Advances on EGFR mutation for lung cancer
Introduction
Lung cancer is among the most commonly diagnosed cancers worldwide, representing the first cause of cancer-related death in both the U.S. and Europe (1,2). Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers, being often diagnosed at an advanced stage when treatment options are limited. First-line chemotherapy for NSCLC patients with advanced disease is generally platinum-based, yielding a median overall survival of 8-11 months (3). Unfortunately, the addition of a targeted agent to a platinum-based chemotherapy backbone either in combination regimens and or as sequential treatment has only marginally improved overall prognosis of patients with advanced disease (4-6). Against this background, the recent recognition that certain genetic abnormalities play a major role in the oncogenic process of NSCLC, has allowed in some cases for appropriate selection of patients candidate to targeted therapies based on well-defined biological characteristics (7,8).
EGFR as a target in NSCLC
Since its identification in 1986, the epidermal growth factor receptor (EGFR) has emerged as a crucial factor for the development and growth of human malignancies, including lung cancer (9). In fact, EGFR signal transduction network plays an important role in multiple tumorigenic processes such as proliferation of cancer cells, angiogenesis, and metastasization. Consistently, EGFR aberrant activation has been shown to be prognostic in NSCLC, which provided a solid rationale for the development of EGFR-targeting strategies for NSCLC (10).
EGFR belongs to the Erb family of transmembrane receptor tyrosine kinases which includes also HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4). Upon ligand binding, EGFR undergoes homo- or hetero-dimerization with other receptors of the same family with subsequent autophosphorylation and activation of the intracellular tyrosine-kinase (TK) domain, recruitment of second messengers and intensification of the anti-apoptotic signaling (11). Interestingly, no ligand has been identified for the HER2 orphan receptor while no kinase activity has been documented for HER3, which allow both HER2 and HER3 to be actively involved in EGFR-mediated signaling as preferred hetero-dimerization partners of EGFR itself. There are several ways through which EGFR can be aberrantly activated including receptor overexpression, gene amplification and gene mutation (10). However, because of its crucial role as oncogenic determinant, the presence of an activating (meaning ligand-independent activation of the TK) EGFR mutation in NSCLC carries major therapeutic implications. The present review will focus on the most recent acknowledgements on EGFR gene mutations in NSCLC, also discussing their potential applicabilities in the clinic.
EGFR gene mutations in NSCLC
In 2004, the identification of somatic mutations of the EGFR gene in NSCLC has led to the recognition of a biologically distinct disease entity which has been termed 'oncogene addicted' to reflect its dependence on EGFR-mediated pro-survival signalling (12-14). Consistently, EGFR-mutated NSCLC patients represents a subgroup which seems to experience a more indolent course of disease irrespective of treatment (15,16). However, the clinical relevance of detecting an activating EGFR mutation in NSCLC as assessed by DNA gene sequencing cannot be understated given the exquisite sensitivity that EGFR-mutated NSCLCs show to the 'reversible' EGFR-TK inhibitors (-TKIs) gefitinib or erlotinib (to which we will also refer to as 'first-generation' EGFR-TKIs) (7), which allows patients to experience a particularly extended survival in the presence of EGFR-TKI treatment, thus in contrast with the historical data reported for NSCLCs when considered as a single disease entity (3).
Importantly, although the incidence of EGFR mutations is higher in patients with certain clinical characteristics such as never smoking history, Asian ethnicity (where they can be found in up to 30% of advanced NSCLCs as opposed to 15% for the western population), female sex and adenocarcinoma histology (17), it is not possible to rule out the possibility of an EGFR mutation solely on the basis of clinical characteristics (18-22). This concept is the basis for testing for an EGFR mutation all NSCLC tissues (preferentially adenocarcinoma) irrespective of clinical characteristics in order not to exclude from a very active targeted treatment patients who are discovered to carry an EGFR mutation.
Specific activating EGFR mutations are either short, in-frame nucleotide deletions, in-frame duplications/insertions or single-nucleotide substitutions clustered around the adenosine triphosphate (ATP) binding pocket of the TK domain (23). To date, in-frame deletions in exon 19 around the LeuArgGluAla motif (del19) at residues 746-750 (the most common being del E746_A750) and exon 21 Leu858Arg (L858R) point mutation are the best characterized mutations, together representing 85-90% of all EGFR mutations in NSCLC (23). The frequency of classic EGFR mutations seem to differ according to ethnic backgrounds. In fact, EGFR genotyping from large prospective studies have shown a higher frequency of del19 mutation compared with L858R for European patients (18,22), whereas the incidence of del19 mutation appear to be only slightly superior in Asiatic patients (19-21). Interestingly, clinical data seem to indicate that patients harboring the del19 mutation are more susceptible to the activity of a reversible EGFR-TKI compared to those carrying the L858R mutation (24). However, the molecular mechanisms underlying this apparent inter-mutation discrepancy in drug sensitivity are not clearly understood, possibly being related to a higher EGFR-dependence of the tumor owing to common association of del 19 mutations with EGFR amplification (25). Moreover, it cannot be excluded that gefitinib or erlotinib possess a different inhibitory effect on del19 mutation favoring the erlotinib, as suggested by biochemical studies (26).
Nevertheless, activating EGFR mutations other than del19 or L858R have been described, usually defined as 'other uncommon mutation'. However, their ability to predict sensitivity to a reversible EGFR-TKI is less striking compared with del 19 or L858R mutations. A recent report exploring the sensitivity of uncommon EGFR mutations to gefitinib or erlotinib showed that two types of uncommon EGFR mutations, namely point mutations in position Gly719 of exon 18 (G719) and Leu861Gln mutation in exon 21 (L861) may have unaltered sensitivity to a reversible EGFR-TKI, being associated with clinical responses in approximately half of cases (27). On the other hand, exon 20 insertions have been associated with primary resistance to EGFR-TKIs (28). However, owing to their rarity, it is not possible to draw definitive conclusions on the true relationship between uncommon EGFR mutations and sensitivity to gefitinib or erlotinib and even case reports may orientate in the decision making process of patients with uncommon activating mutations of the EGFR gene (29).
EGFR gene mutations and sensitivity to gefitinib or erlotinib
Gefitinib or erlotinib are orally bioavailable anilinoquinazoline small molecules that act by selectively and reversibly blocking the phosphorylation of the EGFR-TK domain through competition with ATP for binding at the active site of EGFR itself (30). Early phase III studies comparing gefitinib or erlotinib to placebo in chemotherapy pretreated NSCLCs showed a survival improvement for individuals receiving the EGFR-TKI (31,32) which, in case of gefitinib, was statistically significant only for patients with certain clinical characteristics such as never-smoking history and Asian ethnicity (31). However, only one of these two trials, namely the BR.21 study, showed for erlotinib a statistically significant improvement in overall survival (OS) for the whole population (6.7 months versus 4.7 months, respectively, HR=0.70, P<0.001). Therefore, based on these data, erlotinib was granted approval by American and European regulatory agencies for use as second or third-line therapy after failure of cytotoxic chemotherapy.
Nevertheless, since their identification, activating EGFR gene mutations have emerged as the most important predictor of response to reversible EGFR-TKIs (12-14). From that moment on, several retrospective and prospective studies confirmed that patients carrying an EGFR mutation were particularly sensitive to a first-generation EGFR-TKI, with responses observed in up to 90% of cases (33). Recently, four large phase III trials comparing a reversible EGFR-TKI to standard platinum-based chemotherapy in untreated advanced NSCLCs biologically selected for the presence of an activating EGFR mutation clearly stated the superiority of gefitinib or erlotinib over chemotherapy in terms of response rates (RR) and progression-free survival (PFS) (Table 1) (19-22). Also, as expected, gefitinib and erlotinib were associated with a significantly lower incidence of grade ≥3 adverse events. Notably, the fact that OS was not statistically in favor of gefitinib or erlotinib does not come as a surprise given the high rate of cross-over to an EGFR-TKI in the experimental arm at the time of disease progression. In addition, the particularly long median survival (>24 months) experienced by EGFR-mutated patients treated with a reversible EGFR-TKI might have led to miscalculation of the optimal sample size required to detect a statistically significant difference in survival (35).
At the present time, no head-to-head randomized comparison exists between gefitinib and erlotinib for EGFR-mutated advanced NSCLCs. However, although preclinical studies have shown a differential sensitivity to gefitinib or erlotinib according to the type of activating EGFR mutation expressed by the tumor (del19 or L858R) (26), indirect evidence suggests that it is unlikely that this difference would translate into a clinically meaningful benefit in favor of one of the two agent (Table 1) (19-22). Interestingly, a recent randomized phase III study compared a new reversible EGFR-TKI, icotinib, to gefitinib in chemotherapy pretreated advanced NSCLCs showing comparable efficacy in the EGFR-mutated subgroup of patients (36).
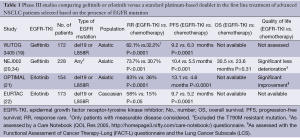
Full table
Importantly, if EGFR-mutated patients benefit much from first-line treatment with gefitinib or erlotinib, the replacement of chemotherapy with a reversible EGFR-TKI as front-line therapy in biologically unselected patients with unknown EGFR mutation status is associated with a worse clinical outcome in terms of both PFS and OS (37,38). Moreover, selection of patients candidate to gefitinib or erlotinib according to clinical characteristics known to be associated with enrichment for the presence of an activating EGFR mutation is per se not sufficient to identify individuals who benefit the most from up-front therapy with a reversible EGFR-TKI (39,40). This question was matter of the IPASS and First-SIGNAL trials in which gefitinib was compared with standard chemotherapy in East-Asian advanced NSCLC patients with adenocarcinoma histology who were only (First-SIGNAL) or mostly (IPASS) never smokers. Although in both studies gefitinib was associated with a significant improvement in the primary PFS endpoint (HR=0.74, P<0.0001 and HR=0.81, P=0.044, for IPASS and First-Signal respectively), this benefit was shown to be driven by the high proportion of EGFR-mutated patients present in the studies population, since the analysis of EGFR wild type patients showed a significantly longer PFS in favor of chemotherapy.
These data strongly support the use of a reversible EGFR-TKI in EGFR-mutated advanced NSCLC patients and allowed recent approval of gefitinib by the European Medicines Agency with this indication. As for erlotinib, it is likely that its current indication will soon be extended to include also treatment-naïve patients with activating EGFR mutations.
Mechanisms of resistance to gefitinib or erlotinb
Unfortunately, approximately 20% to 30% of EGFR-mutated patients do not undergo tumor shrinkage on a first generation EGFR-TKI (19-22,39,40). Moreover, virtually all EGFR-mutated patients who initially benefit from gefitinib or erlotinib eventually develop progressive disease, usually after approximately a year since treatment initiation. Since no standard treatment exists for EGFR-mutated patients who progress while on a reversible EGFR-TKI, strict criteria for definition of acquired resistance have been proposed for better interpretation of clinical trials investigating novel agents in this setting (41). Against this background, the identification of the molecular mechanisms that underlie either primary or acquired resistance to gefitinib or erlotinib is of crucial importance in order to prevent, delay or overcome resistance to treatment. To date, a few mechanisms of resistance to reversible EGFR-TKIs have been identified (Table 2). Preclinically, primary resistance has been associated with in-frame insertion mutations in exon 20 (42). Consistent with these data, most patients with tumors harboring exon 20 insertions have been shown to be resistant to gefitinib (43). As for acquired resistance, in approximately 50% of patients this can be attributed to the occurrence of a secondary threonine-to-metionine missense mutation in codon 790 (T790M) in exon 20 of the EGFR gene, which is located in the "critical" catalytic region of the ATP binding pocket of the EGFR-TK domain (44-47). The way through which the T790M mutation induce resistance to gefitinib or erlotinib is thought to be due to an increased binding affinity between EGFR and ATP rather than to a decreased affinity between EGFR and EGFR-TKI (57). Nevertheless, recent evidence suggests that the T790M mutation might pre-exist in minor clones in almost all reported cases of T790M-related acquired resistance, becoming evident during exposure to a reversible EGFR-TKIs as a result of evolutionary selection during treatment (58). Importantly, a poorer clinical outcome is usually experienced by patients with pre-treatment T790M compared with those without it (58). However, an interesting prospective clinical study suggested that EGFR-mutated patients with T790M-related acquired resistance may have a more favorable prognosis as opposed to non-T790M resistant patients, which might have important clinical implications for the design of clinical trials in this setting (59). Notably, although extremely rare, T790M mutations may exist as major clones irrespective of EGFR-TKI administration in certain patients, thus being implicated also in primary resistance (48,49). More recently, three other less common secondary mutations have been identified as 'de novo' alterations in patients with acquired resistance to first generation EGFR-TKIs, namely the D761Y (exon 19), L747S (exon 19) and T854A (exon 21) mutations (46,50,51).
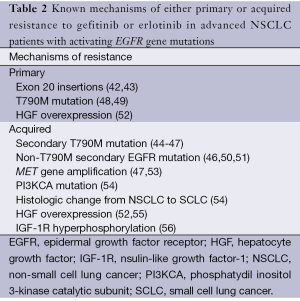
Full table
On the other hand, amplification of the MET proto-oncogene, which encodes a transmembrane TK receptor for the hepatocyte growth factor (HGF) and is involved with invasion, metastasis and angiogenesis in tumors, has been implicated in approximately 20% of the cases of acquired resistance to gefitinib and erlotinib (47,53). MET amplification causes resistance through activation of HER3, which in turn sustains the activity of the phosphatydilinositol 3-kinase (PI3K)/Akt downstream signalling pathway (47). Therefore, even with gefitinib inhibiting the phosphorylation of HER3 by EGFR, the proliferation signal is not inhibited because of the maintenance of the phosphorylation of HER3 by MET. Interestingly, similarly to the T790M mutation, MET gene amplification might be the result of selection of minor clones of pre-existing MET amplified tumor cells becoming dominant during exposure to an EGFR-TKI (52). Occasionally, resistant tumors with MET amplification may have a concurrent secondary T790M mutation (53,59).
A recent study identified mutations in the catalytic subunit of PI3K and phenotipic change into small cell lung cancer (SCLC) as two other mechanism of acquired resistance to reversible EGFR-TKIs in EGFR-mutated patients (54). Intriguingly, the latter mechanism might have important clinical implications since it implies that a rebiopsy at the time of progression would result into significant change in disease management. However, it is still not known whether this phenotipic change reflects the selection of a population of SCLC from a histologically mixed tumor following eradication of the majority of NSCLC clones. Even more intriguingly, EGFR mutations are maintained in SCLCs arising in EGFR-TKIs resistant patients, although the relevance of this phenomenon is uncertain given that EGFR-mutated SCLCs do not seem to be addicted to EGFR pro-survival signalling (60).
Finally, HGF overexpression has been advocated as another possible mechanism of acquired resistance (52,55), probably acting by inducing downstream signal activation independently of HER3 or EGFR (55). Notably, HGF overexpression is likely to be implicated also in primary resistance to a reversible EGFR-TKI in patients with activating EGFR gene mutations (52).
In conclusion, these proposed mechanism of resistance, strongly encourage the use in the clinic of certain strategies to prevent/overcome resistance to reversible EGFR-TKIs in advanced NSCLC patients with activating EGFR gene mutations. Among these, the use of irreversible EGFR-TKIs or combination regimens of an EGFR-TKI with a MET-inhibitor appear to be the most appealing ones.
Irreversible EGFR-TKIs
Similarly to gefitinib or erlotinib, irreversible EGFR-TKIs are anilinoquinazoline inhibitors that, however, unlike them, irreversibly bind EGFR to the amino acid position 797 which enables blockade of EGFR kinase activity even in the presence of an EGFR T790M mutation (61-63). In addition to irreversible binding, simultaneous blockade of two or more members of the EGFR family represents another key feature through which these agents might prove clinically active in delaying/preventing resistance to first-generation EGFR-TKIs.
The dual irreversible EGFR/HER2 inhibitor afatinib (BIBW 2992) is among the most promising drugs for use in the setting of gefitinib- or erlotinib-resistant NSCLCs. Recently, a large randomized phase IIb/III trial comparing afatinib versus placebo was conducted in advanced adenocarcinomas of the lung who had progressed after ≤2 lines of chemotherapy (including at least one platinum-based regimen) and ≥12 weeks of treatment with gefitinib or erlotinib (64). Interestingly, afatinib showed signs of activity by significantly prolonging PFS over placebo in this population of patients with clinically acquired resistance to a reversible EGFR-TKI (3.3 months versus 1.1 months, respectively, HR=0.38, P<0.0001) (64). More importantly, this benefit was particularly evident when the analysis was restricted to key subgroup populations that were likely to be enriched for the presence of EGFR mutations such as those who had experienced prior response or treatment duration ≥48 weeks with a reversible EGFR-TKI (4.4 months vsersus 1.0 month, respectively, HR=0.28) (65).
More recently, afatinib was tested in combination with the anti-EGFR monoclonal antibody cetuximab, based on the solid preclinical background that this combination would overcome resistance to gefitinib or erlotinib in EGFR-mutated NSCLCs (66,67). Crucial prerequsites for trial participation were the presence of EGFR-mutated tumors with clinically acquired resistance to gefitinib or erlotinib (stable disease ≥6 months or prior response to gefitinib or erlotinib) and acquisition of tumor tissue at baseline for molecular analysis. Of note, out of the 47 patients so far enrolled, the afatinib/cetuximab combination reported a RR of 40% with an overall disease control rate (RR + stable disease) of 92% (67). Improtantly treatment activity seemed to be independent of the presence of the T790M mutation.
Current areas of research of afatinib in advanced NSCLC include its use in EGFR-mutated and gefitinib or erlotinib-naïve patients where a RR of 61% with an outstanding median PFS of 14 months was observed in a recently conducted phase II study (68). Also, two relevant phase III studies are currently being run in order to compare afatinib with platinum-based chemotherapy in EGFR-mutated advanced adenocarcinomas of the lung (33).
Dacomitinib (PF-00299804) is another irreversible EGFR-TKI under clinical testing for advanced NSCLC, which acts also as inhibitor of other EGFR family members, namely HER2 and HER4. As monotherapy in clinically (adenocarcinoma, never or light smokers) or biologically (presence of EGFR mutation) selected treatment-naïve advanced NSCLC patients it showed a RR of 45% (69). More importantly, in a subset analysis of 29 evaluable patients with EGFR-mutation positive disease, 51% of responses were observed, including one case of exon 20 insertion, and some degree of tumor shrinkage was observed overall in >90% of EGFR-mutated patients (69).
Currently a double-blind, randomized phase III study is being conducted in chemotherapy-pretreated advanced NSCLC to compare dacomitinib to erlotinib, the primary endpoind being PFS (70). Notably, collection of tissue samples is mandatory for study inclusion, this in order to molecularly characterized whether exists a group of patients (iEGFR mutated or not) who derive more benefit from dacomitinib than from erlotinib.
MET-inhibitors
Importantly, because MET amplification and T790M mutation often occur in the same patient, probably the best strategy is to combine the second-generation irreversible EGFR-TKIs with MET inhibitors. Preclinically, in MET amplified NSCLC cell lines treatment resistance could be suppressed by the addition of erlotinib to a MET inhibitor (71). There are several ways to inhibit the MET signaling pathway, including anti-MET antibodies, inactivation of MET ligand, namely the hepatocyte growth factor (HGF) or inhibition of MET kinase activity. Currently, the anti-MET monoclonal antibody MetMab and the MET-TKI tivantinib have been tested in randomized phase II studies of chemotherapy pretreated advanced NSCLCs, which were hypothesis-generating for identifying biomarkers of sensitivity to MET inhibition such as MET expression by immunohistochemistry and MET gene copy number as assessed by fluorescence in situ hybridizaton (72,73). However none of the ongoing studies with these agents has been thought for the EGFR-mutated NSCLC population undergoing resistance to a reversible EGFR-TKI.
Conclusions
EGFR-mutated NSCLC is a totally distinct disease entity whose EGFR "addiction" is maintained despite progression and/or prior exposure to a first-generation EGFR-TKIs (67,74,75). Therefore, therapeutic advances beyond gefitinib and erlotinib should keep focusing on EGFR blockade, possibly by means of revealing novel mechanisms of EGFR-interference or biological combinations of EGFR-targeting agents. Future scenarios include the possibility to develop therapeutic strategies that can delay further the onset of treatment resistance to EGFR-TKIs such as covalent pyrimidine EGFR inhibitors (76). These agents are 30 to 100-fold more potent against EGFR T790M, and up to 100-fold less potent against EGFR wild type, thus possibly resulting in greater efficacy and better tolerability compared with quinazoline-based inhibitors such as gefitinib, erlotinib or afatinib. To conclude, in recent years the rapid clinical development of EGFR targeting drugs for EGFR-mutated NSCLC represents a proof of concept of how important can be the discovery of a target to which the tumor is addicted for proliferation and survival. Against this background only rationally designed clinical trials can help research move faster toward a personalized therapeutic approach based on patients’ biological characteristics.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010;46:765-81. [PubMed]
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 2008;13:5-13. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Coudert B, Ciuleanu T, Park K, et al. Survival benefit with erlotinib maintenance therapy in patients with advanced non-small-cell lung cancer (NSCLC) according to response to first-line chemotherapy. Ann Oncol 2012;23:388-94. [PubMed]
- Metro G, Cappuzzo F. New targeted therapies for non-small-cell lung cancer. Therapy 2009;6:335-50.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest New Drugs 1999;17:259-69. [PubMed]
- Metro G, Finocchiaro G, Toschi L, et al. Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC). Rev Recent Clin Trials 2006;1:1-13. [PubMed]
- Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol 2006;33:369-85. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005;23:5900-9. [PubMed]
- Shepherd FA, Tsao MS. Unraveling the mystery of prognostic and predictive factors in epidermal growth factor receptor therapy. J Clin Oncol 2006;24:1219-20. [PubMed]
- Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol 2006;11:190-8. [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Gervais R, Vergnenegre A, et al. Spanish Lung Cancer Group. Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: interim results of the European erlotinib versus chemotherapy (EURTAC) phase III randomized trial [abstract]. J Clin Oncol 2011;29:s7503.
- Murray S, Dahabreh IJ, Linardou H, et al. Somatic mutations of the tyrosine kinase domain of epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical database. J Thorac Oncol 2008;3:832-9. [PubMed]
- Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res 2009;15:5267-73. [PubMed]
- Sholl LM, Yeap BY, Iafrate AJ, et al. Lung adenocarcinoma with EGFR amplification has distinct clinicopathologic and molecular features in never-smokers. Cancer Res 2009;69:8341-8. [PubMed]
- Carey KD, Garton AJ, Romero MS, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res 2006;66:8163-71. [PubMed]
- Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 2011;17:3812-21. [PubMed]
- Januszkiewicz L. [Commentary to the article: Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomized controlled trials. Lancet Oncology, 2010; DOI:. Kardiol Pol 2010;68:1183-5. [PubMed]
- De Pas T, Toffalorio F, Manzotti M, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol 2011;6:1895-901. [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Metro G, Crinò L. The LUX-Lung clinical trial program of afatinib for non-small-cell lung cancer. Expert Rev Anticancer Ther 2011;11:673-82. [PubMed]
- Yoshizawa H, Kobayashi K, Inoue A, et al. QOL analysis from NEJ 002 study comparing gefitinib to chemotherapy for non-small cell lung cancer with mutated EGFR Ann Oncol 2010;21:s3159.
- Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101:1642-9. [PubMed]
- Sun Y, Shi Y, Zhang L, et al. A randomized, double-blind phase III study of icotinib versus gefitinib in patients with advanced non-small cell lung cancer (NSCLC) previously treated with chemotherapy (ICOGEN) J Clin Oncol 2011;29:s7522.
- Gridelli C, Ciardiello F, Feld R, et al. International multicenter randomized phase III study of first-line erlotinib (E) followed by second-line cisplatin plus gemcitabine (CG) versus first-line CG followed by second-line E in advanced non-small cell lung cancer (aNSCLC): The TORCH trial J Clin Oncol 2010;28:s7508.
- Thomas M, Reuss A, Fischer JR, et al. Innovations: Randomized phase II trial of erlotinib (E)/bevacizumab (B) compared with cisplatin (P)/ gemcitabine (G) plus B in first-line treatment of advanced nonsquamous (NS) non-small cell lung cancer (NSCLC) J Clin Oncol 2011;29:s7504.
- Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080-7. [PubMed]
- Lee JS, Park K, Kim SW, et al. A randomized phase III study of gefitinib (IRESSA™) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung J Thor Oncol 2009;4:s4.
- Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60. [PubMed]
- Greulich H, Chen TH, Feng W, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med 2005;2:e313. [PubMed]
- Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res 2008;14:4877-82. [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [PubMed]
- Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 2006;12:6494-501. [PubMed]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [PubMed]
- Toyooka S, Kiura K, Mitsudomi T. EGFR mutation and response of lung cancer to gefitinib. N Engl J Med 2005;352:2136. [PubMed]
- Shih JY, Gow CH, Yang PC. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med 2005;353:207-8. [PubMed]
- Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 2007;4:1669-79; discussion 1680.
- Bean J, Riely GJ, Balak M, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res 2008;14:7519-25. [PubMed]
- Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 2010;17:77-88. [PubMed]
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7. [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [PubMed]
- Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 2008;68:9479-87. [PubMed]
- Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest 2008;118:2609-19. [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [PubMed]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77. [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [PubMed]
- Shiao TH, Chang YL, Yu CJ, et al. Epidermal growth factor receptor mutations in small cell lung cancer: a brief report. J Thorac Oncol 2011;6:195-8. [PubMed]
- Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A 2005;102:7665-70. [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [PubMed]
- Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924-32. [PubMed]
- Miller VA, Hirsh V, Cadranei J, et al. Phase IIB/III double-blind randomized trial of afatinib (BIBW 2992, an irreversible inhibitor of EGFR/HER1 and HER2) + best supportive care (BSC) versus placebo in patients with NSCLC failing 1-2 lines of chemotherapy and erlotinib or gefitinib (LUX-LUNG 1). Ann Oncol 2010;21(8 Suppl):LBA 1(abstract).
- Miller VA, Hirsh V, Cadranei J, et al. Subgroup analysis of LUX-Lung 1: A randomized phase III trial of afatinib (BIBW 2992) + best supportive care (BSC) versus placebo + BSC in patients with NSCLC failing 1-2 lines of chemotherapy and erlotinib or gefitinib. Chicago multidisciplinary symposium in thoracic oncology;2010.
- Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest 2009;119:3000-10. [PubMed]
- Janjigian YY, Groen HJ, Horn L, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib J Clin Oncol 2011;29:s7525.
- Yang CH, Shih JY, Su WC, et al. A phase II of afatinib (BIBW 2992) in patients with adenocarcinoma of the lung and activating EGFR mutations Ann Oncol 2010;21:s367.
- Mok T, Spigel DR, Park K, et al. Efficacy and safety of PF-00299804 (PF299), an oral, irreversible, pan-human epidermal growth factor receptor (pan-HER) tyrosine kinase inhibitor (TKI), as first-line treatment (tx) of selected patients (pts) with advanced (adv) non-small cell lung cancer (NSCLC) J Clin Oncol 2010;28:s7537.
- ARCHER 1009: A phase 3 study Of PF-00299804, A pan-HER inhibitor, vs. Erlotinib in the treatment of advanced Non-Small Cell Lung Cancer. [Last accessed 1 December 2011]. Available online: http://clinicaltrials.gov/ct2/results?term=NCT01360554.
- McDermott U, Pusapati RV, Christensen JG, et al. Acquired resistance of non-small cell lung cancer cells to MET kinase inhibition is mediated by a switch to epidermal growth factor receptor dependency. Cancer Res 2010;70:1625-34. [PubMed]
- Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC J Clin Oncol 2011;29:s7505.
- Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 2011;29:3307-15. [PubMed]
- Yamamoto N, Katakami N, Atagi S, et al. A phase II trial of afatinib (BIBW 2992) in patients (pts) with advanced non-small cell lung cancer previously treated with erlotinib (E) or gefitinib (G) J Clin Oncol 2011;29:s7524.
- Oxnard GR, Janjigian YY, Arcila ME, et al. Maintained sensitivity to EGFR tyrosine kinase inhibitors (TKIs) in EGFR-mutant lung cancers that recur after adjuvant TKI J Clin Oncol 2011;28:s7029.
- Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009;462:1070-4. [PubMed]


