Ratio of maximum standardized uptake value to primary tumor size is a prognostic factor in patients with advanced non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-associated death in the world (1). Most non-small cell lung cancer (NSCLC) patients are diagnosed at a relatively late stage, and platinum-based first line chemotherapy is prescribed as a part of standard treatment for advanced NSCLC patients. However, the factor that may predict survival and treatment response is limited.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is a well-established technique for diagnosis and staging in cancer (2,3). The association between higher maximum standardized uptake value (SUVmax) in 18F-FDG PET/CT and poor prognosis or treatment response in cancer patients has been reported in several prior studies (4,5). SUV, a semi-quantitative measurement of FDG uptake, in the primary tumor site of NSCLC has been demonstrated to be correlated with proliferation (4,5) and aggressiveness (6,7).
In 2013, Stiles et al. (8) established the SUVmax to tumor size ratio (SUVmax/tumor size) in his study. SUVmax/tumor size was revealed to be associated with survival in 530 patients who were undergoing resection and histologically diagnosed NSCLC, and was stronger than SUVmax alone. However, the association between SUVmax or SUVmax/tumor size and therapy response or survival in advanced NSCLC patients is still unclear.
The aim of this study is to evaluate the predicting and prognostic significance of pretreatment SUVmax or SUVmax/tumor size in advanced NSCLC patients.
Patients and methods
Study population
The retrospective study protocol was approved by the Hospital Ethics Committee. Patients hospitalized from January 2007 to July 2011 in Department of Respiratory Medicine were included. Inclusion criteria were: (I) histologically or cytologically diagnosed NSCLC; (II) had a pretreatment 18F-FDG PET/CT scanning; (III) in stage IIIB and IV, including those in stage IIIA but not able to surgery or not accept the operation; (IV) had no history or concurrent diagnosis of another type of cancer; (V) overall survival (OS) >3 months; (VI) the clinical data should be available.
Ratio of SUVmax to primary tumor size
Scans were performed by a dedicated 16-slice whole-body PET/CT scanner after the patients injected with pyrogen-free 18F-FDG 10 to 15 mCi. SUVmax values were obtained by drawing the regions of interest over the most intense slice of the primary tumor by correcting for the injected dose and the patient’s weight. The tumor diameter in the primary site was also analyzed.
Therapy response and survival analyses
Therapeutic response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) by CT scans or 18F-FDG PET/CT performed after two cycles of chemotherapy. Clinical responses were classified as disease control rate (DCR) and progressive disease (PD). OS was defined as the time in months between the pathological diagnosis and the date of death, progression-free survival (PFS) as the time between pathological diagnosis and progression disease, and post-progression survival (PPS) as the time between progression disease and the date of death. Patients who were alive were censored at the time of the last clinical follow-up.
Statistical analyses
Statistical analyses were performed using the SPSS statistical software program (version 18.0 for windows). The continuous variables SUVmax, tumor size, and SUVmax/tumor size were dichotomized by a median split. Survival was calculated with the Kaplan-Meier method, and groups were compared with the log-rank test. Multivariate analysis was carried out with the Cox proportional hazards model. A significance level of 0.05 was used for covariate entry. P values less than 0.05 were considered to be statistically significant.
Results
Patient characteristics
Total 237 consecutive advanced NSCLC patients were enrolled. However, 49 patients were excluded because they took no therapy, transferred to other hospitals or died within 3 months. In 188 patients who had pretreatment 18F-FDG PET/CT, six patients with concurrent chemo-radiotherapy and one patient with a second primary extra-pulmonary cancer were excluded. Final 181 patients were included in the further analysis.
The characteristics of the 181 patients were listed in Table 1, with a mean age of 60.6 years (range, 29-87 years). A total of 116 patients were males (64.1%). The number of patients in stage III and IV was 44 and 137, respectively. All 181 patients received first-line therapy, including 157 patients received platinum-based chemotherapy according to the tumor histology and left 24 patients with unknown mutation status received EGFR-TKI therapy. After two courses of treatment, patients took whole body tumor scan and clinical response was evaluated. Among these patients, 139 (76.8%) patients got DCR and 42 (23.2%) patients had PD.
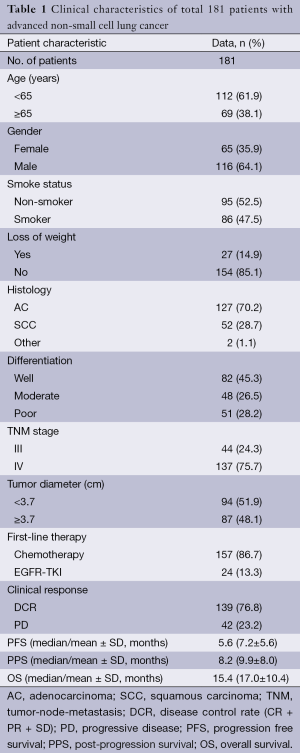
Full table
There were 59 (32.6%) patients survived till April 1st, 2013. In all patients, the median PFS was 5.6 months, the median PPS was 8.2 months and the median OS was 15.4 months.
SUVmax and SUVmax/tumor size analyses
In the study population, the median tumor SUVmax was 8.0 (range, 1.3-25.4), and the median SUVmax/tumor size was 2.2 (range, 0.5-7.5). The distribution of clinical characteristics for SUVmax subgroup and SUVmax/tumor size subgroup is presented in Table 2.
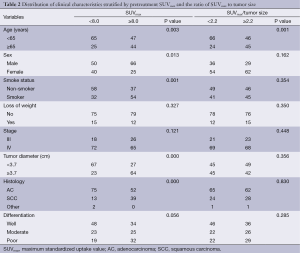
Full table
Patients with pretreatment SUVmax ≥8.0 had a higher prevalence of age ≥65 years (P=0.003), males (P=0.013), smokers (P=0.001), tumor diameter ≥3.7 cm (P=0.000) and squamous cell carcinoma (P=0.000), while patients with higher pretreatment SUVmax/tumor size only tended to be older (≥65 years) (P=0.001).
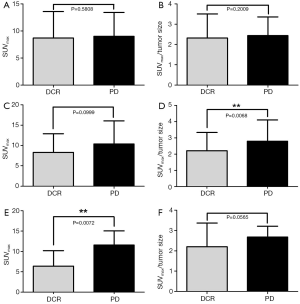
Either the SUVmax or the SUVmax/tumor size were no significantly different in therapeutic response (P=0.5808 and P=0.2009, respectively). In EGFR-TKI subgroup, SUVmax had significant difference between DCR and PD (P=0.0072), while in the group of chemotherapy treatment, SUVmax/tumor size was statistically different in DCR and PD (P=0.0068) (Figure 1).
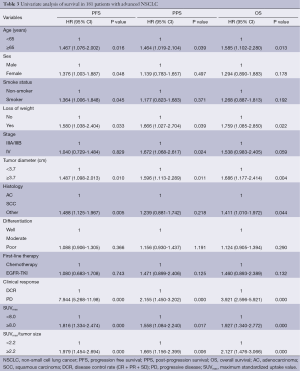
Univariate survival analyses
In univariate analyses, as for the primary outcome OS, age (P=0.013, HR =1.585) and tumor diameter (P=0.004, HR=1.686), loss of weight (P=0.022, HR =1.759), histology (P=0.044, HR =1.411), clinical response (P=0.000, HR =3.921), SUVmax (P=0.001, HR =1.927), SUVmax/tumor size (P=0.000, HR =2.127) were significant prognostic factors (Table 3).
Full table
SUVmax (P=0.000, HR =1.876) and SUVmax/tumor size (P=0.000, HR =1.979) were significant prognostic factors for PFS, together with sex (P=0.048, HR =1.376), age (P=0.016, HR =1.467), smoke status (P=0.045, HR =1.364), loss of weight (P=0.033, HR =1.580), tumor diameter (P=0.010, HR =1.487), histology (P=0.005, HR =1.488), and clinical response (P=0.000, HR =7.944).
And for PPS, age (P=0.039, HR =1.464), loss of weight (P=0.039, HR =1.666), tumor diameter (P=0.011, HR =1.596), stage (P=0.024, HR =1.672), clinical response (P=0.000, HR =2.155), SUVmax (P=0.017, HR =1.558) and SUVmax/tumor size (P=0.006, HR =1.665) were significant prognostic factors.
Kaplan-Meier survival curves showed significant differences in OS, PFS and PPS when patients stratified by SUVmax/tumor size or SUVmax, suggesting that SUVmax/tumor size or SUVmax was correlated with survival in advanced NSCLC patients (Figure 2).
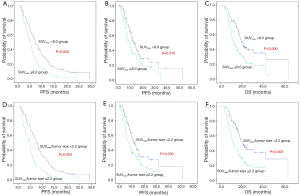
Multivariate survival analyses
The statistical analysis data indicated that only clinical response to first-line therapy (P=0.000, OR =6.555) was independent prognostic factors for PFS, stage (P=0.028, OR =1.673) was associated with PPS independently, and for OS, SUVmax/tumor size ratio (P=0.050, OR =1.656) and clinical response (P=0.002, OR =2.803) were independent prognostic factors (Table 4).
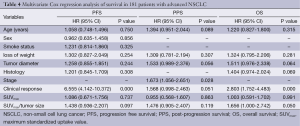
Full table
Discussion
Our study is the first clinical study to evaluate the prognostic value of SUVmax/tumor size in advanced NSCLC patients. SUVmax/tumor size is demonstrated to be significantly correlated with survival of patients in this present study. As a promising functional marker, SUVmax/tumor size is an available factor for predicting outcome in advanced NSCLC patients.
As we known, a tumor did not always have a uniform shape and a homogeneous composition, so tumor diameter could not represent the real tumor burden. Other functional parameters, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were suggested to have prognostic value in previous studies (9,10). MTV and TLG were integrated both tumor volume and biologically relevant metabolic data and was defined as the mean standardized uptake value multiplied by the MTV. However, the volumetric functional assessment could only be made consistently with the advance of image analysis tools and 3-dimensional display techniques. SUVmax/tumor size, taken the real tumor burden the tumor diameter together, is much more simple to perform than MTV or TLG and have the efficiency in clinic, making the result more feasible and credible (11,12).
Stiles et al. (8) provided the evidence in his study that SUVmax/tumor size was a stronger independent predictor of survival than SUVmax alone. However, all the patients enrolled in that study were at an early stage (IA-IIIA). In current research, we demonstrated that SUVmax/tumor size ratio was only affected by age. In survival analysis, SUVmax/tumor size was an independent predictor of OS, PFS and PPS.
In addition, high value of FDG uptake suggested more vigorous tumor cell metabolism and more rapid growth and there were many studies identified the relationship between functional parameters in PET/CT and therapeutic response in several tumors (13). We also analyzed the relationship between SUVmax or SUVmax/tumor size and the response of first-line therapy. However, the correlation was not significant in this study.
This study had its limitations. First, it was retrospective research and study population was from just a single center. Second, the use of EGFR-TKI was proved to be associated with survival in NSCLC (14,15), and 100 patients in our study had used iressa or tarceva, which might affect the survival. Despite these limitations, we included patients strictly and made a relatively large patient cohort and the current study provided important insight into the prognostic importance of pretreatment SUVmax/tumor size.
Taken together, our results is first to demonstrate the SUVmax to primary tumor size in 18F-FDG PET/CT is associated with survival in patients with advanced NSCLC, and might be an important indicator rather than SUVmax alone. Although it was a respective study, our study indicated the potential usefulness of a new predictor for advanced NSCLC patients. To confirm these findings, additional larger, prospective and randomized studies were needed.
Acknowledgements
Funding: This work was supported by grants from the National Natural Scientific Foundation of China (No. 81370172, No. 81302032, No. 81170064 and No. 81401903) and Clinical Science and Technology Project of Jiangsu Province (No. BL2013026).
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:137S-46S. [PubMed]
- Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000;343:254-61. [PubMed]
- Vesselle H, Grierson J, Muzi M, et al. In vivo validation of 3'deoxy-3'-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 2002;8:3315-23. [PubMed]
- Vesselle H, Schmidt RA, Pugsley JM, et al. Lung cancer proliferation correlates with (F-18)fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res 2000;6:3837-44. [PubMed]
- Usuda K, Saito Y, Sagawa M, et al. Tumor doubling time and prognostic assessment of patients with primary lung cancer. Cancer 1994;74:2239-44. [PubMed]
- Arai T, Kuroishi T, Saito Y, et al. Tumor doubling time and prognosis in lung cancer patients: evaluation from chest films and clinical follow-up study. Japanese Lung Cancer Screening Research Group. Jpn J Clin Oncol 1994;24:199-204. [PubMed]
- Stiles BM, Nasar A, Mirza F, et al. Ratio of positron emission tomography uptake to tumor size in surgically resected non-small cell lung cancer. Ann Thorac Surg 2013;95:397-403; discussion 404. [PubMed]
- Huang W, Zhou T, Ma L, et al. Standard uptake value and metabolic tumor volume of 18F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2011;38:1628-35. [PubMed]
- Hyun SH, Choi JY, Kim K, et al. Volume-based parameters of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann Surg 2013;257:364-70. [PubMed]
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Lee HY, Hyun SH, Lee KS, et al. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 2010;17:2787-94. [PubMed]
- Thongprasert S, Duffield E, Saijo N, et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol 2011;6:1872-80. [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [PubMed]


