
Evaluation of the lung immune prognostic index in advanced non-small cell lung cancer patients under nivolumab monotherapy
Background
Although lung cancer remains as the leading cause of cancer-related death worldwide (1), over the last years several advances have been made for the management of this aggressive disease. Immunotherapy, particularly the blockade of the programmed cell death-1 (PD-1)/programmed death ligand 1 (PD-L1) axis, has been recently established as a standard treatment in the advanced non-small cell lung cancer (NSCLC) setting. For instance, nivolumab and atezolizumab have shown a median overall survival (mOS) benefit in previously treated metastatic NSCLC patients compared to docetaxel (2-4). In the same context, but focused on the PD-L1 positive NSCLC subset, pembrolizumab has also shown a mOS gain as first and second-line of therapy (5,6). Moreover, either the addition of pembrolizumab or atezolizumab to standard chemotherapy in previously untreated metastatic NSCLC or of durvalumab after chemoradiotherapy in stage III NSCLC have recently demonstrated a significant improvement in survival outcomes independently of the PD-L1 status (7-10).
To date, despite the huge efforts made looking for an appropriate biomarker to guide immunotherapy candidate selection, only PD-L1 expression determined by immunohistochemistry has been validated in some clinical scenarios. Between all the anti-PD-1 or PD-L1 [PD-(L)1] antibodies approved and available in the advanced NSCLC armamentarium, only pembrolizumab has focused its clinical development on the PD-L1 positivity (5). Overall, subgroup analyses of different randomized clinical trials demonstrate better outcomes in this biomarker-positive population (2-6), but in any case, these data do not support denying this therapeutic strategy to those NSCLC patients with a PD-L1 negative status. Maybe the inherent temporospatial heterogeneity of PD-L1, together with the differences on technical issues and scoring systems, are some of the factors behind its inconsistency as a predictive biomarker (11).
In the era of precision medicine, liquid markers appear as a promising alternative to overcome the limitations of tissue-based biomarkers. Over the last years, several groups have demonstrated the prognostic and predictive value of several inflammatory-related markers, such as the neutrophil to lymphocyte ratio (NLR), derived neutrophil to lymphocyte ratio (dNLR) and more recently, the lung immune prognostic index (LIPI) (12). In 2018 Mezquita et al. (12) have described a new categorical blood-based biomarker, the LIPI, which integrating baseline dNLR and LDH, was able to stratify NSCLC patients under anti-PD-(L)1 treatment according to survival outcomes. Recognizing the importance of validating biomarkers in the real-world clinical scenario, in this study we investigate for the first time to the best of our knowledge the prognostic and predictive utility of the LIPI in a multicenter nivolumab-based cohort.
Patients and methods
Study design and data collection
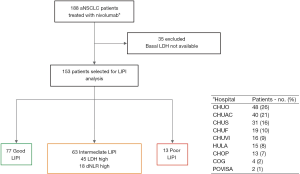
We conducted a multicenter retrospective study of a cohort of 188 patients with advanced NSCLC treated with nivolumab in the second line of therapy or beyond in the context of expanded access program between August 2015 and January 2017 from 9 Galician medical centers (Figure S1).
Complete blood cell counts and LDH level at baseline before nivolumab therapy (within 30 days before the first infusion) were extracted from electronic medical records. Demographic, clinical and pathological data were also collected.
Tumour responses were assessed by the investigators according to Response Evaluation Criteria in Solid Tumors guidelines version 1.1 every 10±2 weeks or before if for medical reasons was indicated.
This study was approved by the Galician Research Ethics Committee (GGC-NIV-2018-01) and conducted in accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki.
Statistical analysis
Disease control rate (DCR) was defined as the proportion of patients who achieved a complete or partial response and a stable disease, and overall response rate (ORR) as the proportion of patients who achieved a complete or partial response. Overall survival (OS) was calculated from the date of nivolumab initiation until death resulting from any cause or last known follow-up for patients alive. Progression-free survival (PFS) was calculated from the date of nivolumab initiation until disease progression or death resulting from any cause or last known follow-up for patients with no disease progression. Patients who died before radiologic assessment were consider as not evaluable for response. dNLR was calculated as neutrophil count/(white blood cell count − neutrophil count) and categorized as high (dNLR >3) or low (dNLR ≤3). LIPI was calculated as previously described by Mezquita et al. (12) based on the baseline dNLR (high, 1 factor; low, 0 factors) and LDH level (> upper limit of normal, 1 factor; ≤ upper limit of normal, 0 factors), establishing 3 groups: good, 0 factors; intermediate, 1 factor; poor, 2 factors.
Comparisons between patient characteristics were performed using χ2 (discrete variables) and one-way analysis of variance (continuous variables). For time-to-event analyses, survival estimates were calculated by the Kaplan-Meier method, and groups were compared with the log-rank test. The impact of the baseline LIPI on survival (PFS and OS), and DCR and ORR was assessed by Cox and logistic regression (enter method) models respectively, adjusted for baseline dNLR and LDH level, and other major covariates. All P values were 2-sided, and those less than 0.05 were considered statistically significant. Statistical analyses were conducted using the Medcalc version 17.9.7 (Broekstraat, Belgium).
Results
Baseline characteristics and outcomes
Baseline characteristics and outcomes of the entire cohort were described previously by Areses Manrique et al. (13). Forty-one percent (n=77) of the patients had a good (0 factors) LIPI, while 33.5% (n=63) and 6.9% (n=13) had intermediate (1 factor) and poor (2 factors) LIPI respectively. Remaining patients (n=35; 18.6%) have not sufficient data to be classified according to the LIPI.
Between the 153 LIPI-classified patients, median OS was 12.9 months [95% confidence interval (CI), 10.7–20.8 months] and median PFS was 5.8 months (95% CI, 4.2–7.1 months). No significant differences were observed between the LIPI groups according to clinicopathologic characteristics (Table 1).
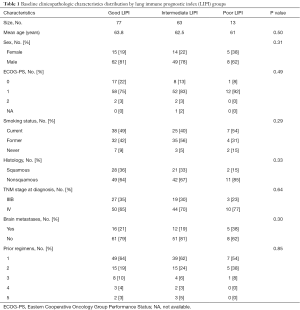
Full table
LIPI utility in prognostication
Median OS for poor, intermediate, and good LIPI patients was 3.4 months (95% CI, 1.9–6.5 months), 7.3 months (95% CI, 4.4–12.9 months), and 20.8 months (95% CI, 14.9–not reached months) respectively (P<0.0001; Figure 1A). Median PFS was 2.8 months (95% CI, 1.8–3.9 months), 5.1 months (95% CI, 3.2–8.5 months), 6.6 months (95% CI, 4.7–8.7 months) for poor, intermediate and good LIPI patients respectively (P=0.07; Figure 1B).
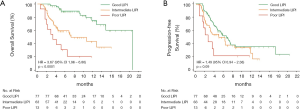
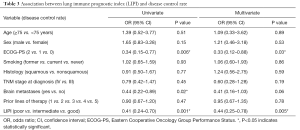
Worse LIPI was significantly associated with shorter OS in univariate [hazard ratio (HR) =3.12, 95% CI, 2.12–4.60; P<0.0001] and multivariate (HR =3.67, 95% CI, 1.96–6.86; P<0.0001) analyses (Table 2).
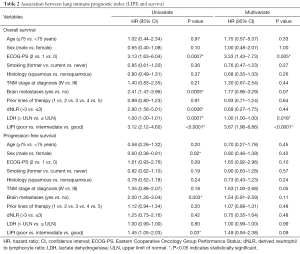
Full table
As expected, we found that worse LIPI was also associated with shorter PFS (HR =1.45, 95% CI, 1.05–2.03; P=0.03), despite the fact that this correlation did not reach statistical significance in multivariate analysis (HR =1.49, 95% CI, 0.94–2.38; P=0.09) (Table 2).
LIPI and DCR association
DCR for poor, intermediate, and good LIPI patients was 23%, 46% and 66% respectively (P=0.004) (Figure 2).
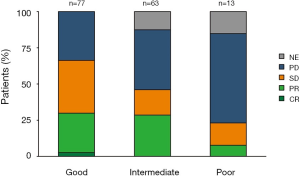
ORR for poor, intermediate, and good LIPI patients was 8%, 29% and 30% respectively (P=0.2) (Figure 2).
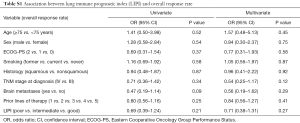
Worse LIPI was associated with lower DCR in univariate [odds ratio (OR) =0.41, 95% CI, 0.24–0.70; P=0.001] and multivariate (OR =0.44, 95% CI, 0.25–0.78; P=0.005) analyses (Table 3). However, there was not a significant association between the LIPI and ORR (Table S1).
Full table
Full table
Discussion
With the arrival of immune checkpoint blockade (ICB) as a standard of care treatment for various cancer types, and considering that ICB benefits only to a limited proportion of patients, the development of markers for helping in candidate selection has become a priority for the oncology community.
Knowing the relevance of biomarker validation in the real-world setting, in this study we investigated the utility of the LIPI as a prognostic and predictive biomarker in a cohort of 153 patients with advanced NSCLC treated with nivolumab in the second line of therapy or beyond. We report that worse LIPI at the start of nivolumab therapy is associated with decreased OS, PFS, and DCR. To the best of our knowledge, this is the first study that evaluates the LIPI utility in a cohort of patients treated with only one specific anti-PD-1 antibody. The results of our study confirm the data previously reported by others in mixed anti-PD-(L)1 cohorts and in one pooled analysis of anti-PD-L1 (atezolizumab)-based clinical trials regarding the LIPI value (12,14,15). Although initially the LIPI was postulated as an ICB specific biomarker (12), this specificity was not confirmed in subsequent exploratory retrospective pooled analyses of data from advanced NSCLC clinical trials evaluating ICB (15,16). In these studies, the LIPI showed its prognostic utility not only in the ICB subset but also in the chemotherapy and targeted therapy ones (15,16).
Recently, the use of steroids (≥10 mg of prednisone or equivalent) prior to anti-PD-(L)1 monotherapy initiation has been associated with smaller ORR, PFS, and OS in NSCLC patients. Being steroids one of the major causes of neutrophilia and lymphopenia in patients with cancer, their use, through the increase of the dNLR (17), can be reflected in a LIPI worsening. This situation, among others, illustrates the huge potential of the LIPI as an inexpensive real-time biomarker to complement other static markers such as PD-L1 or even tumor mutation burden (TMB).
As with PD-L1, the role of TMB as a predictive biomarker for ICB has been retrospectively evaluated in different clinical trials, but conclusive studies confirming its clinical significance are needed (18). With the results of our study, together with the body of evidence available to date, retrospective testing of the LIPI in all the ICB-based randomized clinical trials already run looks mandatory. If its role in prognostication and/or prediction is confirmed in randomized cohorts, and following the renal-cell carcinoma (RCC) experience with the International Metastatic RCC Database Consortium risk score, the LIPI should be integrated into the design of coming clinical trials as a stratification or inclusion criterion.
We acknowledge that one of the limitations of our study, together with the limited sample size, is its retrospective nature. Due to the lack of baseline LDH level in several cases, the LIPI was only calculable for 82% of patients from the entire cohort. On the other hand, being unnecessary the PD-L1 status for prescribing nivolumab in the context of the expanded access program, it was not available for any case. Furthermore, as LDH determination was not centrally performed, methodology and reference range vary between laboratories. Finally, as the radiological assessment was performed locally with a time range of ±2 weeks, PFS estimation can be influenced by this variability.
In summary, the results of our study confirm the utility of the LIPI in prognostication and disease control prediction in advanced NSCLC patients treated with nivolumab in the second line of therapy or beyond. Further retrospective and prospective analyses of the LIPI in anti-PD-(L)1-based randomized clinical trials are warranted.
Acknowledgments
The authors thank Raquel Romero Van der Schoot for technical support in data management.
Footnote
Conflicts of Interest: Juan Ruiz-Bañobre - Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Ipsen, PharmaMar. Speakers’ Bureau: Roche. María C. Areses-Manrique - Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Takeda, Lilly, Bristol-Myers Squibb. Francisco J. Afonso-Afonso - Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Bristol-Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim. Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer. Margarita Amenedo - Consulting or Advisory Role: Boehringer Ingelheim, Clovis Oncology. Speakers' Bureau: AstraZeneca; PharmaMar, Roche, Pierre Fabre, Lilly. Travel, Accommodations, Expenses: Roche, Lilly. José Luis Fírvida-Pérez - Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Boehringer Ingelheim, MSD Oncology, Takeda, Lilly, Bristol-Myers Squibb. Rosario García-Campelo - Consulting or Advisory Role: Roche/Genentech, MSD Oncology, AstraZeneca, Bristol-Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim. Speakers’ Bureau: Roche, AstraZeneca, Bristol-Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim, MSD Oncology. Jorge García-González - Consulting or Advisory Role: Bristol-Myers Squibb, MSD Oncology, Roche/Genentech, Lilly, Boehringer Ingelheim, AstraZeneca, Pierre Fabre. Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Roche/Genentech. Sergio Vázquez - Consulting or Advisory Role: Pfizer, Astellas, Janssen, MSD Oncology, Bayer, Roche, Bristol-Myers Squibb, Boehringer Ingelheim, AstraZeneca, Ipsen, Novartis, Eusa Pharma, Eisai and Sanofi. Speakers’ Bureau: Lilly, Astellas, Bayer, Roche, Boehringer Ingelheim, Ipsen, Novartis, AstraZeneca and Sanofi. Travel, Accommodations, Expenses: Pfizer, Roche and AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol 2018;29:953-8. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Areses Manrique MC, Mosquera Martínez J, García González J, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res 2018;7:404-15. [Crossref] [PubMed]
- Mielgo-Rubio X, Sereno Moyano M, Sotelo-Lezama M, et al. P1.01-69 Validation of the Lung Immune Prognostic Index (LIPI): Useful to Identify Resistance to PD-1 Checkpoint Inhibitors in Pretreated Lung Cancer. J Thorac Oncol 2018;13:S488-9. [Crossref]
- Sorich MJ, Rowland A, Karapetis CS, et al. Evaluation of the Lung Immune Prognostic Index for Prediction of Survival and Response in Patients Treated With Atezolizumab for NSCLC: Pooled Analysis of Clinical Trials. J Thorac Oncol 2019;14:1440-6. [Crossref] [PubMed]
- Kazandjian D, Gong Y, Keegan P, et al. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non-Small Cell Lung Cancer. JAMA Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019;4:e000457. [Crossref] [PubMed]
- Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44-56. [Crossref] [PubMed]


