepatocyte growth factor (HGF), has been demonstrated to be correlated with poor clinical outcome (77). Previous studies showed that approximately 20% patients with acquired resistance had c-MET amplification (78). In Japanese population, the prevalence of primary MET amplification in NSCLC adenocarcinoma ranged from 4% to 5% (79,80). In Chinese NSCLC patients, c-MET amplification was detected in 4.5% (20/448) of patients and the frequency of c-MET amplification was little higher in adenocarcinoma (5.5%, 6/110) (53,55).
BRAF
BRAF is a member of the RAF kinase family. In NSCLC, BRAF mutations are mutually exclusive to EGFR and KRAS mutations, and are found in 1-3% of tumors, most of which are adenocarcinoma (81,82). In a large retrospective analysis of BRAF mutations in Caucasian NSCLC patients, BRAF mutations were identified in 4.9% (36/739) of lung adenocarcinoma and in 0.3% (1/307) of SQCC, more specifically, the presence of V600E BRAF mutations was approximately 9% in female adenocarcinoma and all non-V600E mutations that were detected in adenocarcinomas were found in smokers (83). In Chinese lung adenocarcinoma patients, BRAF mutations were only found in 1.2% (14/1,139) of 1,139 adenocarcinomas (54), which is less common than Caucasian population. Other studies consistently revealed that BRAF mutations were very rare in Chinese population, with a prevalence ranging from 1.5% to 3.0% (53,60,84).
HER2
HER2 is a member of the EGFR family of receptor tyrosine kinases, which also includes EGFR (HER1), HER3 and HER4. Disappointingly, contrast to the successful use of trastuzumab (a monoclonal antibody against HER2) in overexpression/amplification of HER2 breast cancer, HER2-targeted agents, such as trastuzumab and pertuzumab, failed to demonstrate clinical benefit in NSCLC with HER2 overexpression. Interestingly, several case reports and small sample-sized studies showed promising efficacy of HER2 inhibitors in HER2-mutant NSCLC patients. However, the frequency of HER2 mutations are very low (1.6%, 11 of 671) in lung cancer, more frequently in those who are never smokers, adenocarcinoma and female (85-87). In Chinese population, the prevalence of HER2 mutations ranged from 2.4% to 5.94% (54,58,73,84), a little more common than Caucasian population.
Fibroblast growth factor receptor (FGFR)
As mentioned above, the majority of genomic analysis was mainly focused on adenocarcinoma and identification of therapeutic targets for SQCC had lagged behind lung adenocarcinoma. Recently, FGFR has been demonstrated to be a druggable target in SQCC and several multikinase and selective FGFR inhibitors (e.g., ponatinib, sorafenib, dovitinib, BGJ398 and AZD4547, etc.) are being evaluated in clinical trials. Weiss et al. found that focal FGFR1 amplification was associated with response to treatment with small molecule FGFR TKIs with a presence of approximately 20% in SQCC but not in other lung cancer subtypes (88). FGFR gene fusions, which could exhibit oligomerization capability, resulting in FGFR TK activation sensitive to FGFR TKIs (89), were also described in NSCLC. Wang et al. more recently identified FGFR fusions in Chinese NSCLC and found that 11 of 312 (3.5%) SQCC harbored FGFR fusions (90). Notably, six of 1,016 (0.6%) patients with lung adenocarcinoma were also identified with FGFR fusions. Further analysis showed that patients who were smokers, with larger tumor (>3 cm), and poorly differentiation were more likely to harbor FGFR fusions.
Discoidin domain receptor-2 (DDR2)
The DDR2 is a receptor TK (RTK), which acts as collagen receptor, resulting cell migration, proliferation and survival (91). Preliminary study revealed that DDR2 mutations may be potential oncogenic driver in SQCC with a presence of 3.8% of SQCC and tumors harboring DDR2 mutations were sensitive to the multitarget kinase inhibitor dasatinib, which also target DDR1 and DDR2 (92). Consistently, DDR2 mutations were only identified in SQCC in Chinese population (53) and similarly, approximately 3.3% to 4.6% SQCC patients harbored DDR2 mutations (53,93). However, in pure SQCC, the incidence of DDR2 mutations was very low, with a presence of only 0.5% (1/215) (61).
Conclusions and future perspective
To date, for the majority advanced NSCLC patients, targeted therapy plays a more and more important role in the treatment. A lot of efforts have been made by Chinese researchers with respect to lung cancer molecular epidemiology. As EGFR mutations are the most common driver gene and EGFR-TKIs have been widely used in patients with activating EGFR mutations, EGFR mutation analysis should be conducted first in Chinese patients. If patients were in absence of an EGFR mutation, then should be screened for ALK, ROS1, RET fusions and c-MET amplification in Chinese patients. Although the frequencies of certain driver genes are quite low, however, with an estimated 600,000 new cases of lung cancer per year in China, there are still quite a lot patients could derive clinical benefits from corresponding targeted therapy. Crizotinib, an ALK/ROS1/MET inhibitor, which has been approved as first-line therapy for ALK-positive advanced NSCLC and also showed activity in ROS1-rearranged and c-MET amplification NSCLC patients, highlights the development of “precise medicine” in NSCLC. Moreover, several multi-targeted kinase inhibitors that inhibit RET, BRAF, FGFR, and DDR2, such as vandetanib, sorafenib, dovitinib, vemurafenib, and sunitinib etc., have showed anti-tumor activity in preclinical studies. Moreover, clinical trials evaluating the efficacies of these drugs in NSCLC patients harboring certain oncogenic drivers are ongoing. The further mature data may provide rationale to screen certain driver genes in the clinical practice in the future. Hence, to better understand lung cancer molecular epidemiology is becoming increasing important at present.
In the near future, with the development of advanced biotechniques, such as high-throughput multiplex genotyping, it is feasible to simultaneously sequence and measure copy numbers of multiple genes from only nanograms of cancer cell DNA. Therefore, we may not be entangled in deciding which driver gene to detect.
Overall, as mentioned above, the incidence and mortality of lung cancer in China are increasing rapidly over the past 30 years. We are aware of more effective interventions, such as control smoking prevalence and air pollution by Chinese government, screening for lung cancer and conducting standardized clinical management protocols by Chinese physicians, are still urgently needed to reduce the incidence and improve the survival of Chinese lung cancer patients. The time is now.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117-26. [PubMed]
- Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014;26:48-58. [PubMed]
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [PubMed]
- Lam WK, White NW, Chan-Yeung MM. Lung cancer epidemiology and risk factors in Asia and Africa. Int J Tuberc Lung Dis 2004;8:1045-57. [PubMed]
- Yang GH, Ma J, Liu N, et al. Smoking and passive smoking in Chinese, 2002. Zhonghua Liu Xing Bing Xue Za Zhi 2005;26:77-83. [PubMed]
- Anderson Johnson C, Palmer PH, Chou CP, et al. Tobacco use among youth and adults in Mainland China: the China Seven Cities Study. Public Health 2006;120:1156-69. [PubMed]
- Wang DM, Chen BJ, Li WM, et al. Risk factors on lung cancer: a meta-analysis. Chin J Evid-Based Med 2010;10:1446-9.
- WHO. Quantification of the Disease Burden Attributable to Environmental Risk Factors. China Country Profile. Geneva, Switzerland: World Health Organization, 2009.
- Wang DM, Chen BJ, Li WM, et al. Risk factors on lung cancer: a meta-analysis. Chin J Evid-Based Med 2010;10:1446-9.
- Zhao Y, Wang S, Aunan K, et al. Air pollution and lung cancer risks in China--a meta-analysis. Sci Total Environ 2006;366:500-13. [PubMed]
- Kim C, Gao YT, Xiang YB, et al. Home kitchen ventilation, cooking fuels, and lung cancer risk in a prospective cohort of never smoking women in Shanghai, China. Int J Cancer 2014. [Epub ahead of print]. [PubMed]
- Brenner AV, Wang Z, Kleinerman RA, et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int J Epidemiol 2001;30:118-24. [PubMed]
- Wang H, Yang L, Zou L, et al. Association between chronic obstructive pulmonary disease and lung cancer: a case-control study in Southern Chinese and a meta-analysis. PLoS One 2012;7:e46144. [PubMed]
- Wang ZL. Association between chronic obstructive pulmonary disease and lung cancer: the missing link. Chin Med J (Engl) 2013;126:154-65. [PubMed]
- Hosgoodiii HD, Chapman RS, He X, et al. History of lung disease and risk of lung cancer in a population with high household fuel combustion exposures in rural China. Lung Cancer 2013;81:343-6. [PubMed]
- Hu Z, Wu C, Shi Y, et al. A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet 2011;43:792-6. [PubMed]
- Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20. [PubMed]
- Shen L, Yin Z, Wu W, et al. Single nucleotide polymorphism in ATM gene, cooking oil fumes and lung adenocarcinoma susceptibility in Chinese female non-smokers: a case-control study. PLoS One 2014;9:e96911. [PubMed]
- Ouyang FD, Yang FL, Chen HC, et al. Polymorphisms of DNA repair genes XPD, XRCC1, and OGG1, and lung adenocarcinoma susceptibility in Chinese population. Tumour Biol 2013;34:2843-8. [PubMed]
- Guo S, Li X, Gao M, et al. The relationship between XRCC1 and XRCC3 gene polymorphisms and lung cancer risk in northeastern Chinese. PLoS One 2013;8:e56213. [PubMed]
- Hsia TC, Liu CJ, Chu CC, et al. Association of DNA double-strand break gene XRCC6 genotypes and lung cancer in Taiwan. Anticancer Res 2012;32:1015-20. [PubMed]
- Yin J, Vogel U, Ma Y, et al. HapMap-based study of a region encompassing ERCC1 and ERCC2 related to lung cancer susceptibility in a Chinese population. Mutat Res 2011;713:1-7. [PubMed]
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220-41. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Chang S, Ren M, Ren J, et al. Estimates and prediction on incidence, mortality and prevalence of lung cancer in China in 2008. Zhonghua Liu Xing Bing Xue Za Zhi 2012;33:391-4. [PubMed]
- The Ministry of Health of the People’s Republic of China. Third National Retrospect Spot-check of Death-Causation. Beijing, China: Peking Union Medical College Press, 2008:22.
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Giovino GA, Mirza SA, Samet JM, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 2012;380:668-79. [PubMed]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [PubMed]
- Zhang J, Ou JX, Bai CX. Tobacco smoking in China: prevalence, disease burden, challenges and future strategies. Respirology 2011;16:1165-72. [PubMed]
- Yang G, Fan L, Tan J, et al. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA 1999;282:1247-53. [PubMed]
- Xu Y, Xu S, Wu Q, et al. Tobacco knowledge among adults in Zhejiang Province, China. PLoS One 2013;8:e59172. [PubMed]
- Jiang Y, Ong MK, Tong EK, et al. Chinese physicians and their smoking knowledge, attitudes, and practices. Am J Prev Med 2007;33:15-22. [PubMed]
- Han R, Zheng RS, Zhang SW, et al. Trend Analyses on the Differences of Lung Cancer Incidence Between Gender, Area and Average Age in China During 1989-2008. Zhongguo Fei Ai Za Zhi 2013;16:445-51. [PubMed]
- Zhang J, Chen SF, Zhen Y, et al. Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer 2010;116:3656-62. [PubMed]
- Toyoda Y, Nakayama T, Ioka A, et al. Trends in lung cancer incidence by histological type in Osaka, Japan. Jpn J Clin Oncol 2008;38:534-9. [PubMed]
- Thun MJ, Lally CA, Flannery JT, et al. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst 1997;89:1580-6. [PubMed]
- Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005;117:294-9. [PubMed]
- Wang N, Chen WQ, Zhu WX, et al. Incidence trends and pathological characteristics of lung cancer in urban Beijing during period of 1998-2007. Zhonghua Yu Fang Yi Xue Za Zhi 2011;45:249-54. [PubMed]
- Zhang LM. An analysis of epidemic trends of lung cancer from 1991 to 2005 in Dalian city, Liaoning province. China Cancer 2008;17:84-7.
- Jia XS, Zhang DR, Wang EH, et al. The clinical comparison between lung cancer in 80’, 20th century and that in 90’ in 1224 cases of shenyang province. Chin J Pathol 2001;30:332-35.
- Zou XN, Lin DM, Wan X, et al. Histological subtypes of lung cancer in Chinese males from 2000 to 2012. Biomed Environ Sci 2014;27:3-9. [PubMed]
- Tindle HA, Rigotti NA, Davis RB, et al. Cessation among smokers of “light” cigarettes: results from the 2000 national health interview survey. Am J Public Health 2006;96:1498-504. [PubMed]
- Stellman SD, Muscat JE, Thompson S, et al. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer 1997;80:382-8. [PubMed]
- Marugame T, Sobue T, Nakayama T, et al. Filter cigarette smoking and lung cancer risk; a hospital-based case--control study in Japan. Br J Cancer 2004;90:646-51. [PubMed]
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol 2007;25:561-70. [PubMed]
- Couraud S, Zalcman G, Milleron B, et al. Lung cancer in never smokers--a review. Eur J Cancer 2012;48:1299-311. [PubMed]
- He Y, Li S, Ren S, et al. Impact of family history of cancer on the incidence of mutation in epidermal growth factor receptor gene in non-small cell lung cancer patients. Lung Cancer 2013;81:162-6. [PubMed]
- An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One 2012;7:e40109. [PubMed]
- Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 2014;84:121-6. [PubMed]
- Xia N, An J, Jiang QQ, et al. Analysis of EGFR, EML4-ALK, KRAS, and c-MET mutations in Chinese lung adenocarcinoma patients. Exp Lung Res 2013;39:328-35. [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [PubMed]
- Wang J, Dong Y, Cai Y, et al. Clinicopathologic characteristics of ALK rearrangements in primary lung adenocarcinoma with identified EGFR and KRAS status. J Cancer Res Clin Oncol 2014;140:453-60. [PubMed]
- Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 2011;6:e28204. [PubMed]
- Ren S, Kuang P, Zheng L, et al. Analysis of driver mutations in female non-smoker Asian patients with pulmonary adenocarcinoma. Cell Biochem Biophys 2012;64:155-60. [PubMed]
- Li H, Pan Y, Li Y, et al. Frequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking dose. Lung Cancer 2013;79:8-13. [PubMed]
- Pan Y, Wang R, Ye T, et al. Comprehensive analysis of oncogenic mutations in lung squamous cell carcinoma with minor glandular component. Chest 2014;145:473-9. [PubMed]
- Ou SH, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 2012;17:1351-75. [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Fan L, Feng Y, Wan H, et al. Clinicopathological and Demographical Characteristics of Non-Small Cell Lung Cancer Patients with ALK Rearrangements: A Systematic Review and Meta-Analysis. PLoS One 2014;9:e100866. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia 2008;10:298-302. [PubMed]
- Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. [PubMed]
- Wang Z, Zhang X, Bai H, et al. EML4-ALK rearrangement and its clinical significance in Chinese patients with advanced non-small cell lung cancer. Oncology 2012;83:248-56. [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [PubMed]
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [PubMed]
- Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res 2012;18:4570-9. [PubMed]
- Cai W, Li X, Su C, et al. ROS1 fusions in Chinese patients with non-small-cell lung cancer. Ann Oncol 2013;24:1822-7. [PubMed]
- Li F, Feng Y, Fang R, et al. Identification of RET gene fusion by exon array analyses in “pan-negative” lung cancer from never smokers. Cell Res 2012;22:928-31. [PubMed]
- Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375-7. [PubMed]
- Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352-9. [PubMed]
- Cai W, Su C, Li X, et al. KIF5B-RET fusions in Chinese patients with non-small cell lung cancer. Cancer 2013;119:1486-94. [PubMed]
- Park S, Choi YL, Sung CO, et al. High MET copy number and MET overexpression: poor outcome in non-small cell lung cancer patients. Histol Histopathol 2012;27:197-207. [PubMed]
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7. [PubMed]
- Onitsuka T, Uramoto H, Ono K, et al. Comprehensive molecular analyses of lung adenocarcinoma with regard to the epidermal growth factor receptor, K-ras, MET, and hepatocyte growth factor status. J Thorac Oncol 2010;5:591-6. [PubMed]
- Onozato R, Kosaka T, Kuwano H, et al. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol 2009;4:5-11. [PubMed]
- Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 2002;62:6997-7000. [PubMed]
- Naoki K, Chen TH, Richards WG, et al. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res 2002;62:7001-3. [PubMed]
- Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011;29:3574-9. [PubMed]
- Fang R, Zheng C, Sun Y, et al. Integrative genomic analysis reveals a high frequency of LKB1 genetic alteration in Chinese lung adenocarcinomas. J Thorac Oncol 2014;9:254-8. [PubMed]
- Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005;65:1642-6. [PubMed]
- De Grève J, Teugels E, Geers C, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 2012;76:123-7. [PubMed]
- Mazières J, Peters S, Cortot A, et al. Lung cancer harboring HER2 mutation: epidemiological characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [PubMed]
- Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010;2:62ra93. [PubMed]
- Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov 2013;3:636-47. [PubMed]
- Wang R, Wang L, Li Y, et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of Non-small Cell Lung Cancer. Clin Cancer Res 2014;20:4107-14. [PubMed]
- Olaso E, Labrador JP, Wang L, et al. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem 2002;277:3606-13. [PubMed]
- Hammerman PS, Sos ML, Ramos AH, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov 2011;1:78-89. [PubMed]
- Miao L, Wang Y, Zhu S, et al. Identification of novel driver mutations of the discoidin domain receptor 2 (DDR2) gene in squamous cell lung cancer of Chinese patients. BMC Cancer 2014;14:369. [PubMed]