Robot-assisted thoracoscopic surgery versus thoracotomy for c-N2 stage NSCLC: short-term outcomes of a randomized trial
Introduction
Robot-assisted thoracoscopic surgery (RATS) was first used for non-small cell lung cancer (NSCLC) in 2002 (1,2) and had been rapidly gaining popularity worldwide attributing to its minimal invasiveness. The increasing data show that RATS lobectomy is safe and effective for treating patients with early-stage NSCLC, but fewer and retrospective studies on RATS to treat patients with c-N2 NSCLC require robust evidence to evaluate its safety and effectiveness (3). Thus, this multicenter randomized controlled trial (RCT) was designed in China to identify the safety and effectiveness of RATS for treating patients with c-N2 NSCLC by compared with thoracotomy, which is golden standard for surgical therapy of c-N2 NSCLC patients. The required follow-up period has not yet been reached; data from secondary endpoints were collected and analyzed here to estimate short-term perioperative outcomes of RATS.
Methods
Study design
This noninferiority, phase 3, multi-center RCT was approved by the ethics board of Shanghai Chest Hospital (KS1735) and registered at Chinese Clinical Trial Registry (ChiCTR-INR-17012777). This study was launched in January 2016.
Subjects
The patients aged from 18 to 75 years who were clinically diagnosed c-N2 NSCLC according to American Joint Committee on Cancer Tumor-Node-Metastasis classification exhibited as a suspicious pulmonary lesion with enlarged mediastinal lymph nodes (diameter bigger than 1 cm on computed tomography scan) were considered as candidate (4). The subjects enrolled in this study must enable to give written informed consent, and their organ function must be adequate to tolerate pulmonary resection. The patients were ineligible if they accompanied with invasion into neighboring organs (such as hilum), extensive pleural adhesion, earlier thoracotomy, high-dose radiation on the chest, history of other malignancies in the past 5 years except for nonmelanoma skin cancer, cervix cancer in situ, or early-stage prostate cancer. The ineligibility criteria also included predicted postoperative forced expiratory volume in 1 second (FEV1) or diffusing capacity of lung for carbon monoxide value less than 40%, pregnant or lactating female patients, inability to obtain consent. And, intraoperative pleural adhesion or technical challenge to achieve hemostasis needed conversion from RATS to thoracotomy. Furthermore, the following criteria were defined as exclusion in present study, including occult pleural metastasis; major protocol violation; clinician decides the patient should not continue the trail according to individual condition; patients withdraw from the trial; histologic finding is not NSCLC.
All participants gave written informed consent according to International Conference on Harmonisation (5), and then, were registered by the local investigator in each participating institution on the trial’s website to ensure allocation concealment. Following a list of randomization number generated in the trial statistician’s computer with stratification for the participating center, the subjects enrolled in present study were randomly and equally assigned to RATS and thoracotomy groups. The allocation was done by telephone by the trial coordinator. Neither subjects nor any investigators were masked to treatment allocation.
Preoperative work-ups and perioperative care, surgical procedure
Before the operation, history, physical examination, hematologic and biochemical tests, pulmonary function test, computed tomography of chest and abdomen, brain magnetic resonance imaging and diagnostic bronchoscopy were carried out according to the protocol. In all cases, a radical lobectomy combined with mediastinal lymph node dissection was performed. The RATS was operated using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) and followed the strict definition of the Cancer and Leukemia Group B 39802, which included anatomic lobectomy, visualization only by thoracoscope and non-rib-spreading technique (6) (Figures 1,2). The number of ports as defined in the trial.
On the other hand, subjects in the control group underwent conventional lobectomy with a rib-spreading thoracotomy of 15 to 20 cm. In present study, the hilar and mediastinal lymph nodes were routinely dissected, and a minimum of three mediastinal lymph nodes (N2) stations was harvested. In detail, station 2, 3a, 4, 7, 8, 9, 10, and 11 lymph nodes were dissected in right lobectomy, while station 4, 5, 6, 7, 8, 9, 10 and 11 lymph nodes were dissected in left lobectomy. The occurrence of macroscopic residual cancer in resection margin or unresectable malignant lymphadenopathy was defined as incomplete resection. After surgery, histologic examination was routinely performed to identify at least the histologic type, tumor size, the extent of invasion (pleural, neural, lymph vascular, and bronchial involvement), adequacy of surgical margins, and lymph node metastatic status. Within the first 2 years after treatment, patients were reviewed in outpatient clinic in 3-month intervals. Then, the surveillance was scheduled on a 6-month basis for the next 3 years.
Statistical analysis
Before the operation, clinical characteristics of patients were recorded, including gender, smoking history, comorbidities, FEV1, diffusing capacity for carbon monoxide (DLCO) and tumor location. And, these characteristics were submitted to analyze to identify whether baseline factors were well balanced. In addition, data related to operation were collected and compared statistically, including intervention, operative time (minutes), intraoperative blood loss (mL), chest tube duration (day), drainage at postoperative day one (ml) and total drainage (mL), length of hospital day (LOS), death (within 28 days), complications, visual analogue score (VAS) at postoperative day one to five (POD1-5) and overall cost (¥). Furthermore, pathologic variables were also recorded and analyzed, including histologic subtype, tumor size, pathologic stage, surgical margin, sampled lymph node stations, number of lymph nodes and number of N2 lymph nodes. Data from variables related to perioperative care were not collected because of their potential mild variation among the three centers. To verify difference of complications, grading was used and described as follows. Postoperative hemorrhage of up to 750 mL was grade 1, 750 to 1,500 mL was grade 2, 1,500 to 2,000 mL was grade 3, and more than 2,000 mL was grade 4. Pneumonia was graded based on CURB-65 score [confusion, BUN >19 mg/dL, respiratory rate >30, systolic blood pressure (BP) <90 mmHg or diastolic BP 60 mmHg, age >65] with score 0 to 1 being mild, score of 2 being moderate, and score of 3 or more being severe. Air leak was considered mild when present only on cough, moderate when present with speaking and severe when present with breathing. All the other complications were confirmed by laboratory or radiologic findings.
Statistical analysis was performed by SPSS 16.0 (SPSS Inc. Chicago, IL, USA). The differences between two groups were analyzed using Student t, c2, and Fisher exact tests or Wilcoxon rank-sum test. Data were shown as median (25th to 75th percentile) when they were not normally distributed. Patients who suffered from severe adverse events may be taken off the trial for safety but will continue their follow-up for analysis of primary endpoints. The two-sided P value of less than 0.05 was considered as statistically significant.
Results
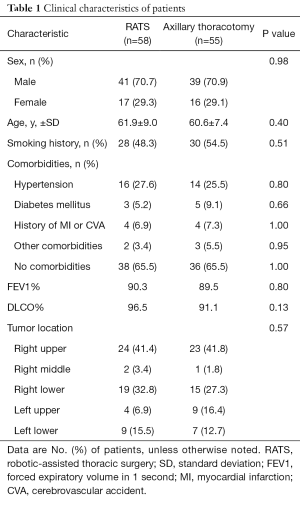
This trial was performed in three leading thoracic referral centers in China from January 2016 to December 2018. Among total of 113 recruited patients, 90 participants were from Shanghai Chest Hospital, 13 patients were from Shanghai Ruijin Hospital, and 10 subjects were from Shanghai Huadong Hospital. By a list of randomization number, 58 subjects were assigned to the RATS group, and 55 patients were assigned to axillary thoracotomy group. In present study, RATS surgery was converted to thoracotomy in five subjects due to extensive pleural adhesion (two cases) and equipment issues (three cases). Thus, data from 53 cases in RATS and 55 cases in axillary thoracotomy were collected to analysis. The mean age of patients was 61.9 years in the RATS group and 60.6 years in the axillary thoracotomy group (P=0.40). The significant difference was undetectable in term of gender, body surface, smoking status, pulmonary disorders, and other comorbidities (Table 1). In other word, baseline factors were well balanced between the two groups.
Full table
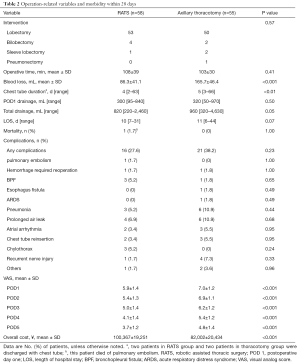
The distribution of different pulmonary resections in RATS group was comparable to those in axillary thoracotomy group (P=0.57). In all cases, the mediastinal lymph node was completely dissected. The median operative time was 108 minutes in the RATS group and 103 minutes in axillary thoracotomy group (P=0.41). However, aid of da Vinci Surgical System led to lower intraoperative blood loss (P<0.001), chest tube duration (P<0.01) and VAS at postoperative day one to five (POD1-5) (P<0.001). Addition, robotic aid generated lower pleural drainage at postoperative day one and total pleural drainage (P=0.05). The median days of hospitalization in RATS patients were slight less than those in axillary thoracotomy subjects, but statistical significance was undetected. Apart from one subject who died of pulmonary embolism, no operation-related death was documented for the whole cohort. As shown in Table 2, morbidities of postoperative complications in subjects who underwent RATS were not statistically less than those in participants underwent axillary thoracotomy (P=0.23).
Full table
Further analyses that included the five conversions in the RATS group were performed, and the results were in line with those that excluded the conversions (Table 2). Postoperative examination showed that adenocarcinoma was dominant histologic subtype in both groups, followed by squamous cell carcinoma. Statistical difference was not present in the distribution of pathologic stages (P=0.72). Incidental stage III disease was identified in 20 patients (35.1%) in RATS group and 26 (49.0%) subjects in axillary thoracotomy group, and one stage IV patient in RATS group was intraoperatively found to be adenocarcinoma of with pleural seeding. Moreover, cancer residual margins were found in one subject who underwent RATS and three patients who underwent axillary thoracotomy (P=0.56). Both surgical approaches generated comparable outcomes in sampled lymph node station (P=0.31), number of lymph node (P=0.79) and number of N2 lymph nodes (P=0.38) (Table 3).
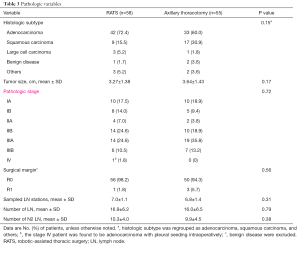
Full table
Discussion
Earlier evaluation of robotic-assisted lobectomy for lung cancer has documented that surgical robot can confer decreased incidence of complications and enhanced outcomes of various statistical indictors during intraoperative and perioperative period (6-16). Park et al. found that robotic surgery holds unique advantages for patients suffered from locally advanced NSCLC and achieve disease-free survival rate and overall survival rate similar to open surgery (17). Another study showed that surgical robot could remove 9.4cm lung cancer tissue under the condition of negative incision margin and thorough systematic lymph node dissection (18). Pieces of evidence above mentioned suggested that RATS should be feasible for local advanced NSCLC. However, feasibility of RATS for c-N2 NSCLC needs to be evaluated. Thus, outcomes of RATS lobectomy for c-NSCLC were compared to axillary thoracotomy in this multicenter RCT.
This trial revealed that usage of da Vinci Robot Surgical System resulted in lesser intraoperative blood loss, which reflects the difficulty and proficiency of the operation, as well as the severity of the trauma. This advanced outcome should attribute to better immunization and full exposure of anatomical structures and usage of disposable cutting and suturing instruments.
The main complications occurred in two cohorts were a pulmonary infection, atrial fibrillation, and air leakage. Earlier studies showed that RATS was associated with relieving pain, reduced phlegm, diminished inflammatory factors and reduced pulmonary infection. Moreover, clearer 3D visualization and exposure under RATS enable to reduce the damage of blood vessels and lung tissues, thereby, reducing the chances of atrial fibrillation and air leakage. However, the abovementioned complication occurred in patients who underwent RATS surgery and displayed comparable incidence to those underwent axillary thoracotomy, which could be variance of small samples.
During lobectomy for lung cancer, the lymph node was previously suggested to be sampled according to different lobe. Only suspected metastatic lymph nodes should be excised and fast-frozen pathological assay to determine the extent of dissection. However, complexity of lymphatic drainage in the lung could lead to micro-metastasis and jumping metastasis, therefore, systematic lymph nodes dissection is a preferred choice. And, lymph node sampling did not improve the complications of surgery. So, direct dissection of systematic mediastinal lymph node was performed in both surgical operations.
The thoroughness of lymph node dissection is pivotal to the comprehensive treatment of lung cancer. It can stage, judge the prognosis, guide the next step of treatment, improve the local control rate, and prolong the disease-free survival time. However, capability of surgical robot dissects thoroughly mediastinal lymph nodes comparable to thoracotomy has always been the focus of debate, especially to the cases of cN2 NSCLC. An earlier cadaver study indicated that thorough lymph node dissection was carried out in VATS followed by conventional thoracotomy (19). Another study showed that only 2% to 3% of the remaining lymph nodes were cleaned by VATS and then further thoracotomy (20). Various studies have documented that VATS can confer the similar capability of mediastinal lymph node dissection compared to thoracotomy, including number of dissections and the number of groups (21). Here, we found that RATS and axillary thoracotomy generated same outcome of lymph node dissection, suggested that surgical robot can thoroughly dissect the lymph nodes as thoracotomy does. So, further thoracotomy was unnecessary for dissection of mediastinal lymph nodes.
Unplanned intraoperative conversion to thoracotomy is an essential issue for the feasibility of RATS. The meta-analysis by Liang et al. (22) indicated that conversion rate to open surgery was significantly lower in patients who underwent RATS than those underwent VATS [10.3% vs. 11.9%; odds ratio (OR) 0.57, P<0.001]. In this trial, five subjects who initially underwent RATS were converted to axillary thoracotomy due to either of extensive pleural adhesion or equipment issues, but not due to intraoperative bleeding. The conversion to open surgery in this trial occupied 8.62% of cases, which was slightly lower earlier analysis. These findings suggested that unplanned intraoperative conversion would be rare event and should not withhold the surgeons from adopting the new techniques with complete establishment of learning curve for RATS lobectomy and standardized procedure.
To our knowledge, this phase 3 multicenter clinical trial is the first randomized study to evaluate the safety and short outcomes of RATS in treatment of cN2 NSCLC by comparing to axillary thoracotomy. It should be noticed that superiority of RATS for quicker recovery might be concealed in this study because the “length of stay” in this study did not reflect patients’ true recovery from operation. Preoperative workups and assessment of protocol before operation took additional days and included in days of hospitalization. Also, analysis together data from five conversion subjects with other RATS patients did not change perioperative outcomes but should be negative factors for other outcomes, such as postoperative pain, acute inflammatory reaction, respiratory function, and quality of life. The “modified intension-to-treat analysis” that allow exclusion of the conversion patients for sub analysis of our clinical trial should be done in further.
In conclusion, RATS is safe and effective to treat patients with cN2 NSCLC owing to its similar short-term outcomes of thorough dissection of lymph node and occurrence of postoperative complications compared to axillary thoracotomy and may be superior due to its lesser intraoperative blood loss. However, long-term follow-up is warranted to verify the superior or equivalent oncologic outcome of RATS lobectomy. The report could be expected by the end of 2020.
Acknowledgments
Funding: This work was supported by Shanghai Hospital Development Center (Grant Number: SHDC12016113), National Natural Science Foundation of China (No. 81702251).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics board approved the study of Shanghai Chest Hospital (KS1735). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Melfi FM, Ambrogi MC, Lucchi M, et al. Video robotic lobectomy. Multimedia Man Cardiothorac Surg 2005;2005:mmcts.2004.000448.
- Kim HK, Choi YS, Kim J, et al. Outcomes of unexpected patho1ogic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:1288-93. [Crossref] [PubMed]
- Greene FL, Page DL, Fleming ID, et al. Lung. In: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002:165-77.
- Branch SK. Guidelines from the International Conference on Harmonisation (ICH). J Pharm Biomed Anal 2005;38:798-805. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Videoassisted thoracic surgery lobectomy: report of CALGB 39802–a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 2004;25:844-51. [Crossref] [PubMed]
- Zhao X, Qian L, Lin H, et al. Robot-assisted lobectomy for nonsmall cell lung cancer in China: initial experience and techniques. J Thorac Dis 2010;2:26-8. [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve andcomplications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Anderson CA, Hellan M, Falebella A, et al. Robotic-assisted lung resection for malignant disease. Innovations (Phila) 2007;2:254-8. [Crossref] [PubMed]
- Giulianotti PC, Buchs NC, Caravaglios G, et al. Robot-assisted lung resection: outcomes and technical details. Interact Cardiovasc Thorac Surg 2010;11:388-92. [Crossref] [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-13. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Hoksch B, Ablassmaler B, Walter M, et al. Radical thoracoscopic lobectomy with lymphadenectomy in a cadaver model. Can J Surg 2002;45:376-80. [PubMed]
- Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect. Ann Thorac Surg 2002;73:900-4. [Crossref] [PubMed]
- Watanabe A, Mishina T, Ohori S, et al. Is video-assisted thoracoscopic surgery a feasible approach for clinical NO and postoperatively pathological N2 non-small cell lung cancer. Eur J Cardiothorac Surg 2008;33:812-8. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]